Abstract
In the present study, the whole-cell patch-clamp technique was applied to follow the inhibitory effect of genistein — a tyrosine kinase inhibitor and a natural anticancer agent—on the activity of voltage-gated potassium channels Kv1.3 expressed in human T lymphocytes (TL). Obtained data provide evidence that genistein application in the concentration range of 1–80 μM reversibly decreased the whole-cell potassium currents in TL in a concentration-dependent manner to about 0.23 of the control value. The half-blocking concentration range of genistein was from 10 to 40 μM. The current inhibition was correlated in time with a significant decrease of the current activation rate. The steady-state activation of the currents was unchanged upon application of genistein, as was the inactivation rate. The inhibitory effect of genistein on the current amplitude and activation kinetics was voltage-independent. The current inhibition was not changed significantly in the presence of 1 mM of sodium orthovanadate, a tyrosine phosphatase inhibitor. Application of daidzein, an inactive genistein analogue, did not affect significantly either the current amplitudes or the activation kinetics. Possible mechanisms of the observed phenomena and their significance for genistein-induced inhibition of cancer cell proliferation are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An important role of ion channels in cancer was proven by research in the last few years. It was shown that ion channels, particularly potassium channels, participate in cancer development and apoptosis (Pardo, 2004; Wang, 2004). Potassium channel blockers may cause an inhibition of cell proliferation in different cancer cell lines (Rouzaire-Dubois & Dubois, 1990; Rouzaire-Dubois, Gerard & Dubois, 1993; Abdul & Hoosein, 2002). It is also known that some compounds able to reverse multidrug resistance of cancer cells, like, for example, verapamil or cyclosporin A, inhibit the activity of voltage-gated potassium channels (Panyi et al., 1996; Rybalchenko et al., 2001). As a consequence, potassium channels may be considered as a promissive target for cancer chemotherapy (Conti, 2004).
Genistein, an isoflavone abundant in some vegetables, known as a potent and relatively specific inhibitor of protein tyrosine kinase (PTK — Akiyama et al., 1987), has been reported to be a natural anticancer agent. Genistein inhibits cancer cell proliferation and promotes apoptosis in colon cancer HT-29 cells (Yu et al., 2004) and inhibits angiogenesis in bladder cancer cells (Su et al., 2005). Genistein was also reported to be an efficient inhibitor of multidrug resistance-associated protein MRP1 (ABCB1), one of the main multidrug resistance transporters overexpressed in some human cancer cells (Bobrowska-Hägerstrand et al., 2001).
It is also known that genistein inhibits the activity of many different types of ion channels including potassium channels. The inhibitory effect of genistein on the channels may be due to the inhibition of PTK—such as it occurs in case of delayed rectifier and A-type voltage-gated potassium channels in mouse Schwann cells (Peretz, Sobko & Attali, 1999) and human “ether-a-go-go” related K+ channels (Cayabyab & Schlichter, 2002). However, genistein may also inhibit potassium channels in a PTK-independent manner. Such an inhibition occurs in case of cardiac delayed rectifier potassium channels (Hool, Middleton & Harvey, 1998; Washizuka et al., 1998), voltage-gated potassium channels in rat and rabbit pulmonary artery cells (Smirnov & Aaronson, 1995) and ATP-sensitive K+ channels in rabbit portal vein-smooth muscle (Ogata et al., 1997). Finally, the mechanism of genistein action may also be complex and include both the PTK- dependent and PTK- independent pathway, such as in the case of cloned human voltage-gated potassium channels Kv1.4 (Zhang & Wang, 2000).
To the group of voltage-gated potassium channels affected by genistein belong also Kv1.3 channels. These channels are expressed abundantly in human T lymphocytes (TL), where they play an important physiological role in setting the cell resting membrane potential, cell mitogenesis and volume regulation (Cahalan, Wulff & Chandy, 2001; Chandy et al., 2004). Kv1.3 channels are also involved in apoptosis of the TL cell line Jurkat (Gulbins et al., 1997; Storey et al., 2003) and malignant lymphocytes (Wang et al., 1992). Kv1.3 channels are also present in rat central nervous system, especially in olfactory bulb neurons, where they play a modulatory role in action potential generation (Veh et al., 1995, Kupper, Prinz & Fromherz 2002; Colley, Tucker & Tadool, 2004;). The channels are also expressed in human alveolar macrophages (Mackenzie, chirakkal & North, 2003), rat choroid plexus epithelial cells (Speake, Kibble & Brown, 2004), epithelial cells from rabbit kidney and colon (Grunnet et al., 2003), human gliomas (Preußat et al., 2003), melanoma cells (Artym & Petty, 2002) and in rat prostate cancer cell lines (Fraser et al., 2003). An expression of voltage-dependent Kv 1.3 channels was up-regulated for example in prostate and breast cancer (Abdul & Hoosein, 2002; Abdul, Santo & Hoosein, 2003). There is also evidence that the activity of Kv1.3 channels regulates energy homeostasis, body weight and peripheral insulin sensitivity in mice (Xu et al., 2003, 2004).
The evidence that genistein might affect the activity of Kv1.3 channels came from experiments performed by Fadool and co-workers (Fadool et al., 1997). The authors showed that application of 25 μM genistein relieved the inhibition of the currents caused by v-Src protein kinase. The effect began after ca 7 minutes of genistein application and was saturated after about 12 minutes. However, the current recovery was not complete—the amplitudes reached a little bit more than half of the control value. The authors suggested that this might probably be due to a blocking effect of genistein on Kv1.3 channels, however, no data about the channel inhibition by genistein was presented. In contrast, application of daidzein, an inactive analogue of genistein, at the same concentration was ineffective. It was concluded that the relief of the v-Src-induced current inhibition by genistein was due to the inhibition of the protein tyrosine kinase (Fadool et al., 1997).
Since genistein is a powerful modulator of a variety of potassium channels, it was of interest to study the inhibitory effect of genistein on Kv1.3 channels in detail. This was the aim of our study. Because these channels are expressed endogenously and predominantly in human T lymphocytes (Cahalan et al., 2001), these cells were used as a model system in our experiments. Obtained data provide evidence that genistein inhibits the activity of Kv1.3 channels similarly to the related Kv1.4 channels (Zhang & Wang, 2000). However, the mechanism of inhibition of Kv1.3 channels in T lymphocytes by genistein includes most probably only the tyrosine kinase-independent pathway. Application of daidzein did not produce any blocking effect on the currents.
Preliminary results were published as an abstract (Teisseyre & Michalak, 2004).
Materials and Methods
Cell Separation, Solutions and Pipettes
Human TL were separated from peripheral blood samples from 10 healthy donors using a standard method described elsewhere (Hirano et al., 1977). After separation, cells were cultured for at least 24 hours in the standard RPMI-1640 Medium (SIGMA) supplemented with 5% vol/vol fetal calf serum (SIGMA).
For experiments, the cells were placed in the external solution containing (in mM): 150 NaCl, 4.5 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, pH=7.35 adjusted with NaOH, 300 mOsm. The pipette solution contained (in mM): 150 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 EGTA; pH=7.2 adjusted with KOH, and osmomolarity at 300 mOsm. The concentration of free calcium in the internal solution was below 100 nM, assuming the dissociation constant for EGTA at pH=7.2 of 10−7 M (Grissmer, Nguyen & Cahalan, 1993). Such a low calcium concentration was applied in order to prevent the activation of calcium-activated IKCal channels (Grissmer, Nguyen & Cahalar 1993). The reagents were provided by the Polish Chemical Company (POCH, Gliwice, Poland), except for HEPES and EGTA, which were purchased from SIGMA. Genistein and daidzein were kindly provided by Dr.Y. Shirataki (Faculty of Pharmaceutical Sciences, Josai University, Sakado, Saitama, Japan). Dishes with cells were placed under an inverted Olympus IMT-2 microscope. External solutions containing genistein were applied using a fast perfusion system RSC 200 (Bio-Logic, Grenoble, France). Pipettes were pulled from a borosilicate glass (Hilgenberg, Germany) and fire-polished before the experiment. The pipette resistance was in the range of 3–5 MΩ.
Electrophysiological Recordings
Whole-cell potassium currents in TL were recorded applying the patch-clamp technique (Hamill et al., 1981). The currents were recorded using an EPC-7 Amplifier (List Electronics, Darmstadt, Germany), low-pass filtered at 3 kHz, digitized using the CED Micro 1401 (Cambridge, UK) analogue-to-digital converter with a sampling rate of 10 kHz. A standard protocol of depolarizing voltage stimuli containing 7 pulses in the range from −60 mV to +60 mV (20 mV increment) was applied every 10 s; pulse duration was 40 ms and holding potential, −90 mV. In measurements of the time dependence of genistein application on the current amplitude and activation kinetics, a modified sequence was applied: 7 depolarizing pulses of +60 mV each were applied every 30 s, pulse duration was 20 ms and holding potential −90 mV. The influence of genistein application on the inactivation kinetics was investigated applying a different sequence containing 7 depolarizing pulses of+60 mV each applied every 30 s, pulse duration was 1000 ms and holding potential −90 mV. The linear (ohmic) component of the current was subtracted off-line from the final record. In some experiments, a sequence containing 10 voltage ramps applied every 20 s depolarizing the cell membrane from −100 mV up to +40 mV, ramp duration 340 ms, holding potential −90 mV, were applied. The linear (ohmic) component of the current was subtracted off-line from the final record. The data were analyzed using the WCP J. Dempster Program.
The data are given as the mean ± standard error. All experiments were carried out at room temperature (22–24 °C).
Data Analysis
Since the number of active channels varied significantly among the cell population, the steady-state activation of the channels was presented in terms of a normalized relative chord conductance (gKnorm) defined by an equation: gKnorm = gK/gK60, where: gK is chord conductance, gK60 is chord conductance at the membrane potential = +60 mV. The chord conductance was calculated according to the definition: gK = Ip/(V-Vrev), where: Ip = amplitude of the current, V = membrane potential, Vrev = reversal potential of the current, assumed to be –75 mV. The voltage dependence of steady-state activation was fitted by a Boltzmann function given by an equation: gKnorm (V) = l/[l+exp−(V−Vn)/kn], where −Vn is the activation midpoint, kn the steepness of the curve. The activation kinetics was fitted by applying a power function, given by an equation: I(t) = Ip[1−exp(−t/τn)]2, where τn is the time constant of activation.
Abbreviations: 4-AP, 4-Aminopyridine; gK, chord conductance; gK60, chord conductance recorded at +60 mV; gKnorm, relative chord conductance normalized to 1 at +60 mV; Ip, current amplitude; Iprel, relative peak current; Inorm, current normalized to 1 at +60 mV; kn, steepness of the voltage dependence; PTK, protein tyrosine kinase; TL, human T lymphocyte; V, membrane potential; Vn, activation midpoint; Vrev – reversal potential of the current; τn, activation time constant.
Results
An example of the whole-cell currents recorded in a TL applying a standard protocol of depolarizing stimuli described in Materials and Methods is presented in Fig. 1A. The currents were evoked upon a membrane depolarization to potentials higher (i.e., more positive) than −40 mV. The currents were completely blocked upon application of 5 mM 4-aminopyridine (4-AP, Fig. 1B), which selectively blocks Kv1.3 channels in TL (Zegarra- Moran et al., 1999). This indicates that the recorded currents were predominantly due to the activation of Kv1.3 channels.
(A) Example of the whole-cell currents in a TL, recorded applying a standard protocol of depolarizing stimuli (see Materials and Methods). For clarity, only the first 30 ms of the records are shown. (B) Relative peak current (Iprel) vs membrane potential obtained under control conditions (empty squares, n = 6) and upon application of 5 mM 4-AP (filled squares, n = 6). Data points were connected with a point-to-point line.
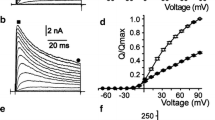
Figure 2 depicts the effect of application of 40 μM genistein on the whole-cell potassium currents recorded in a TL, applying the standard protocol of depolarizing stimuli. Apparently, the current amplitudes were reduced to less than half of the control value (Fig. 2A,B). Moreover, the current activation was considerably slower upon genistein application than under control conditions (Fig. 2B,D). The inhibitory effect of genistein was reversible—a small reduction of current amplitudes before and after application of genistein (compare Fig. 2A and C) was rather due to the “run-down” of the currents often observed in cells exhibiting large whole-cell potassium currents.
Effects of application of 40 μM genistein on the whole-cell potassium currents in TL: control recorded upon application of a standard sequence of depolarizing stimuli (A), currents recorded in the presence of genistein (B) and after wash-out (C). (D) Normalized currents recorded at +60 mV under control conditions and in the presence of genistein.
The steady-state activation curve obtained from measurements carried out under control conditions and in the presence of 40 μM genistein, plotted as a function of membrane potential, is shown in Fig. 3A. The activation midpoint (Vn) value was −18.03 ± 1.47 mV (n = 10) under control conditions and –15.71 ± 2.65 mV in the presence of genistein (n = 10). The difference between these values was statistically insignificant (P > 0.05, Student’s t-test). The steepness parameter (kn) value was 8.07 ± 1.48 mV (n = 10) for control conditions and 10.37 ± 2.3 mV (n = 10) upon application of genistein. The difference was statistically insignificant (P > 0.05, Student’s t-test). Figure 3B shows the amplitude ratio of the currents recorded in the presence of 40μM genistein and under control conditions (Igen/Ictrl) as a function of membrane potential. Apparently, the inhibition of current amplitudes by genistein was voltage-independent in the whole range from –20 mV to +60 mV.
(A) Steady-state activation in terms of a normalized chord conductance as a function of membrane potential: filled squares, control condition (n = 10), empty squares, application of 40 μM genistein (n = 10). Data points were fitted by the Boltzmann function (see Materials and Methods). (B) Current amplitude ratio as a function of membrane potential (n = 10). Data points were connected by a point-to-point line.
Figure 4A depicts the values of activation time constants for records obtained under control conditions and in the presence of 40μM genistein as a function of membrane potential. The time constants were markedly higher upon genistein application than under control conditions. Figure 4B shows the ratio of activation time constants under control conditions and upon genistein application (τctrl/τ gen ) as a function of membrane potential. Similarly to what was observed for the amplitudes, the acceleration of current activation rate upon genistein application did not depend on the membrane potential in the range from 0 to +60 mV.
(A) Activation time constants as a function of membrane potential: open squares, control conditions (n = 10); filled squares, application of 40 μM genistein (n = 10). (B) Time constant ratio as a function of membrane potential (n = 10). Data points in (A) and (B) were connected by a point-to-point line.
The ratio of current amplitudes (I gen /Ictrl) and the ratio of activation time constants upon application of 5 μM genistein and under control conditions (τgen/τctrl) as a function of time after genstein application are depicted in Fig.5. It is clearly visible that the decrease of the current amplitude was correlated in time with increase of the activation time constant. The figure shows also that the inhibitory effect of genistein was time-dependent and it reached the maximum after about 1 minute of isoflavone application.
Figure 6 depicts the inactivation phase of normalized currents recorded under control conditions and upon application of 10 μM genistein, applying a modified sequence containing long-lasting (1 s) depolarizing potentials from –90 mV to +60 mV. Currents recorded under control conditions and in the presence of genistein were nearly the same. This indicates that the inactivation kinetics was not changed upon genistein application.
The dose-response curve for genistein is shown in Fig. 7. From this plot we can see that genistein at 1 μM concentration inhibited the current amplitudes to 0.61 ± 0.025 (n = 5) of control value. The currents were approximately half-blocked in the concentration range from 10 to 40 μM. An application of genistein at a concentration of 10 μM and 40 μM inhibited the peak current to 0.5 ± 0.02 (n = 5) and 0.46 ± 0.05 (n = 10) of the control value, respectively. Further increase of genistein concentration up to 80 μM caused a decrease of the currents to 0.225 ± 0.06 (n = 5) of the control value.
In order to elucidate whether the inhibitory effect of genistein was due to the inhibition of PTK or to a PTK-independent mechanism, experiments with sodium orthovanadate (Na3VO4) were performed. Sodium orthovanadate is a potent inhibitor of the tyrosine phosphatase (Zhang & Wang, 2000). If PTK was involved in the genistein effect on Kv 1.3 activity, an application of sodium orthovanadate would abolish the inhibitory effect of genistein. Our data provide evidence that application of 1 mM sodium orthovanadate alone did not influence the Kv1.3 currents in TL (data not shown). Figure 8 depicts the current-amplitude ratios recorded upon application of 10 μM genistein and when the same concentration of genistein was applied in the presence of 1 mM sodium orthovanadate. In case of genistein applied alone, the current amplitudes were reduced to 0.5 ± 0.02 (n = 5) of the control value. When 10 μM genistein was applied in the presence of 1 mM of sodium orthovanadate, the amplitudes were reduced to 0.55 ± 0.025 (n = 5) of the control value. Results obtained upon co-application of genistein and sodium orthovanadate were not significantly (P > 0.05, Student’s t-test) different from data obtained for genistein alone. These data demonstrate that in case of Kvl.3 channels, the inhibitory effect of genistein occurred predominantly in a PTK-independent manner.
Data available show that daidzein, an inactive analogue of genistein, does not affect the activity of Kv1.4 channels inhibited by genistein (Zhang & Wang, 2000). Moreover, in contrast to genistein, application of daidzein doesn’t relieve the Kvl.3 channel inhibition by v-Src tyrosine kinase (Fadool et al., 1997). In our study, we have also examined whether application of daidzein inhibited Kv1.3 channels. Obtained data showed that application of 40 μM daidzein did not change significantly either the current amplitude (see Fig. 9) or the activation kinetics (not shown) of Kv 1.3 channels in human T lymphocytes. The relative peak current upon application of 40 μM daidzein was 0.99 ± 0.02 of the control value (n = 10).
Discussion
The results of our study provided evidence that application of genistein inhibits the activity of Kv1.3 channels in human T lymphocytes. Obtained data suggest that this inhibition occurs in a PTK-independent manner, first, because application of 1 mM of sodium orthovanadate did not abolish or even diminish the inhibitory effect of genistein. Moreover, if the inhibitory effect involved inhibition of PTK by genistein, it would appear only after about 7–10 minutes of genistein treatment, such as it occurs in case of the genistein-mediated relief of the channel inhibition by v-Src tyrosine kinase (Fadool et al., 1997). In contrast, the inhibitory effect observed in our experiments occurred immediately after cell exposure to genistein and it reached a maximum after about one minute of cell incubation with genistein. This process of channel inhibition is one order of magnitude faster than the process of modulation of Kv 1.3 channel activity observed by Fadool and co-workers (1997) in neuronal cells, which was mediated by genistein-induced inhibition of PTK. Taking into account the fact that in our experiments the time duration of genistein application was never longer than about 5 minutes, the observed effects could not be explained by influence of genistein on PTK activity. The results of our study and of Fadool and coworkers suggest that genistein may exert two opposite effects on Kv1.3 channels—a fast PTK-independent inhibition followed by a relatively slow PTK-dependent stimulation. Such a fast current inhibition followed by a slow stimulation was observed upon genistein application in the delayed rectifier potassium channels in guinea-pig ventricular myocytes (Hool, Middletone & Harvey, 1998).
The inhibitory effect of genistein on Kv1.3 channels reported in this study is similar to the effect exerted on Kv1.4 channels, which are closely related to Kv1.3. In case of Kv1.4 channels, the inhibitory effect occurs in the same concentration range (up to 50 μM), it is also voltage-independent and correlates with a significant slowing of the current activation rate (Zhang & Wang, 2000). Moreover, application of daidzein did not affect significantly the activity of Kv1.4 channels, similarly to what was observed in case of Kv1.3 channels (Zhang & Wang, 2000). However, there are also differences when comparing genistein interactions with Kv1.3 and Kv1.4 channels. First, in the case of Kv1.4 channels, there is a shift of the steady-state activation curve by about 15 mV towards positive membrane potential and this shift is statistically significant (P < 0.05) (Zhang & Wang, 2000). In contrast, our results show that in the case of Kv1.3 channels, the shift is only about 2.3 mV and is statistically insignificant (P > 0.05). Moreover, in the case of Kv1.4 channels, it was shown that the inhibitory effect of genistein was significantly diminished in the presence of 1 mM of sodium orthovanadate (Zhang & Wang, 2000). This may indicate that the inhibition of Kv1.4 channels by genistein is partially due to the inhibition of PTK by this isoflavone (Zhang & Wang, 2000). On the other hand, the application of sodium orthovanadate did not abolish the blocking effect of genistein completely (Zhang & Wang, 2000). Thus, it is suggested that the inhibitory effect on Kv1.4 channels, unlike Kv1.3 channels, is complex and it includes both the inhibition of PTK and other, PTK- independent mechanisms (Zhang & Wang, 2000).
The inhibition of Kv1.3 channels by genistein might be a result of direct interactions with binding sites on the channels or the influence might be indirect and involve changes of properties of lipid phase of the membrane, which, in turn, might affect the channel activity. It was proven that genistein can modulate the properties of phospholipid bilayers (Hwang et al., 2003; Łania-Pietrzak et al., 2005). These changes in properties of lipid membrane may in turn affect the activity of transporting membrane proteins as it was shown, for example, for gramicidin A channels (Hwang, Koeppe & Andersen, 2003). It remains to be elucidated which mechanism predominates in case of Kv1.3 channels.
Results similar to those presented here for Kv1.3 channels in lymphocytes were also obtained in case of voltage-gated potassium channels in rat and rabbit pulmonary artery cells (Smirnov & Aaronson, 1995) and delayed rectifier potassium channels in guinea-pig ventricular myocytes (Washizuka et al., 1998). In both cases, inhibition of channel activity by genistein occurred in a PTK-independent manner and in the same concentration range (10–100 μM) (Smirnov & Aaronson, 1995; Washizuka et al., 1998). The currents were inhibited to a similar extent (to about 0.4 of the control value) and the inhibition was accompanied by a significant decrease of the activation rate (Smirnov & Aaronson, 1995; Washizuka et al., 1998). Thus, the PTK-independent inhibition of potassium channel activity by genistein occurs not only in the case of Kv1.3 channels, but also in other types of channels expressed in pulmonary arteries and in the heart.
The inhibitory effect of genistein on Kv1.3 channels might be of significance for genistein-induced inhibition of proliferation of colon cancer HT-29 cells. Available data provide evidence that application of 15–120 μM genistein inhibits proliferation of the cells in a concentration-dependent manner and the half-blocking concentration range is between 30 and 60 μM (Yu, Li & Liu, 2004). This is similar to the half-blocking concentration range of Kv1.3 channels reported in this study. On the other hand, it is known that Kv1.3 channels participate in the proliferation of TL and the inhibition of Kv1.3 channels inhibits TL proliferation (Cahalan et al., 2001). Since Kv1.3 channels also participate in proliferation of colon cancer cells (Pardo, 2004), it is possible that inhibition of Kv1.3 channels by genistein is involved in the genistein-induced inhibition of proliferation of colon cancer HT-20 cells.
References
Abdul M., Hoosein N. 2002. Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett. 186:99–105
Abdul M., Santo A., Hoosein N. 2003. Activity of potassium channel-blockers in breast cancer. Anticancer Res. 23:3347–3351
Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 262:5592–5595
Artym W., Petty H.R. 2002. Molecular proximity of Kv1.3 voltage-gated potassium channels and ß1-integrins on the plasma membrane of melanoma cells: effects of cell adherence and channel blockers. J. Gen. Physiol. 120:29–37
Bobrowska - Hägerstrand M., Wróbel A., Rychlik B., Bartosz G., Söderstöm T., Shirataki Y., Motohashi N., Molnar J., Michalak K., Hägerstrand H. 2001. Monitoring of MRP-like Activity in Human Erythrocytes—Inhibitory Effect of Isoflavones. Blood Cells, Molecules, and Diseases 27:894–900
Cahalan M., Wulff H., Chandy K. 2001. Molecular properties and physiological roles of ion channels in the immune system. J. Clin. Immunol. 21:235–252
Cayabyab F., Schlichter L. 2002. Regulation of an ERG K+ current by Src Tyrosine kinase. J. Biol. Chem. 277:13673–13681
Chandy K., Wulff H., Beeton, Ch., Pennington M., Gutman G., Cahalan M. 2004. K+ channel as targets for specific immunomodulation. TIPS 25:280–9
Colley B., Tucker K., Fadool D. 2004. Comparison of modulation of Kv1.3 channel by two receptor tyrosine kinases in olfactory bulb neurons of rodents. Rec Channels 10:25-36
Conti M.J. 2004. Targeting K+ channels for cancer therapy. Exp. Ther. Oncol. 4:161–166
Fadool D., Holmes T., Berman K., Dagan D., Levitan I. 1997. Tyrosine phosphorylation modulates current amplitude and kinetics of a neuronal voltage-gated potassium channel. J. Neurophysiol. 78:1563–1573
Fraser S., Grimes J., Diss J., Stewart D., Dolly J., Djamgoz M. 2003. Predominant expression of Kv1.3 voltage-gated K+ channel subunit in rat prostate cancer cell lines: electrophysiological, pharmacological and molecular characterisation. Pfuepgers Arch. 446:559–571
Grissmer S., Nguyen A., Cahalan M. 1993. Calcium-activated potassium channels in resting and activated human T lymphocytes. J. Gen. Physiol. 102:601–630
Grunnet M., Rasmussen H., Hay-Schmidt A., Klaerke D. 2003. The voltage-gated potassium channel subunit, Kv1.3, is expressed in epithelia. BBA 1616:85–94
Gulbins E., Szabo E., Baltzer K., Lang F. 1997. Ceramide-induced inhibition of T lymphocyte voltage-gated potassium channel is mediated by tyrosine kinases. Proc. Natl. Acad. Sci. USA 94:7661–7666
Hamill O.P., Marty A., Neher E., Sakmann B., Sigworth F.J. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers Arch. 39:85–100
Hirano T., Kuritani T., Kishimoto Y., Yamamura Y. 1977. T cell dependency of PWM-induced Ig production by B cells. J Jmmunol. 119:1235
Hool L., Middleton L., Harvey R. 1998. Genistein increases the sensitivity of cardiac ion channels to β-adrenergic receptor stimulation. Circ. Res. 83:33–42
Hwang T., Koeppe R., Andersen O. 2003. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry 42:13646–13658
Kupper J., Prinz A., Fromherz P. 2002. Recombinant Kv1.3 potassium channels stabilize tonic firing of cultured rat hippocampal neurons. Pflügers Arch. 443:541–547
Łania-Pietrzak B., Hendrich A., Żugaj J., Michalak K. 2005. Metabolic O-demethylation does not alter the influence of isoflavones on the biophysical properties of membranes and MRP1-like protein transport activity. Arch. Biochem. Biophys. 433:428–434
Mackenzie A., Chirakkal H., North A. 2003. Kv1.3 potassium channels in human alveolar macrophages. Am. J. Physiol. 285:L862–L868
Ogata R., Kitamura K., Ito Y., Nakano H. 1997. Inhibitory effect of genistein on ATP-sensitive K+ channels in rabbit portal vein smooth muscle. Br. J. Pharmacol 122:1395–1404
Panyi G., Gaspar R., Krasznai J., Ameloot M., Aszalos A., Steels P., Damjanovich S. 1996. Immunosuppressors inhibit voltage-gated potassium channels in human peripheral blood lymphocytes. Biochem. Biophys.Res. Commun. 221:254–258
Pardo L.A. 2004. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 19:285–292
Peretz A., Sobko A., Attali B. 1999. Tyrosine kinases modulate K+ channel gating in mouse Schwann cells. J. Physiol. 519:373–384
Preußat. K., Beetz Ch., Schrey M., Kraft R., Wölfl S., Kalff R, Patt S. 2003. Expression of voltage-gated potassium channels Kv1.3 and Kv1.5 in human gliomas. Neurosci. Lett. 346:33–36
Rouzaire -Dubois B., Dubois J.M. 1990. Tamoxifen blocks both proliferation and voltage-dependent K channels of neuroblastoma cells. Cell. Signal. 2:387–393
Rouzaire-Dubois B., Gerard V., Dubois J.M. 1993. Involvement of K channels in quercetin-induced inhibition of neuroblastoma cell growth. Pfluegers Arch. 423:202–205
Rybalchenko V., Prevarskaya N., Coppenolle F., Legrand G., Lemonnier L., Bourhis X., Skryma R. 2001. Verapamil inhibits proliferation of LNCaP human prostate cancer cells influencing K+ channel gating. Mol Pharmacol 59:1376–1387
Smirnov S., Aaronson P. 1995. Inhibition of vascular smooth muscle cell K+ currents by tyrosine kinase inhibitors genistein and ST 638. Circ. Res. 76:310–316
Speake T., Kibble J., Brown P. 2004. Kv1.1 and Kv1.3 channels contribute to the delayed-rectifying conductance in rat choroid plexus epithelial cells. Am. J. Physiol 286:C611–C620
Storey N.M., Gomez-Angelats M., Bortner C.D., Armstrong D.L., Cidlowski J.A. 2003. Stimulation of Kv1.3 potassium channels by death receptors during apoptosis in Jurkat T lymphocytes. J. Biol. Chem. 278:33319–33326
Su S.J., Yeh T.M., Chuang W.J., Ho Ch.L., Chang K.L., Cheng H.L., Liu H.Sh., Cheng H.L., Hsu P.Y., Chow N.H. 2005. The novel targets for anti-angiogenesis of genistein on human cancer cells. Biochem. Pharmacol 69:307–318
Teisseyre A., Michalak K. 2004. The inhibitory effect of genistein on Kv1.3 channels in human T lymphocytes. Abstracts of the XII Conference of Polish Biophysical Society, Wrocław, Poland, 15-17 September 2004. Curr. Top. Biophys. 28:A–57
Veh R., Lichtinghagen R., Sewing S., Wunder F., Grumbach L, Pongs O. 1995. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur. J. Neurosci. 7:2189–2205
Wang Z. 2004. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pfluegers Arch. 448:274–286
Wang Y., Jia H., Walker A.M., Cukierman S. 1992. K-current mediation of prolactin-induced proliferation of malignant (Nb2) lymphocytes. J. Cell. Physiol 1532:185–189
Washizuka T., Horie M., Obayashi K., Sasayama S. 1998. Genistein inhibits slow component delayed-rectifier K currents via a tyrosine kinase-independent pathway. J. Mol. Cell. Cordial. 30:2577–2590
Xu J., Koni P., Wang P., Li G., Kaczmarek L., Wu Y., Li Y., Flavell R., Desir G. 2003. The voltage-gated potassium channel Kv1.3 regulates energy homeostasis and body weight. Hum. Mol. Genetics. 12:551–559
Xu J., Wang P., Li Y., Kaczmarek L., Wu Y., Koni P., Flavell R., Desir G. 2004 The voltage-gated potassium channel Kv1.3 regulates peripheral insulin sensitivity. Proc. Natl. Acad. Sci. USA 101:3112–3117
Yu Z., Li W., Liu F. 2004. Inhibition of proliferation and induction of apoptosis by genistein in colon cancer HT-29 cells. Cancer Lett. 215:159–166
Zegarra-Moran O., Basola A., Rogolo M., Porcelli A., Rossi B., Galietta L. 1999. HIV-1 Nef expression inhibits the activity of Ca2+-dependent K+ channels involved in the control of the resting potential in CEM lymphocytes. J. Immunol. 162:5359–5366
Zhang Z., Wang Q. 2000. Modulation of a cloned human A-type voltage-gated potassium channel (hKv1.4) by the protein kinase inhibitor genistein. Pfluegers Arch. 440:784–792
Acknowledge
The authors express best thanks to our colleague from the Biophysics Department, Dr. Andrzej Poła, who kindly provided blood samples for lymphocyte isolation. This work was supported by CSR Medical University Grant No 453.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teisseyre, A., Michalak, K. Genistein Inhibits the Activity of Kv1.3 Potassium Channels in Human T Lymphocytes. J Membrane Biol 205, 71–79 (2005). https://doi.org/10.1007/s00232-005-0764-5
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s00232-005-0764-5