Abstract
The mechanism of neurological disturbances in patients with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) is controversial. We studied 12 patients with MELAS using conventional and diffusion weighted MRI (DWI) and MR spectroscopy (MRS), to look at the physiopathology of the stroke-like events. Although conventional MRI showed lesions in all patients, DWI was more sensitive. One patient did not show high signal on DWI 48 h after a from stroke-like episode, but MRS demonstrated a lactate peak in left occipital lobe; 2 weeks after the attack, high signal was demonstrated on the right frontal lobe where MRS had shown a lactate peak. Our findings suggest a possible predictive ability of 1H-MRS, in showing early MELAS lesions and supports the hypothesis that mitochondrial metabolic dysfunction may precedes abnormalities on DWI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like events) is a mitochondrial disorder characterised by nausea, vomiting, seizures, headaches, diabetes mellitus, muscle weakness, exercise intolerance, sensorineural hearing loss, and sudden neurological deficits 1, 2]. Although these neurological disturbances strongly mimic these of stroke, their mechanism is unclear and they are therefore generally termed “stroke-like” events. Two main hypotheses have proffered brain involvement: angiopathy leading to ischaemia or and direct neuronal death resulting from mitochondrial impairment [3].
Although conventional MRI shows signal abnormalities indistinguishable from those seen in ischaemic stroke, newly developed MR techniques applied to the challenge of elucidating the physiopathology [4, 5]. We studied 12 patients with MELAS using conventional and diffusion-weighted MRI (DWI), and MR spectroscopy (MRS) and discuss the physiopathology of the stroke-like events.
Materials and methods
We studies 12 patients with MELAS, one man and 11 women, mean 29.9±8.5 years, clinically diagnosed as having MELAS and confirmed by DNA analyses to have tRNA Leu(UUR) A3243G point mutation [6, 7]. All were examined by a neurologist (Table 1) and had initial and follow-up MRI including DWI and MRS 2 weeks after a stroke-like episode.
Conventional dual-echo fast spin-echo, FLAIR (fluid attenuated inversion recovery) images and DWI were obtained using a 1.5 tesla imager with a circularly polarised head coil. Standard conventional sequences were used. DWI was acquired using a spin-echo echoplanar sequence with diffusion-sensitising gradients switched in slice direction and three different values of b: 30, 300 and 1.100 s/mm2). Sequential sampling of k-space was used with effective echo time 123 ms, bandwidth of 1250 Hz/pixel, and acquisition matrix 128×128, interpolated to 256×256 during image calculation. The DWI sequence provided 20 contiguous 3 mm slices, field of view 230 mm; acquisition time for each sequence was 5 s.
For MRS, we used axial FLAIR images were used to prescribe 2×2×2 cm3 voxels, chosen to include affected and unaffected white matter, as judged from FLAIR images. The point-resolved spectroscopy localisation technique was used with parameters: TR 3000 TE 35 and 140 ms, 128 acquisitions. The spectroscopic data acquisition provides a water-suppressed proton spectrum over a range from 4.3 to 0.1 ppm. Chemical shifts were identified with the N-acetyl-aspartate resonance at 2.02, phosphocreatine and creatine at 3.05 and choline-containing compounds at 3.22 ppm [9]
Results
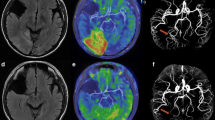
The T2-weighted fast spin-echo and FLAIR images (Fig. 1) showed abnormalities in all patients, FLAIR appearing more sensitive. When new lesions occurred, the brain appeared swollen on T1-weighted images, but this was not always clear. Lesions seen only on DWI were assumed to be new (Fig. 1) [10, 11].
Patient 1. On T2-weighted ( top right) and fluid-attenuated inversion-recovery (FLAIR) ( bottom left) images, high signal was observed in the right frontal (*) and occipital lobe (**) lobes. A left occipital lesion (***) seen better on FLAIR. The right frontal occipital lesions were also visible on a T1-weighted image ( top left), which suggested that they were relatively new. On diffusion weighted imaging (DWI) ( bottom right), only a lesion in the left frontal lobe (*) was visible, suggesting that it was the most recent
MRS at TE 35 ms revealed a large doublet in recent lesions at 1.3 ppm, inverted at TE 140 ms, typical of lactate (Fig. 2). A lactate peak was clearly identified in the most recent lesions and less clearly in lesions known to be present before the first imaging study (Fig. 3). No lactate peak could be identified in a lesion in brain which appeared normal on initial MRI. It has been suggested that these changes in lactate peaks are of diagnostic value in new stroke-like lesions [12, 13, 14]. However, our observations suggest that stroke-like lesions may show a lactate peak for a while after the episode.
We also made a new observation. In a patient with cortical blindness we found two lesions in the occipital lobes in the first images, clearly visible on FLAIR, but only one lesion, presumably newer, was more clearly visible on DWI; it showed increased apparent diffusion coefficients (ADC). FLAIR images obtained 2 weeks later showed a new lesion in the right frontal lobe. On DWI only this lesion, with an increased ADC could be detected and the two older lesions were not visible (Fig. 4). MRS at the time of the first MRI showed lactate peaks in the left occipital lobe, where MRI showed a lesion. However, a lactate peak was also detected in the right frontal lobe, which seemed normal on the first MRI but showed a new lesion on the second (Fig. 5, Table 2).
Patient 1. Serial MRI. The images on the right were obtained 2 weeks after those on the left. The lesion which appeared first (*) on FLAIR ( top left) but not on DWI ( bottom left), while the second lesion (**) was clearly visible on both. We placed four regions of interest (ROI) in the frontal and occipital lobes ( bottom left). After 2 weeks, a third lesion in the frontal lobe (***) was clearly visible on FLAIR ( top right) and on DWI ( bottom right). The first and second lesions were no longer visible on DWI, but were still seen on FLAIR
Patient 1. MRS: the top right image was obtained 2 weeks after the top left. ROI were placed on the DWI in the frontal ( A, C) and occipital ( B, D) lobes. A stroke-like lesion was visible in B but was not in A. However, MRS slowed lactate doublets ( arrowheads) in both (preceding DWI abnormalities), but not in C or D
Discussion
The focal neurological deficits of abrupt onset of MELAS are clinically indistinguishable from strokes [15]. MRI classically shows signal change in both grey and white matter, predominantly in the occipital and parietal lobes, which strongly mimic infarcts. However, their distribution does not usually follow vascular territories and pathological studies do not show lesions of the major cerebral vessels. Fluctuating lesions on MRI are also characteristic (Fig. 4) [16].
The physiopathology of MELAS remains unclear but two main hypotheses have been put forward [10]. The vascular hypothesis holds that metabolic damage to the endothelium leads to small-vessel occlusion and secondary neuronal death [2]. The defect in neuronal metabolism hypothesis states that mitochondrial dysfunction results in anaerobic metabolism and neuronal death from acidosis [17].
Oppenheim et al. [9] reported a patient with MELAS in whom MRI 2 days after a stroke-like episode showed subcortical high signal on FLAIR but normal to high ADC. DWI displays regional mobility of protons and it is claimed that it can help distinguish between cellular and extracellular oedema. In general, in ischaemic infarcts, intracellular diffusion of protons is restricted or reduced, giving dark areas on ADC maps. Oppenheim et al. therefore concluded that increased diffusion supported the metabolic rather than the ischaemic hypothesis. Experimental and human studies suggest that increased ADC reflect cellular oedema, whereas decreased ADC correspond to an increase in the extracellular space [18, 19, 20, 21]. Our observations are in agreement with previous reports and suggest that increased signal on DWI supports the metabolic rather than the ischaemic hypothesis.
Although decreased ADC in early stroke lesions can be a landmark of developing ischaemic strokes, MRI, including DWI, can not predict the occurrence of stroke [22, 23]. One of our patients (Fig. 5), showed a lactate peak in an area appearing normal on DWI; a lesion was, however, evident in the same area on DWI 2 weeks later. This seems to suggest a possible predictive ability of 1H-MRS, in showing MELAS lesions in an early phase and supports the hypothesis that mitochondrial metabolic dysfunction may precede signal change on DWI, although the latter can show ischaemic injury within an hour of symptom onset in human ischaemic stroke [24]. There is thus a possibility that metabolic change detected by 1H-MRS precedes abnormal diffusion. We therefore believe that a combination of DWI and MRS may be used for detecting stroke-like attacks at an early stage.
References
Pavlakis SG, Phillips PC, DiMauro S, et al (1984) Mitochondrial myopathy, encephalopathy, lactic acidosis and stroke like episodes: a distinctive clinical syndrome. Ann Neurol 16: 481–488
Ohama E, Ohara S, Ikuta F, et al (1987) Mitochondrial angiopathy in cerebral blood vessels of mitochondrial encephalomyopathy. Acta Neuropathol 74: 226–233
Molnar MJ, Valikovics A, Molnar S, et al (2000) Cerebral blood flow and glucose metabolism in mitochondrial disorders. Neurology 55: 544–548
Castillo M, Kwock L, Green C (1995) MELAS syndrome: imaging and proton MR spectroscopic findings. AJNR 16: 233–239
Barkovich AJ, Good WV, Koch TK, et al (1993) Mitochondrial disorders: analysis of their clinical and imaging characteristics. AJNR 14: 1119–1137
Goto Y, Nonaka I, Horai S (1097) A new mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS). Biochim Biophys Acta 1097: 238–240
Kotsimbos N, Jean-Francois MJB, Huizing M, et al (1994) Rapid and non-invasive screening of patients with mitochondrial myopathy. Hum Mutat 4: 132–135
Abe K, Terakawa H, Takanashi M, et al (2000) Proton magnetic resonance spectroscopy of patients with parkinsonism. Brain Res Bull 52: 589–595
Oppenheim C, Galanaud D, Samson Y, et al (2000) Can diffusion weighted magnetic resonance imaging help differentiate stroke from stroke-like events in MELAS? J Neurol Neurosurg Psychiatry 69: 248–250
Majoie CB, Akkerman EM, Blank C, et al (2002) Comparison of conventional MR imaging with diffusion-weighted and diffusion tensor imaging: case report. AJNR 23: 813–816
Pavlakis SG, Kingsley PB, Kaplan GP, et al (1998) Magnetic resonance spectroscopy. Use in monitoring MELAS treatment. Arch Neurol 55: 849–852
Yoneda M, Maeda M, Kimura H, Fujii A, Katayama K, Kuriyama M (1999). Vasogenic edema in MELAS: a serial study with diffusion-weighted MR imaging. Neurology 53: 2182–2184
Kamada K, Takeuchi F, Houkin K, et al (2001) Reversible brain dysfunction in MELAS: MEG, and 1H MRS analysis. J Neurol Neurosurg Psychiatry 70: 675–678
Abe K, Inui T, Hirono N, Mezaki T, Kobayashi Y, Kameyama M (1990) Fluctuating MR images with mitochondrial encephalopathy, lactic acidosis, stroke-like syndrome (MELAS). Neuroradiology 32: 77
Clark JM, Marks MP, Adalsteinsson E, et al (1996) MELAS: clinical and pathologic correlations with MRI, Xenon/CT, and MR spectroscopy. Neurology 46: 223–227
Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S (1997) Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology 49: 113–119
Le Bihan D, Breton E, Lallemand D, et al (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161: 401–407
Moseley ME, Cohen Y, Mintorovitch J, et al (1990) Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med 14: 330–346
Schaefer PW, Buonanno FS, Gonzalez RG, et al (1997) Diffusion weighted imaging discriminates between cytotoxic and vasogenic edema in a patient with eclampsia. Stroke 28: 1082–1085
Loubinoux I, Volk A, Borredon J, et al (1997) Spreading of vasogenic edema and cytotoxic edema assessed by quantitative diffusion and T2 magnetic resonance imaging. Stroke 28: 419–426
Kuroiwa T, Nagaoka T, Ueki M, et al (1998) Different apparent diffusion coefficient: water content correlations of gray and white matter during early ischemia. Stroke 29: 859–865
Kucinski T, Vaterlein O, Glauche V, et al (2002) Correlation of apparent diffusion coefficient and computed tomography density in acute ischemic stroke. Stroke 33: 1786–1791
Fiehler J, Foth M, Kucinski T, et al (2002) Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke 33: 79–86
Yoneda Y, Tokui K, Hanihara T, Kitagaki H, Tabuchi M, Mori E (1999) Diffusion-weighted magnetic resonance imaging: detection of ischemic injury 33 minutes after onset in a stroke patient. Ann Neurol 45: 794–797
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abe, K., Yoshimura, H., Tanaka, H. et al. Comparison of conventional and diffusion-weighted MRI and proton MR spectroscopy in patients with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like events. Neuroradiology 46, 113–117 (2004). https://doi.org/10.1007/s00234-003-1138-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-003-1138-2