Abstract
This study was aimed at establishing whether magnetic resonance angiography (MRA) can be applied to planning and performing surgery on ruptured intracranial aneurysms, especially in the early phase, without recourse to intra-arterial digital subtraction angiography (IA-DSA). From February 1998 to August 2001, in all patients presenting with a subarachnoid hemorrhage, MRA was performed first. A three-dimensional time-of-flight MRA protocol with T2-weighted coronal and axial images was used. If MRA demonstrated an aneurysm, surgery was undertaken. IA-DSA was limited to patients with negative or inconclusive MRA findings. We compared MRA images with operative findings in positive patients and with IA-DSA in negatives. IA-DSA was considered the gold standard when MRA findings were inconclusive. In this study, 205 consecutive patients (mean age 52.7 years, 69% women) were included. In 133 patients (64.9%) MRA demonstrated an aneurysm, directly followed by neurosurgical intervention. In 33 patients (16.1%) MRA findings were categorized as inconclusive. In 39 patients (19.0%) MRA results were negative. No false-negative ruptured aneurysms were selected by MRA. In only one patient surgical intervention was performed based on false-positive MRA findings. MRA can replace IA-DSA as a first diagnostic modality in the selection of patients suitable for surgical treatment of ruptured intracranial aneurysms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracranial aneurysms are acquired lesions that are most commonly located at the branching points of the major arteries coursing through the subarachnoid space at the base of the brain [1]. Autopsy and angiographic studies indicate that between 3.6% and 6% of the population harbor an intracranial aneurysm [2].
Subarachnoid hemorrhage (SAH), mostly due to rupture of an intracranial aneurysm, has an incidence of 6–8 per 100,000 person years, peaking in the sixth decade of life and accounting for a quarter of cerebrovascular deaths [3]. Although the management of patients with ruptured intracranial aneurysms has passed through a phase of rapid evolution and modification, the overall morbidity and mortality rates following aneurysm rupture remain high [4, 5]. One of the most important objectives in the management of aneurysmal SAH is to prevent aneurysm re-bleeding and delayed cerebral ischemia caused by arterial vasospasm. Most neurosurgeons, therefore, justify early detection and surgical obliteration of any aneurysm, especially in patients in a good clinical state [6].
Because of its unsurpassed resolution, intra-arterial digital subtraction angiography (IA- DSA) is the gold standard in diagnosing intracranial aneurysms. IA-DSA has been the imaging modality of choice for many years, because it is reliable in the detection of aneurysms or other sources of hemorrhage, and because it can be used as a treatment tool. The latter is particularly advantageous since the recent publication in Lancet of results from the international subarachnoid aneurysm trial (ISAT) [7], showing that endovascular treatment should be the first line of treatment for ruptured cerebral aneurysms that have a suitable morphology.
Despite these advantages of IA-DSA and despite modern advances in the performance of cerebral angiography [8–12], the risk of neurological complications associated with this procedure is not negligible [13–21]. With the development of three-dimensional (3D) magnetic resonance angiography (MRA) and computerized tomographic angiography (CTA), the absolute reliance on IA-DSA for aneurysm detection and surgical planning is changing. Both diagnostic modalities have been used successfully as an alternative to IA-DSA for the surgical management of aneurysmal subarachnoid hemorrhage [22–26]. In the present study, we investigated whether (a) 3D time-of-flight (TOF) MRA is a reliable assessment tool in detecting or ruling out ruptured intracranial aneurysms; and whether (b) 3D TOF MRA can replace IA-DSA in the selection of patients suitable for surgical treatment of ruptured intracranial aneurysms. Furthermore, we checked the clinical outcome of the patients with an MRA-based diagnosis of ruptured aneurysm.
MRA was used as the initial imaging tool in acute SAH for several reasons. During the study period, we had no ability to perform CTA at our department. Furthermore, our study was performed before the results of ISAT were published, and it was not yet known that coiling is the first line of treatment for ruptured aneurysms, which favors first performing IA-DSA instead of MRA. Besides that, in our hospital most ruptured intracranial aneurysms are still treated surgically, and endovascular coiling is restricted to a highly selected patient population, mainly patients with aneurysms of the basilar artery.
Material and methods
Patient population
University Hospital Groningen is the reference center for intracranial aneurysm surgery for the northern provinces of the Netherlands. Starting in February 1998, all patients admitted to our hospital with an SAH consecutively underwent MR angiography as a first diagnostic tool. MRA was performed to select patients suitable for surgical clipping of a ruptured intracranial aneurysm. SAH was suspected on clinical grounds and confirmed by un-enhanced computed tomography (CT) and/or demonstration of blood and blood pigments by lumbar puncture. The study included 205 patients: 63 men and 142 women, with ages ranging 10–79 years (mean 52.7 years (+/- 12.4 SD). The clinical condition at admission of each patient presenting with SAH was categorized according to the original Hunt and Hess grading system [27]. When the condition of the patient changed between admission and operation, we adopted the condition just before surgery for the evaluation (Table 1).
Treatment protocol
All patients were under continuous observation in a neurosurgical intensive care unit until a few days after surgery. Medical consultations for perioperative care were obtained. While awaiting surgery, patients with an SAH were treated with calcium channel blockers. The presence of arterial vasospasm, suspected clinically and confirmed by transcranial Doppler examination, was an indication for triple-H therapy (induced hypervolemia, hemodilution and hypertension), preferably starting after surgery.
CT of the head was performed at admission and repeated after any clinical deterioration. All patients underwent MR angiography within 3 days after admission. If necessary, appropriate sedation was administered before the MR study. IA-DSA was restricted to patients in whom MRA was inconclusive or negative.
The patients were operated for clipping of the aneurysm as soon as possible after the diagnosis was established. Delayed operation was preferred for patients with poor clinical grading or for patients already with signs of vasospasm. Some of the late operations were due to delayed referral to our hospital. In patients with Hunt and Hess grades 3 and 4 and a large life-threatening intracerebral hematoma, an emergency operation was performed. The quality of the recovery in patients with an MRA-based diagnosis of ruptured aneurysm was estimated using the Glasgow Outcome Scale (GOS) at 2 months follow-up [28].
Image acquisition
The T2 and the MRA sequence were carried out on a 1.5-T Siemens Magnetom Vision. The T2-TSE slices (TR (repetition time) / TE (echo time) = 3,500/22–90 ms) in axial and/or coronal planes were constructed with a 512×512 matrix, followed by the 3D-TOF MR angiograms (FISP 3D); TR/TE = 35/6.4 ms; flip angle 20°; matrix=160×512; magnetization transfer (MT) prepulse; time for acquisition (TA) =6.44 min, 24 slices with 1.5 mm effective thickness. No contrast was used. Spin-echo T1-weighted images were also obtained.
The intra-arterial DSA studies were produced on a digital angiography system (Siemens Multiskop with InfiMed image processing) with a 512×512 pixel matrix. Selective three-or four-vessel angiography using a standard projection format (anteroposterior, lateral and reverse-oblique) was performed initially and additional views were performed, if required, to identify the parent vessel and aneurysm neck more clearly. The amount of contrast medium (Omnipaque 300 in a 1:1 dilution) was 12 ml for each series, and the injection rate 10 ml/s when the tip of the catheter was placed proximal to the carotid bifurcation. Injections into the vertebrobasilar system had a rate of 8 ml/s to an overall amount of 8 ml.
Viewing and postprocessing
In order to ensure reproducibility, visualizations of MR data were produced by two experienced neuroradiologists with considerable experience in image postprocessing and who had been informed of the initial CT results, including the sites of SAH and degree of hemorrhage. Both workstation displays and hard copy images were used. Postprocessing consisted of 60° maximum intensity projections (MIP) at six increments for 360° around the head, in both a left-to-right rotation and a head-to-foot rotation. MIP reconstructions were made of the whole data set without editing. Source images were viewed on a routine basis.
The presence of an aneurysm, its site and the parent artery were analyzed. If multiple aneurysms were detected, the usual criteria were applied in an attempt to decide which aneurysm was responsible for the hemorrhage. These criteria included the CT findings (distribution of blood) and the size and irregularity of the aneurysm.
We compared 3D MRA images with surgical findings in positive cases. In negatives, we compared 3D TOF MRA findings with IA-DSA results. If MRA results were considered indeterminate, IA-DSA was the gold standard in the detection or exclusion of aneurysms. IA-DSA was performed and evaluated by the same neuroradiologists. Both the MRA and IA-DSA diagnosis was based on consensus between the two neuroradiologists. All diagnostic findings were discussed with the neurosurgeons, who decided which information was sufficient for them.
Results
In 205 patients the MRA results were categorized into: proven aneurysm (n=133 (64.9%)); indeterminate (n=33 (16.1%)); or negative for aneurysm (n=39 (19.0%)). MRA was classified as inconclusive if the neuroradiologist was not certain about the MRA diagnosis after discussion with the neurosurgeon.
Positive MRA result
In the MRA-proven aneurysms, 133 ruptured aneurysms and 26 associated un-ruptured aneurysms were diagnosed. The distribution of the aneurysm location and size is shown in Table 2. The preoperative and peroperative findings are summarized in Table 3.
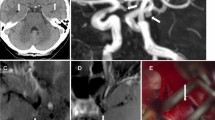
Surgery confirmed 132 ruptured aneurysms that were predicted by MRA, while one proved to be false positive. The patient in whom surgery was based on false-positive MRA findings, presented in Hunt and Hess grade 1 and had had a warning leak a few weeks prior to admission to the hospital. Lumbar puncture demonstrated blood pigments, while CT was negative. Although an aneurysm of the middle cerebral artery was diagnosed on the MR images, a tortuous loop was demonstrated at surgery (Fig 1). Additionally, no surrounding blood or other bleeding source was shown. No IA-DSA examination was performed postoperatively.
At surgery, we checked the presence of 15 of the 26 associated un-ruptured aneurysms detected on MRA. The remaining 11 aneurysms were not verified, because in these patients the surgical approach for the ruptured aneurysms was not suited for the un-ruptured aneurysms. Two aneurysms appeared false positive on MRA (two anterior communicating arteries). One of these lesions proved to be an infundibular dilatation, which was not suitable for surgical clipping. During surgical exploration of the other lesion, torsion of an adhesive anterior communicating complex was shown, with absence of an aneurysm. Two false-negative intracranial aneurysms (one middle cerebral artery and one anterior cerebral artery) were found in two patients. Both aneurysms were smaller than 3 mm in diameter.
Indeterminate MRA result
In 33 patients IA-DSA was performed because of inconclusive MRA findings. In the majority of these patients, IA-DSA was performed, because more information was required regarding the presence or the relative location and orientation of an aneurysm within the skull (Table 4). It is known that the quality of the MRA examination can be degraded because of the inability of the patient to cooperate. At our department, where anesthetic facilities are well-organized, MRA can even be performed on critically ill patients. In only two patients IA-DSA was performed because of considerable motion artifacts on MRA. Because no problems were noticed in even potentially risky patients (Hunt and Hess grades 2 and 3), we have not stated the number of patients that required sedation or anesthesia for performing the MRA examination.
In 24 patients (73%) IA-DSA confirmed the results of MRA. In five patients there was doubt about the presence of an aneurysm (three located on the posterior communicating artery, one on the anterior communicating artery and one on the anterior choroidal artery). Subsequent IA-DSA examination was negative. In four patients MRA had false-negative results (two aneurysms on the sinus cavernosus, one on the anterior choroidal artery and one on the posterior communicating artery). The false-negative aneurysms were all smaller than 5 mm and asymptomatic. Two of them were treated surgically.
IA-DSA showed 28 ruptured and 17 associated un-ruptured aneurysms, confirmed at surgery in 28 patients and was negative in the remaining five patients (Table 2).
Negative MRA result
In the 39 negative cases, IA-DSA was performed as the gold standard, which confirmed the MRA findings in 37 patients (Table 2). In one patient, IA-DSA showed an aneurysm of the posterior communicating artery, presumably the aneurysm that had bled. However, subsequent surgical intervention resulted in a negative exploration. During surgery a small loop of the posterior communicating artery was shown. Additionally, no surrounding blood or other bleeding source was seen.
In another patient, an aneurysm of the posterior communicating artery, which was not considered responsible for the SAH, was diagnosed with IA-DSA. The presence of this asymptomatic aneurysm was checked and confirmed with surgery 2 months after the SAH.
Clinical outcome of patients with an MRA-based diagnosis of ruptured aneurysm
The preoperative Hunt and Hess grades and the clinical outcome as expressed by the GOS are outlined in Table 5. In the majority of patients (n=128) the follow-up was in the University Hospital Groningen. In the remaining five patients the GOS was determined at time of discharge from the hospital, because follow-up was lost (n=3) or performed in another hospital (n=2). Most patients (77%) had been treated within 3 days after the last bleeding. At follow-up 86.5% of patients were in a favorable condition (in Table 5, indicated by “good recovery” and “moderately disabled”). The mortality rate was 4.5%, and the morbidity rate (“severely disabled” and “vegetative state”), 9.0%. There were no complications associated with the performance of 3D MRA.
Vasospasm as a postoperative complication was the main cause of serious failures in the group of patients that was severely disabled at follow-up. Six patients died: two due to fatal vasospasm; one due to arterial vasospasm and pneumonia; one due to carcinoma of the pancreas; one due to myocardial infarction and one due to pulmonary embolism. Permission for autopsy was refused in all six patients.
Summary
MRA had a positive predictive value for detection of ruptured aneurysms for 99% (132/133), with surgery as the gold standard. The negative predictive value of MRA for ruling out ruptured aneurysms was 100%, with IA-DSA as the gold standard. In 16.1% (33/205) of the included patient population, the MRA was considered inconclusive, necessitating further diagnostic investigation by IA-DSA. In this category IA-DSA confirmed MRA in 73% of patients. No mortality or morbidity was associated with the performance of 3D MRA.
Discussion
Imaging tools of intracranial aneurysms
IA-DSA is considered the gold standard for evaluating the intracranial vessels. However, this procedure is invasive and accompanied by radiation exposure and not without risk: cerebral embolus, dissection, rupture of cerebral arteries and hemorrhage, arterial vasospasm, or systemic complaints have been described [13–21]. The risk of complications accompanied by IA-DSA can be avoided if arteriography can be replaced by a reliable noninvasive imaging modality. The diagnostic accuracy of 3D CTA has been shown to be compatible with IA-DSA [29–31]. The results of the study of Matsumoto et al., who performed aneurysm surgery in the acute stage in patients with ruptured cerebral aneurysms by using 3D CTA, were encouraging [26].
Recent blinded-reader studies have reported mean sensitivities of 63–93% for detection of intracranial aneurysms using 3D TOF MRA and/or MIP and mean specificities for exclusion of aneurysms of 92–100% [32–38]. When 3–5 mm was considered the critical size for detection, sensitivities increased to 86–100% [34, 35, 38, 39]. Various reports examined the value of MRA with regard to patients with acute SAH [40–43]. The MRA in all these studies was read on an emergency basis by one or several radiologists and subsequently compared to the IA-DSA results, the latter serving as the reference standard. They established sensitivities for detection of ruptured aneurysms of between 81% and 100%. Wilcock et al. found the specificity to be 100% [43]. In the blinded multireader study of Jäger et al., IA-DSA was deliberately not chosen to represent the reference standard, and the clinical course and surgical findings were used to explain significant differences between the readings of MRA and IA-DSA [44]. Analysis of the data showed that MRA was able to detect aneurysms not seen on IA-DSA. This finding had already been reported by Curnes et al. [45].
A distinct advantage of MRA is that views can be produced in an automated fashion by a technician after the patient has left the imager and can be supplemented by interactive viewing on a workstation, if necessary. MRA can be performed within 20 min, advancing early decision-making. At our department, where anesthetic facilities are well-organized, MRA can even be performed on critically ill patients.
MRA-based surgery of intracranial aneurysms
Unlike previous studies, we included all patients consecutively suitable for neurosurgical intervention as first treatment choice when a probable ruptured intracranial aneurysm was diagnosed by MRA. Only a few studies have been set up to establish whether satisfactory MRA images can be obtained to perform surgery on ruptured intracranial aneurysms without recurring to IA-DSA in the acute phase of illness. In the study of Keogh et al., 21 out of 30 patients for whom a diagnosis of an anterior midline aneurysm was made on MRA were selected to undergo surgery based on MRA images alone [22].
Sankhla et al. studied 51 patients presenting with SAH by MRA. The MRA results were considered satisfactory in 38 patients, and in 20 of them early surgical obliteration was possible based on MRA results [23]. Watanabe et al. performed surgery in 106 patients presenting with SAH, using 3D TOF and MIP [24]. In 48 patients (45.3%) the anatomy around the aneurysms was so typical that clipping was carried out without additional information from IA-DSA and/or computerized tomography angiography. In another study of Keogh et al., 122 patients presenting with SAH were considered for MRA studies [25]. Fifty-five of these patients showing aneurysms on MRA were clinically able to undergo early surgery, and their MRA images were considered satisfactory for surgical planning.
Implications of present study
Loop formation and overlap of vessels have been described as main causes of false-positive and false-negative interpretations [25, 35]. In the present study, this diagnostic limitation of MRA led to two negative surgical explorations, of which one concerned a ruptured aneurysm. Furthermore, in five patients IA-DSA examination had to be performed, because MRA was unable to differentiate a tortuous loop or infundibular dilatation from an aneurysm. Review of source images or images from a selective (partial or target) MIP method, in which part of the source data is processed separately, may help to overcome these problems [34]. In one patient we also faced this diagnostic problem with IA-DSA examination. This has also been described earlier by others [44, 45].
In the present study MRA missed seven asymptomatic aneurysms that were smaller than 5 mm in diameter. Two of these were discovered during surgery and subsequently clipped. Five aneurysms were diagnosed after IA-DSA examination, and three of them were also treated surgically. Decreased MRA signal intensity, which could be explained by stagnant flow near the aneurysm, may render small aneurysms invisible [36]. As mentioned before, 3 mm seems to be the threshold size for detection of aneurysms by MRA.
However, asymptomatic aneurysms smaller than 3 mm are probably not important clinically [46, 47]. More important, no ruptured aneurysms were missed. This result may suggest that a negative MRA in a patient presenting with acute SAH, and thus a high pre-diagnostic suspicion of ruptured aneurysm, is reliable and may replace IA- DSA.
In the present study, in 19% of patients MRA was considered inconclusive, mainly because more information was required regarding the presence or relative location and orientation of the aneurysm. This finding may be influenced by the learning curve of the neuroradiologists with the MRA technique, and, in the beginning, some uncertainty in neurosurgeons about performing surgery based on MRA results alone. In 73% of patients IA-DSA confirmed the MRA results.
The mortality (4.5%) and morbidity (9.0%) rates of the present study are beneficial and acceptable when compared with the results from the study of Edner et al., in which IA-DSA was routine in all cases [48]. In their series, favorable outcomes, as measured with the GOS at 6 months, were 82 out of 122 patients (67%), with 76% treated within 1 week after the last bleeding. The mortality and morbidity rates were16% and 17%, respectively.
Some comments can be made with regard to the present study. The prevalence of ruptured intracranial aneurysms in the total number of patients examined is 78% (160/205), which is comparable with other published data [49]. Hence, our study shows that MRA is useful for diagnosis in patients with a high suspicion of a ruptured aneurysm, i.e., in patients presenting with an SAH. The ability of MRA to correctly detect an aneurysm could change when MRA is used in patients with different pretest probabilities for an intracranial aneurysm. In our study, the radiologist had been informed of the initial CT results. CT findings, although not considered essential, can be of help in confirming the region of interest, by demonstrating the anatomical site of the intracerebral clot or concentration of subarachnoid blood.
In conclusion, in this study we included 205 patients with an SAH for MRA examination and investigated whether MRA could replace IA-DSA in the selection of patients suitable for surgical treatment of ruptured aneurysms. Only one false surgical indication was determined by MRA and no false negatives were selected by MRA. A minority of the MRA results were considered insufficient, making performance of IA-DSA necessary. We conclude, that IA-DSA can be replaced by 3D TOF MRA as the first diagnostic modality in the selection of patients suitable for clipping of ruptured aneurysms.
References
Jennet B, Lindsay KW (1994) An introduction to neurosurgery, 5th edn. Butterworth-Heinemann, Oxford, pp 146–148
Rinkel GJ, Djibuti M, van Gijn J (1998) Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 29:251–256
Linn FH, Rinkel GJ, Algra A, van Gijn J (1996) Incidence of subarachnoid haemorrhage. Role of region, year, and rate of computed tomography: a meta-analysis. Stroke 27:625–629
Hop JW, Rinkel GJ, Algra A, van Gijn J (1997) Case-fatality rates and functional outcome after subarachnoid haemorrhage: a systematic review. Stroke 28:660–664
Hijdra A, Braakman R, van Gijn J, Vermeulen M, van Crevel H (1987) Aneurysmal subarachnoid haemorrhage: complications and outcome in a hospital population. Stroke 18:1061–1067
Thomeer RTWM, Taal JCW, Voormolen JHC, Wintzen AR (1994) Aneurysmal bleeding. A plea for early surgery in good-risk patients. Acta Neurochir (Wien) 128:126–131
ISAT (2002) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 360:1267–1274
Gross-Fengels W, Mödder U, Beyer D, Neufang KF, Godehardt E (1987) Komplikationen brachiocephaler Katheterangiographien bei Verwendung eines nicht-ionischen Kontrastmittels. Radiologe 27:83–88
Mützel W, Speck U (1983) Effects of ionic and non-ionic contrast media after selective peripheral and cerebral arterial injections in rats. Fortschr Röntgenstr 118:62–66
Skalpe IO (1983) The toxicity of nonionic watersoluble monomeric and dimeric contrast media in selective vertebral angiography. Neuroradiology 24:219–223
Skalpe IO, Aulie A (1985) The toxicity of non-ionic watersoluble media in selective vertebral angiography. An experimental study in rabbits with special reference to the difference between monomeric and dimeric compounds. Neuroradiology 27:77–79
Katzen BT (1985) Peripheral, abdominal and interventional applications of DSA. Radiol Clin North Am 23:227–241
Grzyska U, Freitag J, Zeumer H (1990) Selective cerebral intraarterial DSA. Complication rate and control of risk factors. Neuroradiology 32:296–299
Heiserman JE, Dean BL, Hodak JA (1994) Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol 15:1401–1407
Waugh JR, Sacharias N (1992) Arteriographic complications in the DSA era. Radiology 182:243–246
Leffers AM, Wagner A (2000) Neurologic complications of cerebral angiography. A retrospective study of complication rate and patient risk factors. Acta Radiol 41:204–210
Warnock NG, Gandhi MR, Bergvall U, Powell T (1993) Complications of intra-arterial digital subtraction angiography in patients investigated for cerebral vascular disease. Br J Radiol 66:855–858
Cloft HJ, GJ Joseph, JE Dion (1999) Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation. A meta-analysis. Stroke 30:317–320
Jamieson KG (1954) Rupture of an intracranial aneurysm during cerebral angiography. J Neurosurg 11:625–628
Dublin B, Barry N (1980) Cerebral aneurysmal rupture during angiography with confirmation by computed tomography. Surg Neurol 13:19–26
Koenig GH, Marshall WH, Poole GJ, Kramer RA (1979) Rupture of intracranial aneurysms during cerebral angiography: report of ten cases and review of the literature. Neurosurgery 5:314–324
Keogh AJ, Sankhla SK (1996) Magnetic resonance angiography for anterior midline aneurysms. Br J Neurosurg 10(2):143–147
Sankhla SK, Gunawardena WJ, Coutinho CMA, Jones AP, Keogh AJ (1996) Magnetic resonance angiography in the management of aneurysmal subarachnoid haemorrhage: a study of 51 cases. Neuroradiology 38:724–729
Watanabe Z, Kikuchi Y, Izaki K, Hanyu N, Lim FS, Gotou H, Koizumi J, Gotou T, Kowada M, Watanabe K (2001) The usefulness of 3D MR angiography in surgery for ruptured cerebral aneurysms. Surg Neurol 55:359–364
Keogh, Vhora S (1998) The usefulness of magnetic resonance angiography in surgery for intracranial aneurysms that have bled. Surg Neurol 50:122–129
Matsumoto M, Sato M, Nakano M, Endo Y, Watanabe Y, Sasaki T, Suzuki K, Kodama N (2000) Three-dimensional computerized tomography angiography-guided surgery for acutely ruptured cerebral aneurysms. J Neurosurg 94:718–727
Hunt WE, Hess RM (1968) Surgical risk as related to the time of intervention in the repair of intracranial aneurysms. J Neurosurg 28:14–19
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484
Anderson GB, Steinke DE, Petruk KC (1999) Computed tomographic angiography versus digital subtraction angiography for the diagnosis and early treatment of ruptured intracranial aneurysms. Neurosurgery 45:1315–1322
Velthuis BK, Rinkel GJE, Ramos LMP, Witkamp TD, van der Sprenkel JW, Vandertop WP, van Leeuwen MS (1998) Subarachnoid hemorrhage: aneurysm detection and preoperative evaluation with CT angiography. Radiology 208:423–430
Velthuis BK, van Leeuwen MS, Witkamp TE, Ramos LM, van der Sprenkel JW, Rinkel GJ (1999) Computerized tomography angiography in patients with subarachnoid hemorrhage: from aneurysm detection to treatment without conventional angiography. J Neurosurg 91:761–767
Schuierer G, Huk WJ, Laub G (1992) Magnetic resonance angiography of intracranial aneurysms: comparison with intra-arterial digital subtraction angiography. Neuroradiology 35:50–54
Stock KW, Radue EW, Jacob AL, Bao XS, Steinbrich W (1995) Intracranial arteries: prospective blinded comparative study of MR angiography and DSA in 50 patients. Radiology 195:451–456
Korogi Y, Takahashi M, Mabuchi N, Nakagawa T, Fujiwara S, Horikawa Y, Miki H, O’Uchi T, Shiga H, Shiokawa Y (1996) Intracranial aneurysms: diagnostic accuracy of MR Angiography with evaluation of maximum intensity projection and source images. Radiology 199:199–207
Korogi Y, Takahashi M, Mabuchi N, Miki H, Fujiwara S, Horikawa Y, Nakagawa T, O’Uchi T, Watabe T, Shiga H (1994) Intracranial aneurysms: diagnostic accuracy of three-dimensional, Fourier transform, time-of-flight MR angiography. Radiology 193:181–186
Horikoshi T, Fukamachi A, Nishi H, Fukasawa I (1994) Detection of intracranial aneurysms by three-dimensional time-of-flight magnetic resonance angiography. Neuroradiology 36:203–207
Ross JS, Masaryk TJ, Modic MT, Ruggieri PM, Haacke EM, Selman WR (1990) Intracranial aneurysms: evaluation by MR angiography. AJNR Am J Neuroradiol 155:159–165
Adams WM, Laitt RD, Jackson A (2000) The role of MR angiography in the pretreatment assessment of intracranial aneurysms: a comparative study. AJNR Am J Neuroradiol 21:1618–1628
Huston III J, Nichols DA, Luetmer PH, Goodwin JT, Meyer FB, Wiebers DO, Weaver AL (1994) Blinded prospective evaluation of sensitivity of MR angiography to known intracranial aneurysms: importance of aneurysm size. AJNR Am J Neuroradiol 15:1607–1614
Ida M, Kurisu T, Yamashita M (1997) MR angiography of ruptured aneurysms in acute subarachnoid hemorrhage. AJNR Am J Neuroradiol 18:1025–1032
Gouliamos A, Gotsis E, Vlahos L, Samara C, Kapsalaki E, Rologis D, Kapsalakis Z, Papavasiliou C (1992) Magnetic resonance angiography compared to intra-arterial digital subtraction angiography in patients with subarachnoid haemorrhage. Neuroradiology 35:46–49
Anzalone N, Triulzi F, Scotti G (1995) Acute subarachnoid haemorrhage: 3D time-of-flight MR angiography versus intra-arterial digital angiography. Neuroradiology 37:257–261
Wilcock D, Jaspan T, Holland I, Cherryman G, Worthington B (1996) Comparison of magnetic resonance angiography with conventional angiography in the detection of intracranial aneurysms in patients presenting with subarachnoid haemorrhage. Clin Radiol 51:330–334
Jäger HR, Mansmann U, Hausmann O, Partzsch U, Moseley IF, Taylor WJ (2000) MRA versus digital subtraction angiography in acute subarachnoid haemorrhage: a blinded multireader study of prospectively recruited patients. Neuroradiology 42:313–326
Curnes JT, Shogry MEC, Clark DC, Elsner HJ (1993) MR angiographic demonstration of an intracranial aneurysm not seen on conventional angiography. AJNR Am J Neuroradiol 14:971–973
Juvela S, Porras M, Heiskanen O (1993) Natural history of unruptured intracranial aneurysms: a long-term follow-up study. J Neurosurg 79:174–182
McCormick WF, Acosta-Rua GJ (1970) The size of intracranial saccular aneurysms. An autopsy study. J Neurosurg 33:427–442
Edner G, Kagström E, Wallstedt L (1992) Total overall management and surgical outcome after aneurysmal subarachnoid haemorrhage in a defined population. Br J Neurosurg 6:409–420
Wirth F (1985) Surgical treatment of incidental intracranial aneurysms. Clin Neurosurg, Baltimore
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was presented in whole at the following meetings: ECR Vienna March 2003; RSNA Chicago December 2002; and CIRSE Lucerne October 2002
Rights and permissions
About this article
Cite this article
Westerlaan, H.E., van der Vliet, A.M., Hew, J.M. et al. Magnetic resonance angiography in the selection of patients suitable for neurosurgical intervention of ruptured intracranial aneurysms. Neuroradiology 46, 867–875 (2004). https://doi.org/10.1007/s00234-004-1260-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-004-1260-9