Abstract
5-Fluorouracil (5-FU) is a widely used chemotherapy agent for breast cancer, although drug resistance is a critical issue regarding the use of this agent in the disease. Calcium signaling is a well-known main cause of proliferation and apoptosis in breast cancer cells. Although previous studies have implicated TRPV1 inhibitor, anticancer, and apoptotic roles of Hypericum perforatum (HPer) in several cells, the synergistic inhibition effects of HPer and 5-FU in cancer and the stimulation of ongoing apoptosis have not yet been clarified in MCF-7 cells. Therefore, we investigated the apoptotic and antioxidant properties of 5-FU with/without HPer through activation of TRPV1 in MCF-7 cells. The MCF-7 cells were divided into four groups: the control group, the HPer-treated group (0.3 mM), the 5-FU-treated group (25 μM), and the 5-FU+HPer-treated group. The intracellular free calcium ion concentration ([Ca2+]i) increased with 5-FU treatments, but they decreased with the HPer and HPer+5-FU treatments. The [Ca2+]i is further decreased in the four groups by TRPV1 channel antagonist (capsazepine and 0.01 mM) treatments. However, mitochondrial membrane depolarization and apoptosis levels, and the PARP1, caspase 3, and caspase 9 expression levels were increased by 5-FU treatment, although the values were decreased by the HPer and 5-FU+HPer treatments. Cell viability level was also decreased by 5-FU treatment. In conclusion, antitumor and apoptosis effects of 5-FU are up-regulated by activation of TRPV1 channels, but its action was down-regulated by HPer treatment. It seems that HPer cannot be used for increasing the antitumor effect of 5-FU through modulation of the TRPV1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer has the highest incidence of all cancers (28%) and the highest mortality rate worldwide for cancer types in women [1]. Chemotherapeutic agents have been used as the main therapy for treating the disease [2]. One such agent for treating breast cancer is 5-fluorouracil (5-FU) which has been widely used since the 1980s. A well-known first antitumor effect of 5-FU is blockage of DNA synthesis and cell proliferation through binding onto DNA and RNA [3]. Potentiation of apoptosis is a secondary antitumor role of 5-FU in breast cancer cells. However, clinical inadequacy of 5-FU on apoptosis and caspase production was reported due to development of resistance to 5-FU [4], and its role on molecular apoptosis mechanisms in the treatment of breast cancer cells has not been clarified yet. 5-FU is usually used in combination therapies with other antitumor agents or other compounds to increase its antitumor effect [5, 6].
Hypericum perforatum (St John’s wort, HPer) is an ancient antioxidant plant. The main bioactive components of HPer are hyperforin and hypericin [7]. HPer is believed to have a number of health-promoting effects that include antioxidant, anti-carcinogenic, anti-proliferative, and apoptotic activities [8]. Extracts of HPer induced cytotoxic effect through arrest of molecular cell cycles in cultures of cell lung cancer cells [5], HT-29 colon adenocarcinoma cells [9], and of prostate cancer cells [10], and MCF-7 breast cancer cells [6]. The anti-proliferative effects of HPer extract (hyperforin) have been attributed to its direct inhibition of caspase 3 and caspase 9 activations, cell cycle arrest in different cancer cell lines [5, 9]. In addition, HPer may interfere with cancer metastasis because nuclear factor kB (NFkB)-regulated expression of metastatic proteins in different breast cancer cell line were shown to be down-regulated by HPer extracts such as hyperoside and hyperforin [11,12,13].
Several physiological functions and pathophysiological events such as mitochondrial function, breast cancer cell proliferation, and apoptosis are regulated by Ca2+ [14, 15]. To organize such functions, the Ca2+ signal must be precisely regulated. In the Ca2+ signal process, there are well-known channels, namely ligand- and voltage-gated calcium channels. In addition to the well-known cation channels, the transient receptor potential (TRP) channel family has been discovered within recent years. A member of the family is TRP vanilloid 1 (TRPV1) [16]. The channel is activated by different stimuli including capsaicin and oxidative stress although its activity is inhibited by specific antagonists such as capsazepine (CPZP) and 5′-iodoresiniferatoxin [17, 18]. In recent studies, we observed an inhibitor role of HPer on apoptosis, mitochondrial oxidative stress, and the TRPV1 channel in rat neurons [19, 20] and human phagocytic cells [21]. Result of a recent study indicated that 5-FU-induced autophagic death in human hepatocarcinoma cell line (HepG2) is associated with Orai1-mediated store-operated calcium entry [22]. Therefore, 5-FU may decrease cell proliferation, through increase of mitochondrial oxidative stress, apoptosis, and TRPV1 activation. Thus, it important that the relationship between changes in HPer, ROS, [Ca2+]i levels, TRPV1 activation, and 5-FU-related toxicity in MCF-7 breast cancer cells becomes clarified.
Regarding this issue, especially in the case of breast cancer patients, new therapeutic strategies of 5-FU, such as combination therapy, are required. To our knowledge, there is no report on apoptosis, mitochondrial oxidative stress, and Ca2+ signaling through TRPV1 channels in HPer- and 5-FU-treated MCF-7 cell line. Therefore, this study was aimed to investigate if HPer could increase the anticancer effect of 5-FU through up-regulation of mitochondrial oxidative stress, apoptosis, and accumulation of intracellular [Ca2+]i-induced oxidative stress in breast cancer cells.
Materials and methods
Cells and reagents
The Michigan Cancer Foundation-7 breast cancer cell line (MCF-7) was originally obtained from Şap Institute, Ministry of Food, Agriculture and Livestock, Republic of Turkey (Ankara, Turkey). Ethylene glycol-bis(2-aminoethyl-ether)-N,N,N′,N′-tetraacetic acid (EGTA), 5-FU and dimethyl sulfoxide (DMSO), and Roswell Park Memorial Institute (RPMI) 1640 medium were obtained from Sigma-Aldrich Chemical (St. Louis, MO, USA). Fura-2/AM was purchased from Calbiochem (Darmstadt, Germany). Dihydrorhodamine-123 (DHR 123) and Tris–glycine gels were from Molecular Probes (Eugene, OR, USA). Caspase substrates [N-acetyl-Leu-Glu-His-Asp-7-amino-4-methylcoumarin (AC-LEHD-AMC) and N-acetyl-Leu-Glu-His-Asp-7-amino-4-methylcoumarin (AC-LEHD-AMC)] were purchased from Bachem (Bubendorf, Switzerland). Capsaicin and CPZP were obtained from Santa Cruz Inc. (Istanbul, Turkey). A mitochondrial stain 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) was obtained from Santa Cruz (Dallas, TX, USA). Primary antibodies (rabbit anti-caspase 9, mouse anti-caspase 3, rabbit anti-PARP, and mouse monoclonal β-actin) were purchased from Cell Signaling Technology (İstanbul, Turkey) although secondary antibodies (Anti-mouse IgG and Anti-rabbit IgG antibodies conjugated with horseradish peroxidase) were obtained from GE Healthcare (Istanbul, Turkey). Polyvinylidene difluoride membranes were purchased from Millipore Inc. (MA, USA). All organic solvents were also purchased from Santa Cruz Inc. (Istanbul, Turkey).
Hypericum perforatum
Hypericum perforatum extract was purchased from Xi’an Lyphar Biotech Co., Ltd. (Xian City, Shaanxi Province, China). The extract mainly contained ≥0 0.32% total hypericin, 8.0% flavonoids, and 7.0% hyperforin.
Cell culture
MCF-7 cells were cultured in RPMI supplemented with 10% heat-activated fetal bovine serum, containing penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37 °C in a humidified incubator at 37 °C, 5% CO2, and 95% air. The RPMI medium was changed every other day. Preparation details of the cultures in the 96- or 24-well plates were described previously [23].
The cells were counted daily by removing a small volume from the tissue culture flask (filter cap, sterile, 250 ml, 75 cm2), diluting it with an equal volume of trypan blue (0.4%), and tallying viable cells (trypan blue excluding) with a cell counter (Casy Modell TT, Roche, Germany). Cultures were maintained as a suspension without shaking or stirring at a density of 1 × 106 cells per ml by dilution with fresh media [24].
Groups
Cells were seeded in 8–10 flasks at a density of 1 × 106 cells per flask (filter cap, sterile, 250 ml, 75 cm2). All cells were cultured at 37 °C. The cells were divided into four main groups as follows:
-
Control group The cells were not incubated with 5-FU, CPZP, and HPer but were kept in a flask containing the same cell culture medium and conditions for 48 h.
-
5-FU group Cells in the group were incubated with 5-FU (25 μM) for periods of 24 h [25].
-
HPer group Cells in the group were incubated with HPer (0.3 mM) for periods of 24 h [26].
-
5-FU+HPer group Cells in the group were incubated with 5-FU (25 μM) for periods of 24 h and then incubated with HPer (0.3 mM) for a further periods of 24 h.
For the [Ca2+]i level assay, the cells were further treated with capsaicin (1 µM and 10 min incubation) for activation of the TRPV1 channel and when required, they were inhibited with the TRPV1 blocker CPZP (0.01 mM and 30 min incubation). The capsaicin, CPZP, HPer, and 5-FU were dissolved in DMSO and the pH (7.2) was adjusted using KOH. 5-FU was prepared freshly but CPZP and capsaicin were stored at −33 °C. At the end of the incubations, the control and treated cells were used for the analyses of [Ca2+]i levels using a plate reader and for Western blotting.
Intracellular free calcium ion ([Ca2+]i) concentration measurement
The [Ca2+]i concentration in the MCF7 cell was measured as previously described [24]. Briefly, cells were suspended in the standard extracellular buffer solution (in mM: 145 NaCl, 1 MgCl2, 1 CaCl2, 5 KCl, 10 HEPES, 10 d-glucose with the pH adjusted with KOH to 7.2) and 0.1% fatty acid free bovine serum albumin solution containing 2 μM fura2/AM for 45 min at 37 °C and treated with TRPV1 antagonist (CPZP and 0.01 mM) to inhibit Ca2+ entry before stimulation of TRPV1 (capsaicin and 1 µM). Fluorescence emission was monitored in an Eclipsys Spectrofluorometer (Varian Inc, Sydney, Australia) at 505 nm. Excitations in the assay were 340 and 380 nm. Fluorescence intensity of [Ca2+]i level was calculated from the ratio of fura-2 fluorescence from excitation at 340 nm to that from excitation at 380 nm (F340/F380) and they were calibrated according to the method of Grynkiewicz et al. [27]. A total of 3 experiments were performed for measuring the [Ca2+]i concentration.
The [Ca2+]i level was measured using the integral of the rise in [Ca2+]i for 100 s after addition of capsaicin [23]. The [Ca2+]i concentration is reported as nM concentration with sampling at 1-s intervals, as previously described [28, 29].
Cell viability (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2-tetrazolium bromide, MTT) assay
To assess HPer protective effects on cell viability, we evaluated the mitochondrial activity of living cells by an MTT quantitative colorimetric assay. After treatments of 5-FU and HPer, the cells were washed and incubated with fresh medium containing MTT (0.5 mg/ml) at 37 °C for 90 min. Then, the supernatant was discarded and DMSO was added to dissolve the formazan crystals. The absorbance in each well was measured at 490 and 650 nm using a microplate reader (Infinite pro200; Tecan Austria GmbH, Groedig, Austria) [28, 29]. A total of 3 experiments (n = 3) was performed for the cell viability assay. The data are presented as the fold increase over the pretreatment level.
Assay for apoptosis, caspase 3 and 9 activities
The apoptosis analysis was spectrophotometrically performed with a commercial kit according to the instructions provided by Biocolor Ltd. (Northern Ireland) and elsewhere [23, 24]. Briefly, the APOPercentage dye is actively transported into cells when the membrane of apoptotic cell loses its asymmetry and then the dye is staining apoptotic cells red, thus allowing detection of apoptosis by spectrophotometer.
The determinations of caspase 3 and caspase 9 activities were based on a method previously reported [28, 29] with minor modifications. Caspase 3 substrate (AC-DEVD-AMC) and caspase 9 substrate (AC-LEHD-AMC) cleavages were measured with the microplate reader (Infinite pro200) with excitation wavelength of 360 nm and emission at 460 nm. The data were calculated as fluorescence units/mg protein and presented as the fold increase over the pretreatment level.
Intracellular reactive oxygen species (ROS) measurement
DHR 123 is a non-fluorescent, non-charged dye that easily penetrates cell membrane. Once inside the cell, DHR 123 becomes fluorescent upon oxidation to yield rhodamine 123 (Rh 123), the fluorescence being proportional to ROS generation. The fluorescence intensity of Rh123 was measured in the microplate reader (Infinite Pro200). Excitation was set at 488 nm and emission at 543 nm [28]. The data are presented as fold increase over the pretreatment level.
Mitochondrial membrane potential determination
The change in mitochondrial membrane potential caused by 5-FU was assayed using a membrane-permeable dye (JC-1) [30]. The JC-1 dye accumulates in mitochondria in a potential-dependent manner. The green JC-1 signal was measured at the excitation wavelength of 485 nm and the emission wavelength of 535 nm, the red signal at the excitation wavelength of 540 nm and the emission wavelength of 590 nm. Fluorescence changes were analyzed using the microplate reader (Infinite Pro200). The data are presented as the fold increase over the pretreatment level.
Western blot analysis
The total protein in the supernatant was assessed spectrophotometrically (Shimadzu UV-1800, Kyoto, Japan) using Bradford reagent at 595 nm. Equivalent amounts of extracted proteins (50 μg) were separated by 10 or 12% SDS-polyacrylamide gel electrophoresis, and then electro-blotted onto polyvinylidene difluoride membranes. The membranes were blocked using 5% blocking buffer (5% bovine serum albumin in Tris-buffered saline with 0.1% Tween 20) for 1 h at room temperature. Blots were incubated overnight at 4 °C with the following primary antibodies: rabbit anti-caspase 9, rabbit anti-caspase 3 (1:1000), and rabbit anti-PARP (1:1000). The membrane was washed 3 times with Tris-buffered saline containing 0.1% Tween 20 for 10 min and followed by incubation with appropriate secondary antibody for 2 h, and then washed again 3 times with Tris-buffered saline, 0.1% Tween 20. Relative levels of immunoreactivity were quantified using ECL Western HRP Substrate (Millipore Luminate Forte, USA) and visualization was achieved through X-ray film (GE Healthcare, Amersham Hyperfilm ECL, UK) [20]. Rabbit anti-β-actin (1:2000) was used as an internal control for the concentration of proteins loaded. The data are presented as relative density over the pretreatment level (experimental/control).
Statistical analyses
The SPSS statistical program (version 17.0, software, SPSS. Chicago, IL, USA) was used for analyzing the data. All results are expressed as mean ± standard deviation (SD). Statistical significances were detected using Mann–Whitney U test. p values of less than 0.05 were regarded as significant.
Results
Effects of 5-FU and HPer on intracellular [Ca2+]i influx
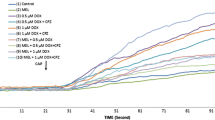
[Ca2+]i influx has been indicated to be important for apoptosis and cancer cell death [22,23,24], although HPer induced Ca2+ influx modulator role in several cells [20, 21, 31]. The line plots and columns of [Ca2+]i as level and influx are presented in Fig. 1a, b, respectively. It is well known that not all cell lines contain capsaicin-sensitive TRPV1 cation channel and they need transfection procedures before studying the TRPV1 channel. However, the MCF-7 cell line has the TRPV1 cation channel without transfection [32]. In the MCF-7 cells, TRPV1 channels were activated by 5-FU but not by HPer incubations. The [Ca2+]i influx was significantly (p < 0.001) higher in the 5-FU group than in the control. However, the [Ca2+]i influx was significantly (p < 0.001) decreased by CPZP and HPer treatments and its influx was lower in the 5-FU+CPZP and 5-FU+HPer groups compared to the 5-FU alone group. The [Ca2+]i influx was also significantly (p < 0.001) lower in the HPer and HPer+CPZP groups than in the 5-FU and 5-FU+HPer+CPZP groups.
In vitro effect of Hypericum perforatum (HPer and 0.3 mM for 24 h) and 5-FU (25 μM for 24 h) on the free intracellular calcium ([Ca2+]i) levels (a) and Ca2+ influx (b) in MCF-7 cell (n = 3 and mean ± SD). The cells are stimulated by capsaicin (CAPSN and 1 µM for 10 min) but they were inhibited by capsazepine (CPZP and 0.01 mM for 30 min). (a) p < 0.001 and (b) p < 0.05 versus control. (c) p < 0.001 versus 5-FU group. (d) p < 0.001 and (e) p < 0.05 versus 5-FU+HPer group. (f) p < 0.001 versus 5-FU+HPer+CPZP group
Apoptosis and cell viability (MTT) results
The mean apoptosis and MTT values in the control, 5-FU, and 5-FU+HPer groups are shown in Fig. 2. The MTT level was significantly (p < 0.001) lower in the 5-FU groups than in the control group, although it was not increased by HPer and CPZP treatments. However, the apoptosis levels were significantly (p < 0.001 and 210%) higher in the 5-FU groups than in the control groups, although the levels were decreased by HPer (≥240%) and CPZP (≥250%) treatments (p < 0.001).
Effect of Hypericum perforatum (HPer and 0.3 mM for 24 h) and 5-FU (25 μM for 24 h) on apoptosis and cell viability (MTT) levels (mean ± SD and n = 3). The cells were stimulated by capsaicin (CAPSN and 1 µM for 10 min) but they were inhibited by capsazepine (CPZP and 0.01 mM for 30 min). Values are expressed as fold increase (experimental/control). (a) p < 0.001 and (b) p < 0.05 versus control. (c) p < 0.001 and (d) p < 0.05 versus 5-FU group. (e) p < 0.001 versus 5-FU+CPZP group. (f) p < 0.001 versus 5-FU+HPer+CPZP group
Caspase 3 and caspase 9 activity results
We investigated the protective effects of HPer and CPZP on the rate of programmed cell death as indicated by caspase values in the 5-FU-incubated cancer cells. The results of caspase 3 and 9 activities in the four groups are shown in Fig. 3. The caspase 3 and 9 activities in the 5-FU group were markedly (p < 0.001) higher than in the control and HPer groups. However, the caspase 3 and 9 activities were markedly (p < 0.001) lower in the 5-FU-HPer and HPer groups than in the 5-FU group only. There were effects on caspase activity of HPer treatments in the cancer cells. There was no statistical change in the caspase activities in CPZP-treated groups. On the other hand, the caspase 3 and 9 activities did not differ between 5-FU and 5-FU+CPZP groups or also between 5-FU+HPer and 5-FU+HPer+CPZP.
Effect of Hypericum perforatum (HPer and 0.3 mM for 24 h) and 5-FU (25 μM for 24 h) treatments on caspase 3 and 9 activities in the MCF-7 cells (mean ± SD and n = 3). The cells were stimulated by capsaicin (CAPSN and 1 µM for 10 min) but they were inhibited by capsazepine (CPZP and 0.01 mM for 30 min). Values are expressed as fold increase. (a) p < 0.001 and (b) p < 0.05 versus control. (c) p < 0.001 versus 5-FU group. (d) p < 0.001 versus 5-FU+CPZP group. (e) p < 0.001 versus 5-FU+HPer+CPZP group
Intracellular ROS production and mitochondrial depolarization (JC-1) levels
In order to determine whether the potentiating effects of HPer and the 5-FU is due to a production of intracellular ROS, fluorescence (JC-1) of oxidation-sensitive intracellular probes (DHR123)-loaded MCF-7 cells were treated with HPer. The mean JC-1 and ROS levels in the four groups are shown in Fig. 4 and it can be seen that the levels of JC-1 and ROS were markedly higher (p < 0.001) in the 5-FU groups than those in the HPer and control groups. However, the JC-1 and ROS levels were markedly decreased (p < 0.001) by HPer treatments. Pretreatment with CPZP did not add to the effect on the JC-1 and ROS levels found in the 5-FU+HPer groups and their levels did not differ in the 5-FU and 5-FU+CPZP or between the 5-FU+HPer and 5-FU+HPer+CPZP groups.
Effect of Hypericum perforatum (HPer and 0.3 mM for 24 h) and 5-FU (25 μM for 24 h) treatments on mitochondrial membrane depolarization (JC-1) and intracellular ROS production in the MCF-7 cells (mean ± SD and n = 3). The cells are stimulated by capsaicin (CAPSN and 1 µM for 10 min) but they were inhibited by capsazepine (CPZP and 0.01 mM for 30 min). Values were expressed as fold increase. (a) p < 0.001 versus control. (b) p < 0.001 versus 5-FU and 5-FU+CPZP groups. (c) p < 0.001 versus 5-FU+HPer and 5-FU+HPer+CPZP groups
Caspase 3, caspase 9, and poly(ADP-ribose) polymerase (PARP) expression levels
Upon apoptotic stimulation, cytochrome c released from mitochondria becomes associated with procaspases 3 and 9/Apaf 1 and then the procaspases are converted to active caspases 3 and 9. In the current study, we assayed active caspase 3 and 9 expression levels as an indicator of apoptosis (Fig. 5). The caspase 3 and 9 expression levels were markedly higher (p < 0.05) in the 5-FU groups than those in the control group. The caspase 3 and 9 expression levels were decreased in the HPer (p < 0.001) and 5-FU+HPer (p < 0.05) groups compared with the 5-FU alone group.
Effect of Hypericum perforatum (HPer and 0.3 mM for 24 h) and 5-FU (25 μM for 24 h) treatments on caspase 3 and 9 expression levels in MCF-7 cells (mean ± SD and n = 3). Values were expressed as relative density over the pretreatment level. (a) p < 0.05 versus groups control. (b) p < 0.001 versus 5-FU and 5-FU+HPer groups
PARP1 is an abundant enzyme present in cells that indicates and signals damage to DNA repair mechanisms. We measured PARP1 expression level as an indicator of damage to DNA repair mechanisms in the four groups (Fig. 6). The PARP1 expression level was significantly (p < 0.05) higher in 5-FU groups than that in the control group. The PARP1 expression level was remarkably decreased in the HPer (p < 0.01) and 5-FU+HPer (p < 0.05) groups compared with the 5-FU group.
Effect of Hypericum perforatum (HPer and 0.3 mM for 24 h) and 5-FU (25 μM for 24 h) treatments on PARP1 expression level in MCF-7 cells (mean ± SD and n = 3). Values were expressed as relative density over the pretreatment level. (a) p < 0.05 versus groups control. (b) p < 0.05 and (d) p < 0.01 versus 5-FU group. (c) p < 0.05 versus 5-FU+HPer group
Discussion
Resistance to chemotherapy can develop during treatment of breast cancers. Therefore, development of an adjuvant therapy with 5-FU in the treatment of breast cancer has great interest. HPer is a traditional medicine that has been used from ancient times in treatments of human neurological disease and now is known to be rich in antioxidants [8]. It has been used for treatment of cancer in a combination with chemotherapeutic agents but not with 5-FU [6, 9, 10]. HPer was tested on apoptosis and cell viability values in cisplatin-exposed MCF-7 cells [6, 33], but its molecular mechanisms of action in the treatment of breast cancer cells have not been clarified yet. The results of the current study clearly indicate that anti-apoptosis and oxidant effects of 5-FU involved activation of TRPV1 channel, although these effects were reduced by HPer treatment through inhibition of the TRPV1.
Oxidative stress induces an irreversible effect on cellular components such as nucleic acids, lipids, and proteins [15] in which the intracellular level of ROS is increased. Chemotherapeutic agents, such as 5-FU, kill cancer cells through over production of intracellular ROS [34]. Overload of Ca2+ entry induces excessive mitochondrial ROS production, mitochondrial membrane depolarization, and caspase 3 and 9 activations [28, 30]. TRPV1 channels are activated by different stimuli including capsaicin and oxidative stress in different cell types including MCF-7 [35]. Therefore, mitochondria are a target for cancer prevention and chemotherapy because chemotherapeutic agents may modulate or interfere with mitochondrial functions to promote mitochondrial membrane permeability and cell death [4, 28, 36]. Chemotherapeutic agent-induced excessive Ca2+ entry through activation of TRPV1 changes membrane structure and functions, promotes excessive intracellular ROS production and depolarization of the mitochondria of intact cells, and induces apoptosis in the MCF-7 cells [23, 24]. In the current study, 5-FU induced excessive Ca2+ entry through activation of TRPV1 and raised intracellular ROS production and depolarization of the mitochondria of intact cells, and induced apoptosis and caspase 3 and 9 activations in the MCF-7 cells. The inhibitor role of HPer on the TRPV1 channel, apoptosis, mitochondrial membrane depolarization, and intracellular ROS production in rat neurons and human neutrophils has been reported [19,20,21, 37]. Similar molecular pathways have now been observed in the current study and we have shown the inhibitory role of HPer in the 5-FU-induced apoptosis, mitochondrial membrane depolarization, intracellular ROS production, caspase 3 and 9 expression levels in MCF-7 cells through inhibition of TRPV1 channel.
The results of the present study show that 5-FU-induced apoptosis and caspase 3 and 9 expression levels were decreased in the MCF-7 cell line by HPer treatment although MTT levels were increased by the treatment. Studies of HPer effects on apoptosis in cancer cell lines are somewhat limited and have produced conflicting results. Similarly, Mirmalek et al. [6] reported that the apoptosis levels indicated by annexin V in MCF-7 cells was lower in a hypericin-treated group (5 μg/ml and 24 h incubation) than in a cisplatin group. The result of another study indicated that a low concentration (100 nM) of hypericin did not inhibit an apoptotic effect in the human fibroblasts cell line, although treatment with normal concentration (10 μM) of hypericin failed to induce apoptosis [38]. However, the results reported by Roscetti et al. [33] indicated that purified hypericin (used in 24 and 48 h incubations) has no inhibitory effect on cell viability and apoptosis in a human erythroleukemic cell line, although a methanolic extract of HPer induced apoptotic effect in the cells. Contrary to our findings, Acar et al. [11] investigated the apoptotic effect of two different doses (1,5 and 7.5 μg/ml) of hypericin in MCF-7 cells and found an apoptotic action of 7.5 μg/ml of hypericin but not by 1.5 μg/ml. Özen et al. [12] investigated the dose-dependent effect of hypericin on apoptosis in blood cancer (HL-60) cells and were unable to demonstrate any apoptotic effect up to a dose of 5 μM dose. It seems that an apoptotic effect of HPer in cancer cells occurs in a dose-dependent manner but can be cell specific.
The critical role of [Ca2+]i levels in regulating cancer cell proliferation has been well documented in breast cancer cells [39]. Activation of the TRPV1 channel induces Ca2+ entry from the extracellular fluid and evokes a transient increase of [Ca2+]i level leading to the stimulation of intracellular ROS production and DNA damage through PARP1 activation [15, 16]. In turn, the increased ROS induces further activation of the TRPV1 channel and increase of [Ca2+]i levels. In mice neurons [40, 41] and pancreatic cancer cells [42], activation of TRPV1 increases Ca2+ entry and apoptosis formation, whereas decrease of [Ca2+]i concentration through TRPV1 treatment results in inhibition of apoptosis [16, 20, 35]. In the present study, we have provided convincing evidence that TRPV1-mediated Ca2+ entry stimulated 5-FU-induced apoptotic cell death in MCF-7 cells via up-regulation of mitochondrial oxidative stress, caspase, and PARP1 by a Ca2+ signaling molecular pathway. The apoptosis level was decreased by HPer through down-regulation of the molecular pathways. Similarly, Eriksson and Eriksson [13] reported possible interactions between hypericin and the Ca2+ SERCA pump.
In conclusion, 5-FU induced anti-apoptotic cell death in MCF7 breast cancer cells through stimulating Ca2+ entry via activation of TRPV1 channels, although HPer induced anti-apoptotic effects through inhibition of the TRPV1 channel. Therefore, stimulation of TRPV1 channel activation increased apoptotic effect of 5-FU in MCF-7 cells. However, blockade of TRPV1-mediated Ca2+ entry through HPer treatment may not be a potential strategy to sensitize breast cancer cells to 5-FU treatment.
Abbreviations
- [Ca2+]i :
-
Intracellular free Ca2+
- 5-FU:
-
5-Fluorouracil
- CAPSN:
-
Capsaicin
- CPZP:
-
Capsazepine
- DHR 123:
-
Dihydrorhodamine-123
- DMSO:
-
Dimethyl sulfoxide
- EGTA:
-
Ethylene glycol-bis[2-aminoethyl-ether]-N,N,N,N-tetraacetic acid
- HPer:
-
Hypericum perforatum
- JC-1:
-
5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- MTT:
-
3-[4,5-Dimethyl-2-thiazolyl]-2,5-diphenyl-2-tetrazolium bromide
- PARP1:
-
Poly(ADP-ribose) polymerase 1
- ROS:
-
Reactive oxygen species
- RPMI:
-
Roswell Park Memorial Institute
- TRP:
-
Transient receptor potential
- TRPV1:
-
Transient receptor potential vanilloid 1
References
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics. CA Cancer J Clin 60:277–300
Nelson SH, Marinac CR, Patterson RE et al (2016) Impact of very low physical activity, BMI, and comorbidities on mortality among breast cancer survivors. Breast Cancer Res Treat 155:551–557
Wyatt MD, Wilson DM 3rd (2009) Participation of DNA repair in the response to 5-fluorouracil. Cell Mol Life Sci 66:788–799
Yang J, Wu Y, Wang X, Xu L, Zhao X, Yang Y (2017) Chemoresistance is associated with overexpression of HAX-1, inhibition of which resensitizes drug-resistant breast cancer cells to chemotherapy. Tumour Biol 39:1010428317692228
Jahani M, Azadbakht M, Norooznezhad F, Mansouri K (2017) L-arginine alters the effect of 5-fluorouracil on breast cancer cells in favor of apoptosis. Biomed Pharmacother 88:114–123
Mirmalek SA, Azizi MA, Jangholi E et al (2016) Cytotoxic and apoptogenic effect of hypericin, the bioactive component of Hypericum perforatum on the MCF-7 human breast cancer cell line. Cancer Cell Int 16:3
Kimira M, Arai Y, Shimoi K, Watanabe S (1998) Japanese intake of flavonoids and isoflavonoids from foods. J Epidemiol 8:168–175
Russo E, Scicchitano F, Whalley BJ et al (2014) Hypericum perforatum: pharmacokinetic, mechanism of action, tolerability, and clinical drug-drug interactions. Phytother Res 28:643–655
Šemeláková M, Mikeš J, Jendželovský R, Fedoročko P (2012) The pro-apoptotic and anti-invasive effects of hypericin-mediated photodynamic therapy are enhanced by hyperforin or aristoforin in HT-29 colon adenocarcinoma cells. J Photochem Photobiol B 117:115–125
Stavropoulos NE, Kim A, Nseyo UU et al (2006) Hypericum perforatum L. extract—novel photosensitizer against human bladder cancer cells. J Photochem Photobiol B 84:64–69
Acar M, Ocak Z, Erdogan K et al (2014) The effects of hypericin on ADAMTS and p53 gene expression in MCF-7 breast cancer cells. J BUON 19:627–632
Özen KP, Şahin F, Avcı ÇB, Hışıl Y, Gündüz C, Saydam G (2007) Hypericium perforatum extract (St. John’s Wort) and hypericin induce apoptosis in leukemic HL-60 cells by effecting h-TERT activity. Turk J Haematol 24:127–133
Eriksson ES, Eriksson LA (2012) Identifying the sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) as a potential target for hypericin—a theoretical study. Phys Chem Chem Phys 14:12637–12646
Ohkubo T, Yamazaki J (2012) T-type voltage-activated calcium channel Cav3.1, but not Cav3.2, is involved in the inhibition of proliferation and apoptosis in MCF-7 human breast cancer cells. Int J Oncol 41:267–275
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Nazıroğlu M (2015) TRPV1 channel: A potential drug target for treating epilepsy. Curr Neuropharmacol 13:239–247
Nazıroğlu M, Övey İS (2015) Involvement of apoptosis and calcium accumulation through TRPV1 channels in neurobiology of epilepsy. Neuroscience 293:55–66
Pecze L, Jósvay K, Blum W et al (2016) Activation of endogenous TRPV1 fails to induce overstimulation-based cytotoxicity in breast and prostate cancer cells but not in pain-sensing neurons. Biochim Biophys Acta 1863:2054–2064
Nazıroğlu M, Çiğ B, Özgül C (2014) Modulation of oxidative stress and Ca(2+) mobilization through TRPM2 channels in rat dorsal root ganglion neuron by Hypericum perforatum. Neuroscience 263:27–35
Uslusoy F, Nazıroğlu M, Övey İS, Sönmez TT (2017) Hypericum perforatum L. supplementation protects sciatic nerve injury-induced apoptotic, inflammatory and oxidative damage to muscle, blood and brain in rats. J Pharm Pharmacol. doi:10.1111/jphp.12741
Nazıroğlu M, Sahin M, Ciğ B, Aykur M, Erturan I, Ugan Y (2014) Hypericum perforatum modulates apoptosis and calcium mobilization through voltage-gated and TRPM2 calcium channels in neutrophil of patients with Behcet’s disease. J Membr Biol 247:253–262
Tang BD, Xia X, Lv XF et al (2017) Inhibition of Orai1-mediated Ca(2+) entry enhances chemosensitivity of HepG2 hepatocarcinoma cells to 5-fluorouracil. J Cell Mol Med 21:904–915
Sakallı Çetin E, Nazıroğlu M, Çiğ B, Övey İS, Aslan Koşar P (2017) Selenium potentiates the anticancer effect of cisplatin against oxidative stress and calcium ion signaling-induced intracellular toxicity in MCF-7 breast cancer cells: Involvement of the TRPV1 channel. J Recept Signal Transduct Res 37:84–93
Koşar PA, Nazıroğlu M, Övey İS, Çiğ B (2016) Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in MCF-7 breast cancer cells: involvement of TRPV1 channels. J Membr Biol 249:129–140
Gao J, Yan Q, Liu S, Yang X (2014) Knockdown of EpCAM enhances the chemosensitivity of breast cancer cells to 5-fluorouracil by downregulating the antiapoptotic factor Bcl-2. PLoS ONE 9:e102590
Leuner K, Li W, Amaral MD et al (2013) Hyperforin modulates dendritic spine morphology in hippocampal pyramidal neurons by activating Ca(2+)-permeable TRPC6 channels. Hippocampus 23:40–52
Grynkiewicz C, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Uğuz AC, Cig B, Espino J et al (2012) Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J Pineal Res 53:91–98
Uğuz AC, Nazıroğlu M, Espino J et al (2009) Selenium modulates oxidative stress induced cell apoptosis in human myeloid HL-60 cells via regulation of caspase-3, -9 and calcium influx. J Membr Biol 232:15–23
Espino J, Bejarano I, Paredes SD et al (2010) Melatonin counteracts alterations in oxidative metabolism and cell viability induced by intracellular calcium overload in human leucocytes: changes with age. Basic Clin Pharmacol Toxicol 107:590–597
Nazıroğlu M, Kutluhan S, Ovey IS, Aykur M, Yurekli VA (2014) Modulation of oxidative stress, apoptosis, and calcium entry in leukocytes of patients with multiple sclerosis by Hypericum perforatum. Nutr Neurosci 17:214–221
Vercelli C, Barbero R, Cuniberti B et al (2014) Transient receptor potential vanilloid 1 expression and functionality in MCF-7 cells: a preliminary investigation. J Breast Cancer 17:332–338
Roscetti G, Franzese O, Comandini A, Bonmassar E (2004) Cytotoxic activity of Hypericum perforatum L. on K562 erythroleukemic cells: differential effects between methanolic extract and hypericin. Phytother Res 18:66–72
Sun Y, Huang L, Mackenzie GG, Rigas B (2011) Oxidative stress mediates through apoptosis the anticancer effect of phospho-nonsteroidal anti-inflammatory drugs: implications for the role of oxidative stress in the action of anticancer agents. J Pharmacol Exp Ther 338:775–783
Çiğ B, Nazıroğlu M (2015) Investigation of the effects of distance from sources on apoptosis, oxidative stress and cytosolic calcium accumulation via TRPV1 channels induced by mobile phones and Wi-Fi in breast cancer cells. Biochim Biophys Acta 1848:2756–2765
Malki A, Mohsen M, Aziz H et al (2016) New 3-cyano-2-substituted pyridines induce apoptosis in MCF 7 breast cancer cells. Molecules 21(2):230
Özdemir ÜS, Nazıroğlu M, Şenol N, Ghazizadeh V (2016) Hypericum perforatum attenuates spinal cord injury-induced oxidative stress and apoptosis in the dorsal root ganglion of rats: involvement of TRPM2 and TRPV1 channels. Mol Neurobiol 53:3540–3551
Hamilton HB, Hinton DR, Law RE et al (1996) Inhibition of cellular growth and induction of apoptosis in pituitary adenoma cell lines by the protein kinase C inhibitor hypericin: potential therapeutic application. J Neurosurg 85:329–334
Nazıroğlu M, Tokat S, Demirci S (2012) Role of melatonin on electromagnetic radiation-induced oxidative stress and Ca2+ signaling molecular pathways in breast cancer. J Recept Signal Transduct Res 32:290–297
Yamaguchi K, Ono K, Hitomi S et al (2016) Distinct TRPV1- and TRPA1-based mechanisms underlying enhancement of oral ulcerative mucositis-induced pain by 5-fluorouracil. Pain 157:1004–1020
Sano T, Utsumi D, Amagase K et al (2017) Lafutidine, a histamine H2 receptor antagonist with mucosal protective properties, attenuates 5-fluorouracil-induced intestinal mucositis in mice through activation of extrinsic primary afferent neurons. J Physiol Pharmacol 68:79–90
Hartel M, di Mola FF, Selvaggi F et al (2006) Vanilloids in pancreatic cancer: potential for chemotherapy and pain management. Gut 55:519–528
Acknowledgements
MN formulated the present hypothesis and was responsible for writing the report. HAD and GN were responsible for analysis of the data. The authors wish to thanks Dr. İshak Suat Övey (Neuroscience Research Center, SDU, Isparta, Turkey) for helping the Western blot analyses and Dr. Peter J Butterworth (Department of Nutrition, King’s College, London, UK) for polishing English.
Funding
There is no financial support for the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Rights and permissions
About this article
Cite this article
Deveci, H.A., Nazıroğlu, M. & Nur, G. 5-Fluorouracil-induced mitochondrial oxidative cytotoxicity and apoptosis are increased in MCF-7 human breast cancer cells by TRPV1 channel activation but not Hypericum perforatum treatment. Mol Cell Biochem 439, 189–198 (2018). https://doi.org/10.1007/s11010-017-3147-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3147-1