Abstract
The aim of this study was to estimate the distribution and density of a representative set of activating and inhibitory receptors on gated natural killer (NK) cells, as well as on their bright and dim subsets, and to correlate the receptor expression with NK cell activity for healthy individuals on CD3−CD16+ NK cells. We show that in 43 healthy controls NK cell activity against K562 target cells was 37.34% (E:T, 80:1) by standard chromium release assay. The expression of receptors on NK cells and their subsets was analyzed by flow cytometry. The cytotoxic CD3−CD16bright NK subset constituted 78.97%, while the regulatory CD3−CD16dim NK subset constituted 21.03% of NK cells. We show the distribution of NKG2D, CD161, CD158a, and CD158b receptors on CD3−CD16+ NK cells in peripheral blood lymphocytes (PBLs), on gated NK cells, and on the CD3−CD16bright and CD3−CD16dim subsets. Contrary to CD158a and CD158b killer immunoglobulin-like receptors (KIRs), there is a significant positive correlation of NKG2D and CD161 expression with NK cytotoxicity. We show the kinetics of change in CD3−CD16+NK/K562 conjugate composition, together with the stronger target binding capacity of CD16bright NK cells. Furthermore, we show that after coculture of PBLs with K562 the expression of CD107a, a degranulation marker, on CD3−CD16+NK cells and subsets is time dependent and significantly higher on the cytotoxic CD3−CD16bright NK subset. The novel data obtained regarding expression of NK cell activating and inhibitory receptors for healthy individuals may aid in detecting changes that are associated with various diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human natural killer (NK) cells are large granular lymphocytes that comprise approximately 15% of peripheral blood lymphocytes (PBLs), lack CD3, and express CD16 and CD56 surface antigens that define the NK cell subset (Cooper et al. 2001a, b; Robertson and Ritz 1990). NK cells are important effectors of the innate immune system that are able to lyse transformed, infected, and allogeneic cells without prior sensitization (Kiessling et al. 1975; Trinchieri 1989; Konjević et al. 1995).
NK cell phenotype and function have been almost exclusively defined with respect to the expression of CD56, an adhesion molecule that is not directly involved in cytotoxicity, and not with respect to CD16 expression (Cooper et al. 2001a, b; Konjević et al. 2003). However, CD16 is one of the most important cytotoxic receptors (Nagler et al. 1989; Sun 2003) that, aside from direct, is also involved in antibody-dependent, cellular cytotoxicity (ADCC) (Lanier et al. 1986), cytokine production, proliferation, and postactivational NK cell apoptotic death (Warren and Kinnear 1999). Moreover, it has been established that CD16, as well as another prominent NK cytotoxic receptor, NKp46, mediates direct killing, i.e., lysis of some virus-infected and tumor cells (Lanier 2003; Mandelboim et al.1999). CD16, as well as NK p46, associates with two cytoplasmatic domains, composed of FcεRI γ or TCR ζ chains (Lanier 2003; Mandelboim et al.1999; Warren and Skipsey 1991). These two intracytoplasmatic chains comprise immunoreceptor tyrosine-based activation motifs (ITAMs), which, upon ligand binding, become phosphorylated and induce signal transduction by activation of nonreceptor tyrosine kineses, such as syk and zeta-chain-associated protein kinase 70 (ZAP-70) (Vivier et al.2004; Lanier 2005).
Moreover, NK cells, based on CD16 cell surface expression, are also divided into two subsets, which are functionally analogous to CD56 subsets. In this sense, CD56dim NK cells have high expression of CD16 and are defined as CD16bright, while CD56bright NK cells have low expression of CD16 antigen and are defined as the CD16dim subset (Nagler et al. 1989). In light of this, the CD16bright-like CD56dim subset has high expression of the main cytotoxic receptor CD16 and is involved in cytotoxicity, while the CD16dim-like CD56bright subset has a regulatory function and produces abundant cytokines (IFN-γ, TNF-α, IL-10, IL-13, and GM-CSF) (Cooper et al. 2001a, b; Biron et al. 1999).
Several new families of activating and inhibitory receptors have recently been identified on NK cells, including natural ctotoxicity receptors (NCRs), c-lectin like receptors, and killer immunoglobulin-like receptors (KIRs), and it has been shown that NK cell activity is determined by the balance of the activating and inhibitory receptors (Farag and Caligiuri 2006). One of the most prominent activating receptors, NKG2D (Raulet 2003), is a c-type lectin that, upon binding stress-induced ligands on transformed cells, such as MHC class I-related molecules, MICA/MICB, and UL16-binding proteins (ULBPs) 1–4 (Diefenbach et al. 2003), induces cytotoxicity by recruiting phosphoinositide (PI)-3-kinases after association of its intracellular domain with DAP10 adaptor protein (Wu et al. 1999). Although NKG2D-mediated responses are beneficial in immune responses against tumors and pathogens, evidence is also emerging that this system may be deleterious by contributing to autoimmunity (Di Santo 2006).
Also, one of the earliest markers of NK cells, CD161 (mouse NKR-P1A analogue) (Bennett et al. 1996), encoded by a single nonpolymorphic gene without a precisely defined signaling pathway in humans (Di Santo 2006), is primarily designated an activating receptor (Lanier 1998; Azzoni et al. 1998). However, recently, upon identification of its lectin-like transcript 1 (LLT1) ligand, its inhibitory potential has been introduced, but it remains controversial (Rosen et al. 2005; Aldemir et al. 2005).
The NK cell KIR repertoire depends on both KIR and HLA gene polymorphisms (Raulet et al. 2001). Clinical studies have correlated KIR gene content with infection, cancer, autoimmunity, pregnancy syndromes, and transplant outcome (Trowsdale and Parham 2004; Yoon et al. 2007; Boyton and Altmann 2007; Martin and Carrington 2008). Consequently, differential expression of these inhibitory receptors by subsets of human NK cells allows them to carefully monitor self (and foreign) MHC molecules and uniquely regulate cytotoxicity when pathological processes perturb MHC expression (Di Santo 2006). KIR is divided into haplotype A and B, with A being more frequent and including inhibitory CD158a (KIR2DL1) and CD158b (KIR2DL2,3) receptors (Trowsdale and Parham 2004), which recognize HLA-Cw4,6,5 and HLA-Cw1,3,7 HLA class I molecules, respectively (Uhrberg et al. 1997; Yawata et al. 2006). These two KIRs inhibit NK cell activity through an immune tyrosine-based inhibitory motif (ITIM) (Long 1999) by recruiting protein tyrosine phosphatases (SHP-1 and SHP-2) responsible for dephosphorylation of surrounding tyrosine kinases and adaptor proteins, including DAP-10. Futhermore, blocking actin cytoskeleton-dependent raft recruitment of different receptors may be a general mechanism by which inhibitory receptors control NK cell activation (Watzl and Long 2003). Moreover, it has recently been shown that higher expression of CD158b on NK cells is implicated in greater susceptibility to melanoma and its metastasis (Naumova et al. 2007).
Despite the fact that formation of NK cell conjugates with tumor cells is a prerequisite for direct cytotoxicity, i.e., necrosis, characterized by exocytosis of perforin and granzyme granules, these NK-K562 target tumor cell conjugates have only been characterized with respect to CD56+ NK cells (Vitale et al. 1991; Jacobs et al. 2001), and not with respect to CD16-defined NK cells, although CD16 is engaged in cell-mediated cytotoxicity.
Furthermore, NK conjugate formation is followed by transient membrane expression of specific CD107a, lysosomal-associated membrane protein 1 (LAMP-1), associated with release of lytic lysosomal vesicles containing perforin, and is strongly upregulated on the surface after cytotoxic degranulation (Rubio et al. 2003; Trzonkowski et al. 2004). Despite the fact that the most potent mediators of natural cytotoxicity are CD16+CD56dim NK cells, the expression of 107a has until now been analyzed almost exclusively on CD56+ NK cells (Gryzwacz et al. 2007).
There is an urgent need for reference values for NK cell receptors, as their changes could confer altered risk in a number of diseases including human immunodeficiency virus (HIV) susceptibility and progression, hepatitis C virus clearance, idiopathic bronchiectasis, autoimmunity, and cancer. Moreover, receptors such as NKG2D, which are beneficial in surveillance against cancer and infections but deleterious in the development of autoimmune disorders, could also, together with their ligands, become targets for therapeutic intervention.
In this study we define in healthy individuals the distribution and density of a representative set of NK cell activating and inhibitory receptors and correlate their percentage with NK cell activity on CD16-defined NK cells and the cytotoxic CD16bright and regulatory CD16dim subsets. Considering that changes in receptor expression modify NK cell activity, these novel data may be useful in detecting predisposition for or existence of various diseases.
Methods
In this study heparinized blood samples were obtained from 43 healthy volunteers (23 males and 20 females) from 23 to 51 years of age with no evidence of any disease or infection. All 43 healthy individuals were evaluated for NK cell activity, 32 individuals for NK cell phenotype, and 14 for conjugate formation with K562 and expression of CD107a degranulation marker. Correlation between NK cell cytotoxicity and the percentage of CD16bright NK cells was simultaneously investigated for 26 individuals.
Peripheral Blood Mononuclear Cell (PBMC) Isolation
PBMCs were isolated using a Lymphoprep (Nypacon, Norway) density gradient, centrifuged at 500 g for 40 min, and washed three times in RPMI 1640 culture medium (CM; Gibco, UK) supplemented with 10% fetal calf serum (FCS; Sigma, USA).
Flow Cytometric Analysis
Surface phenotypes of freshly isolated PBMC subsets were identified using the following combinations of directly labeled monoclonal antibodies (mAbs): CD3PerCP/CD16FITC, CD3PerCP/ CD16FITC/CD161PE, CD3PerCP/CD16PE/CD158aFITC, CD3PerCP/CD16FITC/CD158bPE (Becton Dickinson, San Jose, CA, USA), and CD3PerCP/CD16FITC/NKG2DPE cells (R&D, USA). Samples were prepared as previously described (Jackson and Warner 1986). Briefly, 1.0 × 105 freshly isolated PBMC in 100 μl of RPMI 1640 supplemented with 10% FCS were incubated for 30 min at 4°C with 20 μl of the appropriate mAb combination, washed twice with ice-cold PBS, and fixed with 1% paraformaldehyde prior to FACS analyses. Surface marker expression was quantified on a FACSCalibur flow cytometer (Becton Dickinson). A total of 10,000–50,000 events verified as PBLs, according to their physical characteristics (FSC and SSC), were collected per sample and analyzed using CellQUEST software. Exclusion of nonspecific fluorescence was based on matched isotype mAb combinations conjugated with FITC, PE, and PerCP (Becton Dickinson). In order to define two subsets of low, i.e., CD16dim, and high, i.e., CD16bright, NK cells, CD3−CD16+ NK cells were divided based on the density of the CD16 antigen defined by mean fluorescence intensity (MFI) (Konjević et al. 2009). In order to precisely define the expression of any receptor on CD16+ NK cells for each individual, the flow cytometry data on the percentage of a double-positive subset (e.g., CD3−CD16+CD161+) was divided by the percentage of CD3−CD16+ NK cells and multiplied by 100, according to the following formula:
as previously reported (Konjević et al. 2007), for precise definition of, e.g., the CD3−CD16+CD161+ subset. For the expression of NK cell receptors in the CD16dim or CD16bright NK cell subsets, this formula was used with appropriate substitutions relevant for these NK cell subsets. This calculation gives results identical to those with flow cytometric gating.
The density of expression of any NK receptor (CD16, CD161, NKG2D, CD158a, CD158b) in the CD16dim or CD16bright NK cell subsets was defined by MFI.
CD107 Expression Assay
CD107a (Becton Dickinson) expression was estimated on CD3−CD16+ NK cells and CD16dim and CD16bright NK cell subsets after 30 min and 4 h of PBL stimulation with target K562 tumor cells at an effector-to-target, E (PBL):T (K562), ratio of 3:2 (Rubio et al. 2003).
Natural Cytotoxicity Assay
NK cell specific lysis was determined using a standard cytotoxicity assay (Brown et al. 1985). One hundred microliters of freshly isolated PBMCs, as effector cells, at a concentration of 4.0 × 106/ml CM and two 1:1 dilutions, were mixed with 100 μl of the erythromyeloid cell line K562, at a concentration of 0.05 × 10/6/ml (prelabeled with radioactive chromium-51 (Na2CrO4, As = 3.7 MBq; Amersham, UK)), to form triplicates of three E:T ratios: 80:1, 40:1, and 20:1. The assay was performed in 96-microwell round-bottom plates (Falcon, USA), which were incubated for 4 h at 37°C in a humidified atmosphere containing 5% CO2. Plates were then centrifuged for 3 min at 200 g and the supernatant from each well was used for determination of the amount of 51Cr released from the lysed target tumor cells by a gamma counter (Berthold, FRG), expressed as counts per minute (cpm). The mean percentage cytotoxicity was calculated using the following formula:
Maximal release was obtained by incubation of target K562 cells at the same concentration in the presence of 5% Triton X-100, and spontaneous release was obtained by incubation of the appropriate target tumor cell line in culture medium alone.
Conjugate Forming Assay
Freshly isolated PBMCs from healthy donors were adjusted to 3 × 106cells/ml and stained with CD16PE and CD3 PerCp monoclonal antibodies. One hundred microliters of the PBL suspension was added to 100 μl of washed K562 (1 × 106/ml). The cell mixture was centrifuged at 100×g for 3 min, then one sample was incubated for 30 min and another sample for 4 h at 37°C in a humidified atmosphere in a CO2 incubator. Cells were gently mixed and analyzed by FACS by gating previously on PBLs and excluding CD3+ cells. Because K562 cells display discrete autofluorescence in FL1, conjugates can be identified in green (FITC) and red (CD16PE) (Jacobs et al. 2001).
The fluorescence of pure target cells, lymphocytes, and lymphocytes mixed with target cells stained with matched isotype controls of mAbs directly conjugated with FITC, PE, and PerCP (Becton Dickinson) on FL1, FL2, and FL3 was taken into account as the background control. The background data were subtracted from the analyzed samples and the remaining events were divided according to the autofluorescence of target cells on FL1. K562/lymphocyte conjugates were gated based on their scatter (FSC and SSC) characteristics, showing a size greater than that of lymphocytes and similar to that of target cells. CD3+ cells were excluded by gating on PBMC and K562 (Vitale et al. 1991). By applying the appropriate gating procedure, CD3−/CD16dim+ and CD3−/CD16bright cells were assessed within the conjugates. For the conjugate forming assay, as well as all flow cytometric analysis, we always acquired 50,000 events.
Statistical Analysis
The significance of differences for results obtained for bright and dim CD3−CD16+ NK cell subsets was determined by nonparametric exact Wilcoxon signed-rank test, while the correlation between NK cell activity and receptor expression or density was determined by Pearson’s test. For multiple comparisons, p-values were adjusted accordingly with Bonferroni correction (*α1 < 0.05/3 = 0.0166).
Results
Evaluation of cytotoxic NK cell activity of 43 healthy controls performed against the standard sensitive erythromyeloid K562 tumor target cell line showed 37.34 ± 2.83%, 22.25 ± 2.52%, and 14.47 ± 2.06% cytotoxicity for E:T ratios of 80:1, 40:1, and 20:1, respectively (Fig. 1a).
Evaluation of NK cell cytotoxic activity of 43 healthy controls, performed against the standard sensitive K562 tumor target cell line, for E:T ratios of 80:1, 40:1, and 20:1 (a); percentage of two functionally different NK cell subpopulations, CD3−CD16bright and CD3−CD16dim, in freshly isolated PBLs of 32 healthy individuals (b); and positive correlation of NK cell lysis for E:T ratios of 80:1, 40:1, and 20:1; and percentages of the cytotoxic, CD3−CD16bright, NK cell subset of 26 healthy individuals (c). Results are shown as box plots showing the median, lower and upper quartiles, and range of the data
According to the density of expression of CD16 on NK cells, the percentage of regulatory CD3−CD16dim NK cells for 32 healthy controls was 21.03 ± 2.07%, while the percentage of CD3−CD16bright was 78.97 ± 2.07% (Fig. 1b); it was significantly (p < 0.01, Pearson’s test) correlated with NK cell cytotoxicity for an E:T ratio of 80:1 and also for E:T ratios of 40:1 (p = 0.0009) and 20:1 (p = 0.014) (Fig. 1c). The percentage of CD3−CD16dim NK cell subsets was 21.03 ± 2.07% (Fig. 1b) and did not show any correlation (data not shown) with NK cell cytotoxicity.
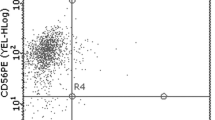
Expression of NKG2D, CD161, CD158a, and CD158b NK cell receptors was estimated as the percentage on CD3−CD16+ NK cells in PBLs and on gated CD3−CD16+ NK cells (Fig. 2a–c). Results obtained for NK cell receptors on CD3−CD16+ NK cells in PBLs showed that NKG2D+ and CD161+ were the most abundant (11.78 ± 1.60% and 5.45 ± 0.1.24%, respectively), while CD158a+ and CD158b+ (1.16 ± 0.21% and 4.84 ± 0.58 %, respectively) were the less abundant populations in CD3−CD16+ lymphocytes. Analysis of these receptors on a gated CD3−CD16+ NK cell population showed that NKG2D had the highest expression (77.87 ± 2.70%), CD161 (37.57 ± 4.40%) and CD158b (38.3 ± 3.02%) had similar, lower levels of expression, and CD158a (12.31 ± 2.18%) had the lowest level.
The percentage of activating, NKG2D and CD161, and KIR inhibitory, CD158a and CD158b, NK cell receptors in freshly isolated CD3−CD16+ NK cells in PBLs (a) and on gated CD3−CD16+ NK cells (b) in healthy controls (n = 32) and representative flow cytometric dot plots of all NK cell receptors on CD3−CD16+ NK cells in PBLs (c). Results are shown as box plots showing the median, lower and upper quartiles, and range of the data
Percentage expression of actived NK cell receptors on the CD3−CD16+ NK cell subset correlated positively with NK cell cytotoxic function, showing a higher significance for NKG2D (p = 0.0005, Pearson’s test) than for CD161 (p = 0.01548) (Table 1), while inhibitory KIRs (CD158a and CD158b) did not correlate with NK cell function.
Analyses of the expression of different NK cell receptors on the CD16dim and CD16bright NK cell subsets (Fig. 3) showed that the expression of NKG2D (70.73 ± 3.24%) as well as CD161 (78.46 ± 2.66%) was significantly higher (p < 0.01, exact Wilcoxon signed-rank test) on the CD16bright than on the CD16dim NK cell subset (29.27 ± 3.24% for NKG2D and 21.54 ± 2.66% for CD161). Further analysis showed that the expression of KIR receptors, CD158a and CD158b, was significantly higher (p < 0.01; 93.18 ± 1.30% and 82.46 ± 2.93%, respectively) on CD16bright, although the receptors were also present on the CD16dim NK cell subset (6.82 ± 1.30% and 17.54 ± 2.93%, respectively).
In Table 2 we list the percentage, range, and minimum and maximum values for all investigated parameters expressed as CD3−CD16+, CD3−CD16dim, or CD3−CD16bright NK or the expression of NK cell activating (NKG2D, CD161) and inhibitory (CD158a, CD158b) receptors in PBLs and CD3−CD16+, CD3−CD16dim, or CD3−CD16bright NK cell subsets. The absolute numbers listed were calculated by multiplying the number of PBLs obtained for each individual with the percentage for each subset obtained by flow cytometry and are expressed as the number of each subset × 109 per liter.
The density of CD16 antigen expression, i.e., mean fluoresence intensity (MFI) was significantly higher (p < 0.01, exact Wilcoxon signed-rank test) on the CD16bright (453.2 ± 36.42) than on the CD16dim (48.88 ± 3.60) NK cell subset. Analysis of the density of expression of the other NK cell receptors showed that the MFI of CD161 and CD158b was significantly higher on the CD16bright NK cell subset (p < 0.05 exact), while NKG2D and CD158a did not show any significant difference in its MFI between the bright and the dim NK cell subsets (Table 3).
Conjugate forming assay was used to measure complexes between CD3−CD16+ NK and K562 tumor target cells utilizing the fact that K562 tumor cells display green (FL-1) fluorescence by flow cytometry. Analysis of NK cell/K562 conjugates over time indicated that after 30 min of incubation, CD16bright, compared to CD16dim, NK cells showed more effective binding, a result that persisted, although at a lower level, after 4 h of incubation and was characterized by poorer detection of conjugates (Fig. 4a–c).
Representative flow cytometric dot plots of NK cell/K562 target tumor cell conjugates over time showing that, compared to the NK subset distribution at the beginning of coincubation (a), the distribution after 30 min shows stronger binding of CD16bright NK cells to K562 target tumor cells, compared to CD16dim NK cells (b), and the change in subset distribution in favor of CD16dim NK cells that is detected after longer, 4-h, coincubation (c). CD3+ cells were eliminated by an appropriate gating procedure
Analysis of the expression of CD107a, a marker that characterizes NK cell degranulation, showed that, compared to the initial value of 16.98 ± 3.74% for gated CD3−CD16+CD107a+ cells, there was a significant (p = 0.0004, Bonferroni correction, exact Wilcoxon signed-rank test) increase, to 36.36 ± 3.74%, after 30 min of contact of NK cells with K562 target cells, which did not differ significantly (52.79 ± 3.60%) even after 4 h of incubation. It was also found that the expression of CD107a showed the same trend of a significant (p = 0.0008) increase during the given incubation period on the gated CD16bright NK cell subset, when initial values (23.04 ± 6.9%) were compared to those after 30 min (55.55 ± 4.38%) and after 4 h (63.48 ± 3.46%) of incubation with K562 tumor cells. Contrary to this, the CD3−CD16dim NK cell subset showed lower expression of CD107a in comparason to the CD3−CD16bright subset, with a significant (p = 0.0004), although transient increase after 30 min (Fig. 5a–c).
Expression of CD107a increases significantly (p < 0.01, exact Wilcoxon signed-rank test) after 30 min and after 4 h of coincubation with K562 target tumor cells compared to the initial value on CD3−CD16+ NK cells in PBLs (a), while analyses pertaining to NK cell subset expression show a 10-fold increase on CD3−CD16bright NK cells and only a 2-fold increase on CD3−CD16dim (b) in investigated healthy controls. Results are shown as mean ± standard error for 14 healthy individuals. Representative flow cytometry dot plots show the change in CD107a expression on gated. CD3−CD16bright and CD3−CD16dim NK cells at 0 min, 30 min, and 4 h of coincubation with K562 target tumor cells (c)
Discussion
Considernig that NK cells consitute the most important effector subset of innate immunity and represent the first line of defense against viruses, parasites, and tumors (Kiessling et al. 1975; Trinchieri 1989), in this study we evaluated NK cell activity, as well as the percentage of two functionally distinct NK cell subsets, i.e., the regulatory CD16dim and the cytotoxic CD16bright subset.
In this study we obtained new data regarding the density of expression (MFI) of CD16 in the CD16bright subset and showed that it is 10 times higher than the density in the CD16dim subset, which is in accord with a study on one individual only (Cooper et al. 2001a) and another on six healthy individuals (Lima et al. 2001) that reported CD16 density in the corresponding CD56 subsets. In light of this, we showed that the percentage cytotoxic CD3−CD16bright NK cell subset correlates positively with NK cell cytotoxic function, which supports the original finding by Nagler et al. (1989)that CD16 is involved in direct cytotoxicity, as the isolated CD16− NK cell subset shows very low levels of cytotoxicity against numerous malignant cell lines.
Our extensive analyses of the distribution of several NK cell receptors show that the most prominent activating receptor, NKG2D (Diefenbach et al. 2003), is also the most abundant receptor when analyzed on NK cells in PBLs and on gated NK cells, which is in agreement with several other studies, although exclusively on gated CD3−CD56+ cell subsets (André et al. 2004; Izumi et al. 2006). We also show ample expression of CD161, another common NK cell receptor, analyszed in the same way, which differs from the higher values for CD161 expression on gated NK cells obtained in a few other studies, probably due to high variation between the minimal and the maximal values in those studies (Pascal et al. 2004; Izumi et al. 2006). Recently, decreased expression of these two activating receptors has been shown to be associated with impaired NK cell cytotoxicity in metastatic melanoma (Konjević et al. 2007).
Our results define the presence of the most frequent inhibitory KIRs, CD158a and CD158b, showing that they are not present on all NK cells, as well as the fact that the expression of CD158b is three times higher than that of CD158a (Pascal et al. 2004). Despite the importance of these KIRs, only one report defines their expression on CD3−CD16+ NK cells (Kogure et al. 2003), while few other studies are based on CD56 NK subsets (Kogure et al. 1999; Zambello et al. 2003; Epling-Burnette et al. 2003; Gazit et al. 2004), the majority of them giving only a qualitative description of KIR expression (Cooper et al. 2001a, b). It is interesting that although the NK cell subset KIR repertoire is determined stochastically (Pascal et al. 2004) and the KIR haplotype frequencies vary, these differences may reflect regional pathogen-driven selection (Boyton and Altmann 2007).
The importance of NKG2D and CD161 NK cell receptors is reflected in their positive correlation with NK cell activity, as opposed to the lack of correlation between the expression of inhibitory CD158a and CD158b KIRs and NK cell cytotoxicity, possibly reflecting the low percentage and enormous diversity of the KIR family. This supports the data that inhibitory KIRs on NK cells serve as a rheostat, regulating and dampening signals transduced through activating receptors without being able to completely terminate NK cell effector function (Lanier 2005).
Regarding the functional dichotomy of NK cells, there are very few reports on healthy individuals that deal with the quantitative distribution of NK cell receptors on CD56, and even less so on CD16, bright and dim NK cell subsets (Cooper et al. 2001b; Lima et al. 2001; Takahashi et al. 2007). Our extensive new results also show significantly higher expression of investigated NKG2D, CD161, CD158a, and CD158b receptors on the CD16bright compared to the CD16dim NK cell subset. The found predominance of KIRs on the cytotoxic CD16bright compared to the CD16dim NK cell subset suggests their regulatory role in NK cell cytotoxicity, which is essential in maintaining tolerance. Furthermore, the importance of KIRs on NK cells not only lies in regulating NK cell cytotoxicity but also, based on the “one cell-one inhibitory receptor” hypothesis, implies that their contact with self MHC class I molecules during development provides a positive signal for NK cell maturation, leading to licensing of fully competent peripheral NK cells (Anfossi et al. 2006).
We also analyzed receptor density on the CD16bright and CD16dim NK cell subsets in order to better define receptor expression. Our data, showing a similar density of NKG2D expression on CD16bright and CD16dim NK cell subsets, are in agreement with one report, however, evaluated on CD56dim and CD56bright NK cells (André et al. 2004) or the entire CD56+ NK population (Epling-Burnette et al. 2004), as well as a significantly higher density of CD161 on the CD16bright (Lima et al. 2001; Tarazona et al. 2002) and, also, a significantly higher density of CD158b on the CD16bright NK cell subset. However, there is no significant difference in the density of the less abundant CD158a receptor, giving new insight into KIR characteristics (Cooper et al. 2001b; Lima et al. 2001).
To our knowledge, there are no reports that deal with the kinetics of NK/K562 tumor cell line conjugate composition with respect to either CD56 or CD16 receptors or functionally diverse bright and dim NK cell subsets. We provide new data for CD3−CD16+/K562 conjugates demonstrating, after 30 min, a stronger binding capacity of CD16bright cells to K562, which we have shown to amply express activating NKG2D receptor, as opposed to CD16dim NK cells. Moreover, in our kinetic study we demonstrate, for the first time, the expected finding that conjugate composition changes with time, so that after a longer (4-h) follow-up, the conjugates lose CD16bright in favor of CD16dim NK cells. These data confirm that following secretion of cytotoxic granules, NK cells become CD16dim or even CD16 negative, as suggested in one previous report (Gryzwacz et al. 2007).
Furthermore, the appearance of increased CD107 on CD3−CD16bright NK cells after 30 min of contact with K562 cells, a finding that is maintained for 4 h, supports the positive correlation shown between this subset and NK cell cytotoxicity. Contrary to this, our finding that the regulatory and weakly cytotoxic CD3−CD16dim NK cells have lower expression of CD107a, despite a transient increase after 30 min, supports their limited cytotoxic potential (Alter et al. 2005). We would like to point out that the lower percentage of CD107a obtained in this study compared to the very few previous studies is probably the consequence of the substantially lower number of investigated healthy individuals (Penack et al. 2005), analysis of CD107a expression on CD3−CD56+ NK cells using varying durations of incubation (Bryceson et al. 2005; Gryzwacz et al. 2007), or diverse E:T ratios (Alter et al. 2004, 2005; Anfossi et al. 2006; Gryzwacz et al. 2007; Bryceson et al. 2005).
Considering that engagement of KIRs with self MHC class I molecules, according to the “licensing hypothesis,” is required for maturation (Raulet 2006; Gasser and Raulet 2006), we show that the KIR-positive CD3−CD16bright NK subset is CD107-high and encompasses mature cytotoxic cells rich in proteolytic granules, as opposed to the KIR-low/negative CD3−CD16dim NK subset, which is defined as regulatory, having fewer granules and, consequently, lower CD107a expression. In this study we confirm the novel hypothesis that potentially autoreactive KIR-low NK cells, detected in investigated healthy individuals as KIR-low/negative CD3−CD16dim NK cells, acquire tolerance and become “hyporesponsive during development,” as shown by their low CD107a expression, reflecting reduced cytotoxicity (Fernandez et al. 2005).
Based on our extensive investigation of NKG2D, CD161, CD158a, and CD158b NK cell receptors on CD3−CD16+ NK cells and CD3−CD16bright and CD3−CD16dim subsets in healthy individuals, we show a significant correlation of NKG2D and CD161 with NK cell cytotoxicity. Moreover, through analysis of the CD107a degranulation marker, we unambiguously show the cytotoxic potential of the CD16bright NK cell subset. These novel data for a set of NK cell activating and inhibitory receptors are significant, as their up- or down-regulation may participate in various pathophysiological conditions.
References
Aldemir H, Prod’homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, Bihl F, Braud VM (2005) Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol 175:7791–7795
Alter G, Malenfant JM, Altfeld M (2004) CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294:15–22
Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, Streeck H, Johnston MN, Staller KD, Zaman MT, Yu XG, Lichterfeld M, Basgoz N, Rosenberg ES, Altfeld M (2005) Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 106:3366–3369
André P, Castriconi R, Espéli M, Anfossi N, Juarez T, Hue S, Conway H, Romagné F, Dondero A, Nanni M, Caillat-Zucman S, Raulet DH, Bottino C, Vivier E, Moretta A, Andre PP (2004) Comparative analysis of human NK cell activation induced by NKG2D and natural cytotoxicity receptors. Eur J Immunol 34:961–971
Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E (2006) Human NK cell education by inhibitory receptors for MHC class. Immunity 25:331–342
Azzoni L, Zatsepina O, Abebe B, Bennett IM, Kanakaraj P, Perussia B (1998) Differential transcriptional regulation of CD161 and a novel gene, 197/15a, by IL-2, IL-15, and IL-12 in NK and T cells. J Immunol 161:3493–3500
Bennett IM, Zatsepina O, Zamai L, Azzoni L, Mikheeva T, Perussia B (1996) Definition of a natural killer NKR-P1A +/CD56−/CD16− functionally immature human NK cell subset that differentiates in vitro in the presence of interleukin 12. J Exp Med 184:1845–1856
Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP (1999) Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol 17:189–220
Boyton RJ, Altmann DM (2007) Natural killer cells, killer immunoglobulin-like receptors and human leucocyte antigen class I in disease. Clin Exp Immunol 149:1–8
Brown RL, Ortaldo JR, Griffith RL, Blanca I, Rabin H (1985) The proliferation and function of human mononuclear leukocytes and natural killer cells in serum-free medium. J Immunol Methods 81:207–214
Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO (2005) Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med 202:1001–1012
Cooper MA, Fehniger TA, Caligiuri MA (2001a) The biology of human natural killer-cell subsets. Trends Immunol 22:633–640
Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA (2001b) Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 97:3146–3151
Di Santo JP (2006) Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol 24:257–286
Diefenbach A, Hsia JK, Hsiung MY, Raulet DH (2003) A novel ligand for the NKG2D receptor activates NK cells and macrophages and induces tumor immunity. Eur J Immunol 33:381–391
Epling-Burnette PK, Painter JS, Chaurasia P, Bai F, Wei S, Djeu JY, Loughran TP Jr (2004) Dysregulated NK receptor expression in patients with lymphoproliferative disease of granular lymphocytes. Blood 103:3431–3439
Farag SS, Caligiuri MA (2006) Human natural killer cell development and biology. Blood Rev 20:123–137
Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH (2005) A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood 105:4416–4423
Gasser S, Raulet DH (2006) Activation and self-tolerance of natural killer cells. Immunol Rev 214:130–142
Gazit R, Garty BZ, Monselise Y, Hoffer V, Finkelstein Y, Markel G, Katz G, Hanna J, Achdout H, Gruda R, Gonen-Gross T, Mandelboim O (2004) Expression of KIR2DL1 on the entire NK cell population: a possible novel immunodeficiency syndrome. Blood 103:1965–1966
Gryzwacz B, Kataria N, Verneris MR (2007) CD56dimCD16 + NK cells downregulate CD16 following target cell induced activation of matrix metaloprteinases. Letter to the Editor. Leukemia 21:356–359
Izumi Y, Ida H, Huang M, Iwanaga N, Tanaka F, Aratake K, Arima K, Tamai M, Kamachi M, Nakamura H, Origuchi T, Kawakami A, Anderson P, Eguchi K (2006) Characterization of peripheral natural killer cells in primary Sjögren’s syndrome: impaired NK cell activity and low NK cell number. J Lab Clin Med 147:242–249
Jackson A, Warner N (1986) Preparation, staining and analysis by flow cytometry of peripheral blood leukocytes. In: Rose N, Friedman H, Fahey J (eds) Manual of clinical laboratory immunology, 3rd edn. American Society for Microbiology, Washington, DC, pp 226–235
Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE (2001) CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol 31:3121–3127
Kiessling R, Klein E, Wigzell H (1975) “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 5:112–117
Kogure T, Fujinaga H, Niizawa A, Hai LX, Shimada Y, Ochiai H, Terasawa K (1999) Killer-cell inhibitory receptors, CD158a/b, are upregulated by interleukin-2, but not interferon-gamma or interleukin-4. Mediators Inflamm 8:313–318
Kogure T, Mantani N, Sakai S, Shimada Y, Tamura J, Terasawa K (2003) Natural killer cytolytic activity is associated with the expression of killer cell immunoglobulin-like receptors on peripheral lymphocytes in human. Mediators Inflamm 12:117–121
Konjević G, Schlesinger B, Cheng L, Olsen KJ, Podack ER, Spuzic I (1995) Analysis of perforin expression in human peripheral blood lymphocytes, CD56+ natural killer cell subsets and its induction by interleukin-2. Immunol Invest 24:499–507
Konjević G, Jović V, Jurisić V, Radulović S, Jelić S, Spuzić I (2003) IL-2-mediated augmentation of NK-cell activity and activation antigen expression on NK- and T-cell subsets in patients with metastatic melanoma treated with interferon-alpha and DTIC. Clin Exp Metastasis 20:647–655
Konjević G, Martinovi KM, Jurisi V, Babovi N, Spuzi I (2009) Biomarkers of suppressed natural killer (NK) cell function in metastatic melanoma: decreased NKG2D and increased CD158a receptors on CD3-CD16+ NK cells. Biomarkers 14:258–270
Konjević G, Mirjacić Martinović K, Vuletić A, Jović V, Jurisić V, Babović N, Spuzić I (2007) Low expression of CD161 and NKG2D activating NK receptor is associated with impaired NK cell cytotoxicity in metastatic melanoma patients. Clin Exp Metastasis 24:1–11
Lanier LL (1998) NK cell receptors. Annu Rev Immunol 16:359
Lanier LL (2003) Natural killer cell receptor signaling. Curr Opin Immunol 15:308–314
Lanier LL (2005) NK cell recognition. Annu Rev Immunol 23:225–274
Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH (1986) The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol 136:4480–4486
Lima M, Teixeira MA, Queiros ML, Leite M, Santos AH, Justica B, Orfao A (2001) Immunophenotypic characterization of normal blood CD56 + lo versus CD56 + hi NK-cell subsets and its impact on the understanding of their tissue distribution and functional properties. Blood Cells Mol Dis 27:731–743
Long EO (1999) Regulation of immune responses through inhibitory receptors. Annu Rev Immunol 17:875–904
Mandelboim O, Malik P, Davis DM, Jo CH, Boyson JE, Strominger JL (1999) Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc Natl Acad Sci USA 96:5640–5644
Martin MP, Carrington M (2008) KIR locus polymorphisms: genotyping and disease association analysis. Methods Mol Biol 415:49–64
Nagler A, Lanier LL, Cwirla S (1989) Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol 143:3183–3191
Naumova E, Mihaylova A, Ivanova M, Mihailova S (2007) Impact of KIR/HLA ligand combinations on immune responses in malignant melanoma. Cancer Immunol Immunother 56:95–100
Pascal V, Schleinitz N, Brunet C, Ravet S, Bonnet E, Lafarge X, Touinssi M, Reviron D, Viallard JF, Moreau JF, Déchanet-Merville J, Blanco P, Harlé JR, Sampol J, Vivier E, Dignat-George F, Paul P (2004) Comparative analysis of NK cell subset distribution in normal and lymphoproliferative disease of granular lymphocyte conditions. Eur J Immunol 34:2930–2940
Penack O, Gentilini C, Fischer L, Asemissen AM, Scheibenbogen C, Thiel E, Uharek L (2005) CD56dimCD16neg cells are responsible for natural cytotoxicity against tumor targets. Leukemia 19:835–840
Raulet DH (2003) Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 3:781–790
Raulet DH (2006) Missing self recognition and self tolerance of natural killer (NK) cells. Semin Immunol. 18:145–150
Raulet DH, Vance RE, McMahon CW (2001) Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol 19:291–330
Robertson MJ, Ritz J (1990) Biology and relevance of human natural killer cells. Blood 76:2421–2438
Rosen DB, Bettadapura J, Alsharifi M, Warren Mathew PA, HS Lanier LL (2005) Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol 175:7796–7799
Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP (2003) Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med 9:1377–1382
Sun PD (2003) Structure and function of natural-killer-cell receptors. Immunol Res 27:539–548
Takahashi E, Kuranaga N, Satoh K, Habu Y, Shinomiya N, Asano T, Seki S, Hayakawa M (2007) Induction of CD16+ CD56bright NK cells with antitumour cytotoxicity not only from CD16− CD56bright NK Cells but also from CD16− CD56dim NK cells. Scand J Immunol 65:126–138
Tarazona R, Casado JG, Delarosa O, Torre-Cisneros J, Villanueva JL, Sanchez B, Galiani MD, Gonzalez R, Solana R, Peña J (2002) Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. J Clin Immunol 22:176–183
Trinchieri G (1989) Biology of natural killer cells. Adv Immunol 47:187–376
Trowsdale J, Parham P (2004) Mini-review: defense strategies and immunity-related genes. Eur J Immunol 34:7–17
Trzonkowski P, Szmit E, Myśliwska J, Dobyszuk A, Myśliwski A (2004) CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol 112(3):258–267
Uhrberg M, Valiante NM, Shum BP, Shilling HG, Lienert-Weidenbach K, Corliss B, Tyan D, Lanier LL, Parham P (1997) Human diversity in killer cell inhibitory receptor genes. Immunity 7:753–763
Vitale M, Zamai L, Neri LM, Manzoli L, Facchini A, Papa S (1991) Natural killer function in flow cytometry: identification of human lymphoid subsets able to bind to the NK sensitive target K562. Cytometry 12(8):717–722
Vivier E, Nunes JA, Vely F (2004) Natural killer cell signaling pathways. Science 306:1517–1519
Warren HS, Kinnear BF (1999) Quantitative analysis of the effect of CD16 ligation on human NK cell proliferation. J Immunol 162:735–742
Warren HS, Skipsey LJ (1991) Phenotypic analysis of a resting subpopulation of human peripheral blood NK cells: the FcR gamma III (CD16) molecule and NK cell differentiation. Immunology 72:150–157
Watzl C, Long EO (2003) Natural killer cell inhibitory receptors block actin cytoskeleton-dependent recruitment of 2B4 (CD244) to lipid rafts. J Exp Med 197:77–85
Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH (1999) An activating immunoreceptor complex formed by NKG2D and DAP10. Science 285:730–732
Yawata M, Yawata N, Draghi M, Little AM, Partheniou F, Parham P (2006) Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med 203:633–645
Yoon SR, Chung JW, Choi I (2007) Development of natural killer cells from hematopoietic stem cells. Mol Cells 24:1–8
Zambello R, Falco M, Della Chiesa M, Trentin L, Carollo D, Castriconi R, Cannas G, Carlomagno S, Cabrelle A, Lamy T, Agostini C, Moretta A, Semenzato G, Vitale M (2003) Expression and function of KIR and natural cytotoxicity receptors in NK-type lymphoproliferative diseases of granular lymphocytes. Blood 102:1797–1805
Acknowledgments
This study was supported by Grant 145056 from the Ministry of Science and Technology of the Republic of Serbia. We wish to thank Mrs. Jasna Popović Basić and Mrs. Miroslava Ćulafić for their help and excellent technical work. We are grateful to Mrs. Dušica Gavrilović for statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Konjević, G., Mirjačić Martinović, K., Vuletić, A. et al. Distribution of Several Activating and Inhibitory Receptors on CD3−CD16+ NK Cells and Their Correlation with NK Cell Function in Healthy Individuals. J Membrane Biol 230, 113–123 (2009). https://doi.org/10.1007/s00232-009-9191-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-009-9191-3