Abstract
Relative cerebral blood flow (CBF) < 30% has been identified as a predictor of infarct core on computed tomography perfusion (CTP). We investigated the relationship between CTP-predicted infarct core and diffusion-weighted imaging magnetic resonance imaging (DWI MRI). We conducted a retrospective analysis comparing infarct core (CBF < 30%; RAPID iSchemaView) and post-revascularization DWI MRI (ADC < 620 cc; RAPID iSchemaView) in patients with internal carotid artery (ICA) or proximal middle cerebral artery (MCA) stroke between November 2016 and May 2019. Included subjects had a modified Thrombolysis in Cerebral Infarction (mTICI) score of 2b or better and presented within 24 h of last known well (LKW) time. Two hundred one cases were identified. Mean duration from LKW time to CTP and MRI was 4.3 and 28.6 h, respectively. Median ischemic core volume was 8 cc, and median MRI infarct volume was 17 cc. CTP core volume showed fair correlation with MRI infarct volume (r = 0.294, p < 0.0001). There was a stronger association between CBF < 30% and DWI MRI in subjects presenting beyond 6 h (r = 0.359, p = 0.011). In a multivariate analysis, greater volumetric difference was associated with younger age (p = 0.001), longer duration from LKW time to revascularization time (p < 0.020), and longer CTP to revascularization time (p < 0.0001). Reduced relative CBF < 30% is a fair measure of infarct size within 24 h of anterior circulation, large artery occlusion (LAO) stroke when adequate reperfusion is achieved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Randomized clinical trials have demonstrated the efficacy of endovascular therapy (EVT) for patients with large artery occlusion (LAO) acute ischemic stroke [1,2,3]. Two early window stroke intervention trials that established the benefit of EVT excluded patients with large core infarct sizes from enrollment [4, 5]. More recently, the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) and DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo (DAWN) studies utilized estimates of ischemic core volume on computed tomography perfusion (CTP) to determine radiographic and clinical mismatch, respectively, for patient selection [2, 3]. Both studies demonstrated high positivity in favor of EVT versus standard medical therapy with astonishingly low numbers needed to treat.
Automated CTP technology offers the potential to efficiently distinguish between irreversibly damaged brain tissue and at-risk, potentially salvageable brain tissue. Reduced relative cerebral blood flow (CBF) can estimate ischemic core size, and values less than 30% have been used for patient selection in clinical trials [2,3,4,5]. While an accurate assessment of ischemic core size is essential prior to EVT between 6 and 24 h, it also may be used as a factor in thrombectomy decisions within 6 h of last known well (LKW) time [6]. In addition, CTP-based estimates of core infarct size have been associated with functional outcome after stroke [7].
Prior studies have demonstrated that reduced relative CBF of less than 30% accurately estimates final infarct volume on diffusion-weighted imaging magnetic resonance imaging (DWI MRI) [8], while others have shown ischemic core overestimation with the same parameter, illustrating the so-called “ghost core infarct” concept [9, 10]. A more recent trial utilized prospectively collected data from the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials (HERMES) and the Tenecteplase Versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK) trials to retrospectively determine DWI and CTP CBF < 30% differences in patients who achieved early, good revascularization of a LAO [11]. CTP core overestimation was rare, and median core underestimation exceeded 25 cm3 (cc). Larger baseline ischemic core volume correlated with larger differences in ischemic core underestimation.
Given its importance with respect to both endovascular treatment candidacy and its association with patient outcomes, we investigated the relationship between CTP-based estimated ischemic core volume and MRI infarct volume in patients with good revascularization of an anterior circulation LAO ischemic stroke within 24 h of LKW time.
Methods
Between November 2016 and May 2019, we conducted a retrospective analysis of patients from a large healthcare system’s stroke network database. We compared core infarct size on CTP (CBF < 30%; RAPID iSchemaView) and post-revascularization DWI MRI (ADC < 620 cc; RAPID iSchemaView) in patients with internal carotid artery (ICA) or middle cerebral artery (MCA), M1 occlusions who achieved good reperfusion.
All data were collected prospectively in a code stroke registry. Subjects were required to have baseline CTP and follow-up MRI scans that were of adequate quality for interpretation. Patients were selected for mechanical thrombectomy based on the endovascular therapy guideline for the healthcare system, which closely mirror those recommended by the American Heart Association/American Stroke Association [6] (Table 1). Post-thrombectomy reperfusion was determined by neuro-interventionalist calculated modified Thrombolysis in Cerebral Infarction (mTICI) scores. Subjects were included if they achieved greater than 50% reperfusion in the affected vascular territory (mTICI 2b, 2c, or 3).
All patient characteristics were de-identified. Study approval was obtained from the Atrium Health Institutional Review Board (IRB), the IRB of the healthcare system’s certified Comprehensive Stroke Center, Carolinas Medical Center (Charlotte, NC). Due to the retrospective study design and use of de-identified data, the requirement for patient consent was waived by the IRB.
Statistical Analysis
Descriptive statistics were reported for demographic and clinical characteristics, including initial National Institutes of Health Stroke Scale (NIHSS) score, comorbidities, intervention therapy, location of LAO, duration from LKW time to imaging/final revascularization, and imaging at baseline and post-intervention (CTP ischemic core volume and MRI infarct volume). The volumetric difference was calculated by subtracting the CTP core infarct size from the post-revascularization DWI MRI volume, with the absolute volumetric difference as the absolute value of the volumetric difference in cc. CTP core infarct sizes that were greater/less than the post-revascularization DWI MRI were defined as an overestimation/underestimation, respectively. Pearson correlations for baseline core infarct size on CTP versus post-intervention infarct volume and volumetric difference were performed. Similarly, Pearson correlations between volumetric difference and duration between LKW to imaging time points were performed, stratified by overestimation and underestimation of final core infarct volume. Finally, multivariable linear regression models were fitted to examine the association between duration from important clinical time points to final revascularization with volumetric difference while adjusting for age, sex, and baseline core infarct volume.
Results
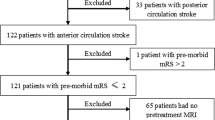
Demographic and clinical characteristics are listed in Table 2. Two hundred one patients met the inclusion/exclusion criteria for the study (Fig. 1). Fifty-two percent were men, median NIHSS was 16, and over 50% were treated with IV alteplase and EVT. Seventy-one percent had an MCA occlusion, 6% an ICA occlusion, and 23% had tandem ICA and MCA occlusions.
One hundred fifty-two subjects presented in the 0–6-h time window, and 49 presented beyond 6 h. CTP and MRI images obtained an average of 4.3 and 28.6 h after LKW time, respectively (Table 2). Revascularization achieved an average of 6.3 h from LKW time (Table 2).
Median CTP ischemic core volume was 8 cc and median MRI infarct volume was 17 cc (Table 3). Median volumetric difference was 9 cc. CTP core underestimation occurred in approximately 63% of subjects, while overestimation was less common, occurring in 22% (Table 3). CTP core size matched MRI infarct volume in the remaining 15%. CTP core size was within 5 cc of MRI infarct volume in 28% of subjects, while 58% of subjects had core size within 20 cc of MRI infarct volume (Table 3).
For the subset of patients with mTICI 2c or TICI 3 scores (124 patients), median CTP ischemic core volume was 5 cc and median final MRI infarct volume was 9 cc. Infarct volume underestimation (51%) was more common than overestimation (27%). Infarct volume was within 5 cc, 10 cc, and 20 cc in 38%, 51%, and 68% of patients, respectively. For the 115 patients achieving mTICI 3, median CTP core volume was also 5 cc and median final MRI infarct volume was 9 cc.
For all subjects, CTP core volume demonstrated fair positive correlation with MRI infarct volume (r = 0.294, p < 0.0001; Table 4). In the 6–24-h time window, the correlation between the two infarct measurements was stronger (r = 0.359; p = 0.0114), though remained in the fair range.
In a multivariate analysis, greater volumetric difference between MRI and CTP volumes was associated with longer duration from LKW time to revascularization time (p = 0.020) and CTP time to revascularization time (p < 0.0001; Table 5). Greater volumetric difference was also associated with younger age (p = 0.001).
An illustrative case example is shown in Fig. 2.
A 61-year-old man with a history of multiple vascular risk factors presented 79 min after his last known well (LKW) time with a left middle cerebral artery stroke syndrome. His initial NIHSS score was 23.
Head CT showed no acute process. CTA head/neck showed distal L MCA, M1 occlusion.
CT perfusion showed core infarct (CBF < 30%, RAPID iSchemaView) of 86 cc and mismatch of 81 cc (above panel).
He was treated with IV alteplase and taken for endovascular therapy, with TICI 3 revascularization achieved less than 3 h from LKW time.
Follow-up brain MRI obtained the next day showed L MCA infarct size of 91 cc (below panel).
Volumetric difference between estimate of his infarct core (CBF < 30%) and infarct size measured by diffusion-weighted MRI was 5 cc.
His strength improved though he was left with a primarily nonfluent aphasia.
Discussion
We compared CTP-based estimates of ischemic core size, represented by relative reduced cerebral blood flow (CBF < 30%), to the gold-standard infarct size measurement of diffusion-weighted MRI (ADC < 620 cc) in patients with successful endovascular reperfusion of an ICA and/or proximal MCA occlusion within 24 h of stroke onset. Our study demonstrates that CTP ischemic core volume has a fair correlation with MRI infarct volume in this subset of patients.
To our knowledge, this is the largest series to investigate the accuracy of CTP-based estimates of infarct volume. In addition, our study is the first to include EVT patients within 24 h of LKW time, adding to the existing literature in earlier stroke time windows.
Previously, Albers et al. [8] demonstrated accurate infarct volume estimates with CTP in subjects enrolled in SWIFT-PRIME. More recently, in a cohort of 59 patients enrolled in DEFUSE 3 who achieved > 90% revascularization, baseline core infarct volumes showed a strong correlation with 24-h infarct volumes [11].
Our results may be explained by infarct growth after CTP. Subjects enrolled in SWIFT-PRIME and DEFUSE 3 were selected by evidence of salvageable brain tissue on CTP, and thus likely had good collateral blood flow status in order to meet study inclusion criteria [12]. Despite including EVT subjects within 24 h, our series included a large number of patients in earlier time windows (mean LKW to revascularization time of 6.3 h; > 75% presenting within 6 h). Patients in our series were selected for EVT based on our healthcare system guideline, which is in accordance with national guidelines that do not include a requirement for salvageable brain tissue on CTP in the 0–6-h time window in order to determine EVT candidacy [6]. Thus, subjects in the early time window included those with both favorable and unfavorable collaterals. As “fast-growing” infarcts are more likely to be present in earlier time windows in patients without advanced imaging selection [12], it is expected that infarct growth was more likely to occur, and at a more rapid pace, in our series than in SWIFT-PRIME or DEFUSE 3. The absence of advanced imaging selection in patients presenting early and the large percentage of 0–6 h patients likely led to infarct growth and a weaker association between CBF < 30% and MRI DWI in our study than previously reported. Moreover, a stronger association between CBF < 30% and DWI MRI is seen in subjects presenting beyond 6 h (Table 4).
Additional supporting evidence for the concept of infarct growth is demonstrated in Table 5. Greater volumetric difference between CTP and MRI strongly correlated with increased LKW to revascularization time and CTP to revascularization time. The greater the time to revascularization, the greater the potential time for infarct growth, increasing volumetric difference. Future efforts may focus on improving imaging to revascularization times, particularly in patients transferred for EVT.
Infarct growth may occur before or after reperfusion, and the concept of variable infarct growth has been demonstrated on MRI imaging [13]. In a substudy of patients enrolled in DEFUSE 2, patients who even had > 90% reperfusion were shown to have a precipitous increase in DWI volume over the initial 20 h after stroke onset [13]. Moreover, infarct growth rate was highest in patients with an unfavorable perfusion profile compared to those with a favorable profile, in keeping with the concept of “fast” versus “slow”-growing infarcts [12].
Median infarct volumetric difference was 9 cc in our study, compared to 13 cc [8], 21.3 cc [11], and 25.4 cc [14] per other groups who utilized the same CBF and DWI threshold parameters. Hoving et al. found that infarct overestimation was uncommon [14]. We similarly found that infarct size underestimation with CTP was more common than overestimation. In line with CTP only showing a fair correlation with DWI MRI in our study, CTP core size was within 10 cc of MRI infarct volume only 40% of the time and within 20 cc in 58% of subjects. Interquartile range for volumetric difference in our study was large, 35 cc. In subjects achieving mTICI 2c or 3, CBF < 30% more closely matched final DWI infarct volume, as we might expect given the reduced likelihood for infarct growth with improved reperfusion.
An area of interest in endovascular stroke care has been the value of EVT for patients presenting early with large, predicted core infarctions on CTP. Although infarct underestimation was more common than overestimation in our study, randomized trials without advanced imaging inclusion criteria demonstrated positivity in favor of thrombectomy [1]. In addition, our study demonstrated that CTP ischemic core volume showed poor correlation with both volumetric difference and absolute volumetric difference (Table 4). In line with guideline recommendations [6] and recommendations from other groups [7], the presence of a large core infarction should not exclusively exclude patients from receiving EVT in early time windows.
Linear regression analysis in our study demonstrated that increasing age was associated with lower volumetric difference (Table 5). While older age has been linked to poor collateral blood flow status in a series of patients with MCA occlusion [15], age has not otherwise been definitely and independently associated with worse collateral status. In our series, patients in the 6–24 h group had a higher median age than those in the 0–6 h group (69 versus 63). Thus, older patients with favorable collaterals were included in our series by meeting the healthcare system endovascular therapy guideline for late window thrombectomy, whereas younger patients enrolled in the 0–6 h window with no requirement for evidence of salvageable brain tissue on CTP had a higher likelihood of having fast-growing infarcts. This may have contributed to older patients having reduced infarct growth prior to revascularization and follow-up MRI, and consequently a stronger association with lower volumetric difference.
Our study has several limitations. Patients were selected for mechanical thrombectomy based on endovascular therapy guidelines for our healthcare system, which closely reflect recommendations from the AHA/ASA. While this limits the subjects studied to those who only received EVT, our purpose was to specifically investigate patients who achieved good revascularization, and this was unlikely to be achieved in the absence of treatment with EVT. As infarct core measurements are meant to represent an estimate of irreversibly damaged brain tissue, comparisons made to DWI MRI after revascularization are thus meant to compare a pre-intervention estimate of infarcted tissue (CBF < 30%) to the gold-standard measurement of infarct size (DWI MRI).
The time delay between initial CTP imaging and revascularization may have led to infarct growth, although mean time between CTP and revascularization in our study was 1.9 h, comparable to other studies [11, 14]. In addition, more delayed MRI imaging might have produced larger infarct sizes, given the potential for infarct growth with increased time from stroke onset [13]. MRI infarct volume assessments are limited by a threshold of ADC < 620, and some areas may simply not be detected due to software limitations.
Various other parameters, namely, different CBF and cerebral blood volume (CBV) thresholds [16,17,18,19,20,21,22,23,24,25], have been investigated as a measure of predicted infarct volume. Notably, CBF < 38% has been demonstrated to correlate strongly with DWI infarct volume, though median time from stroke onset to CT in that series was 185 min, and MRI was performed a median of 36 min after CT [16]. As previously described, more delayed imaging with MRI has the potential to demonstrate larger DWI volume, compared to earlier imaging, even when good revascularization is achieved [13]. We chose to specifically investigate the threshold of CBF < 30%, as this parameter has been used in multiple randomized, clinical trials as an estimate of ischemic core size and part of inclusion criteria for trial enrollment [2,3,4,5].
Infarct growth is impacted by collateral blood flow status, which was not assessed in our study. It has recently been demonstrated that a marker of collaterals on CTP, the hypoperfusion intensity ratio, may be able to quantify the degree of infarct growth [26]. Future studies may investigate the relationship between collateral blood flow status and time on infarct growth using different imaging modalities. Lastly, our study is subject to the inherent limitations of a retrospective analysis.
Conclusion
In our series, reduced relative CBF < 30% showed a fair correlation with MRI DWI volume within 24 h of anterior circulation, LAO stroke when adequate reperfusion was achieved. There was a stronger association between the two estimates in patients presenting in later time windows. When discrepancies existed between CTP and the gold-standard of DWI MRI, CTP underestimation was more common than overestimation. Younger age and longer duration from LKW time and CTP time to revascularization were associated with greater difference between infarct size estimates, which may be related to infarct growth. Additional studies may focus on collateral blood flow status, age, and their relationship to estimates of infarct size.
Data Availability
All data were de-identified and gathered in a code stroke registry for the healthcare system. All data relevant to the study are included in the article. Any additional data are available upon reasonable request. Please address any requests to: Jeremy.Rhoten@atriumhealth.org or Rahul.Karamchandani@gmail.com.
References
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31.
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18.
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21.
Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95.
Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019:STR0000000000000211.
Campbell BCV, Majoie CB, Albers GW, Menon BK, Yassi N, Sharma G, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019;18:46–55.
Albers GW, Goyal M, Jahan R, Bonafe A, Diener H-C, Levy EI, et al. Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann Neurol. 2016;79:76–89.
Martins N, Aires A, Mendez B, Boned S, Rubiera M, Tomasello A, et al. Ghost infarct core and admission computed tomography perfusion: redefining the role of neuroimaging in acute ischemic stroke. Interv Neurol. 2018;7:513–21.
Boned S, Padroni M, Rubiera M, Tomasello A, Coscojuela P, Romero N, et al. Admission CT perfusion may overestimate initial infarct core: the ghost infarct core concept. J Neurointerv Surg. 2017;9:66–9.
Rao V, Christensen S, Yennu A, Mlynash M, Zaharchuk G, Heit J, et al. Ischemic core and hypoperfusion volumes correlate with infarct size 24 hours after randomization in DEFUSE 3. Stroke. 2019;50:626–31.
Albers GW. Late window paradox. Stroke. 2018;49:768–71.
Wheeler HM, Mlynash M, Inoue M, Tipirnini A, Liggins J, Bammer R, et al. The growth rate of early DWI lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion. Int J Stroke. 2015;10:723–9.
Hoving JW, Marquering HA, Majoie CBLM, Yassi N, Sharma G, Liebeskind DS, et al. Volumetric and spatial accuracy of computed tomography perfusion estimated ischemic core volume in patients with acute ischemic stroke. Stroke. 2018;49:2368–75.
Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. 2013;74:241–8.
Cereda CW, Christensen S, Campbell BCV, Mishra NK, Mlynash M, Levi C, et al. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a DWI standard. J Cereb Blood Flow Metab. 2016;36:1780–9.
Bivard A, Levi C, Spratt N, Parsons M. Perfusion CT in acute stroke: a comprehensive analysis of infarct and penumbra. Radiology. 2013;267:543–50.
Bivard A, Spratt N, Levi C, Parsons M. Perfusion computer tomography: imaging and clinical validation in acute ischaemic stroke. Brain. 2011;134:3408–16.
Bivard A, McElduff P, Spratt N, Levi C, Parsons M. Defining the extent of irreversible brain ischemia using perfusion computed tomography. Cerebrovasc Dis. 2011;31:238–45.
Campbell BCV, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–40.
Bivard A, Levi C, Krishnamurthy V, Hislop-Jambrich J, Salazar P, Jackson B, et al. Defining acute ischemic stroke tissue pathophysiology with whole brain CT perfusion. J Neuroradiol. 2014;41:307–15.
Campbell BCV, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke. 2012;43:2648–53.
Kamalian S, Kamalian S, Maas MB, Goldmacher GV, Payabvash S, Akbar A, et al. CT cerebral blood flow maps optimally correlate with admission diffusion-weighted imaging in acute stroke but thresholds vary by postprocessing platform. Stroke. 2011;42:1923–8.
Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–85.
Schaefer PW, Souza L, Kamalian S, Hirsch JA, Yoo AJ, Kamalian S, et al. Limited reliability of computed tomographic perfusion acute infarct volume measurements compared with diffusion-weighted imaging in anterior circulation stroke. Stroke. 2015;46:419–24.
Guenego A, Mlynash M, Christensen S, Kemp S, Heit JJ, Lansberg MG, et al. Hypoperfusion ratio predicts infarct growth during transfer for thrombectomy. Ann Neurol. 2018;84:616–20.
Author information
Authors and Affiliations
Contributions
Conceptualization: Rahul R. Karamchandani, Jeremy B. Rhoten, Dale Strong, Jeffrey Bodle, Andrew U. Hines, and Andrew W. Asimos. Methodology: Rahul R. Karamchandani, Jeremy B. Rhoten, and Dale Strong. Data curation: Dale Strong and Jeremy B. Rhoten. Formal analysis: Dale Strong and Brenda Chang; Writing—original draft: Rahul R. Karamchandani. Writing—review and editing: Rahul R. Karamchandani, Jeremy B. Rhoten, Dale Strong, Brenda Chang, Gary Defilipp, Joe Bernard, Jonathan D. Clemente, Eric Wang, Ross Bellavia, William Stetler, Jeffrey Bodle, Andrew U. Hines, and Andrew W. Asimos. Supervision: Andrew W. Asimos.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Jonathan Clemente serves as a Clinical Trainer and Member of the Medical Advisory Board of IschemaView (Redmon, CA). Dr. Rahul R. Karamchandani, Jeremy B. Rhoten, Dale Strong, Brenda Chang, Dr. Gary Defilipp, Dr. Joe Bernard, Dr. Eric Wang, Dr. Ross Bellavia, Dr. William Stetler, Dr. Jeffrey Bodle, Dr. Andrew U. Hines, and Dr. Andrew W. Asimos declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Due to the retrospective study design and use of de-identified data, the requirement for informed consent was waived by the IRB.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Karamchandani, R.R., Rhoten, J.B., Strong, D. et al. Computed Tomography Perfusion Core Infarct Measurement Compared to Diffusion-Weighted Magnetic Resonance Imaging in Patients with Revascularization of Anterior Circulation, Large Artery Occlusion Ischemic Stroke. SN Compr. Clin. Med. 2, 2730–2737 (2020). https://doi.org/10.1007/s42399-020-00651-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-020-00651-z