Abstract
The main aim of this work is to investigate the influence of different parameters on the stability of spherical CuO nanoparticles dispersed in water, ethylene glycol and 50:50 water/ethylene glycol binary mixture as different base fluids for heat transfer applications. Nano-fluids were prepared using two-step method at weight concentration of 0.1–0.4 %. Time-settlement experiments were established to examine the stability of nano-fluids. Quality tests were also performed to investigate the morphology, purity and size of nanoparticles. In order to stabilize the nano-fluids, stirring, pH control and sonication were utilized. The criteria for assessing the stability of nano-fluids were zeta potential and time-settlement experiments. Results demonstrated that the ethylene glycol can be the best medium for dispersing the CuO nanoparticle in comparison with water and water/EG binary mixture. The best condition for achieving the most stable nano-fluid was also introduced. Role of sonication time, stirring time and addition of surfactant on the stability of nano-fluids were investigated and briefly discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to the potential of energy-saving and wide application of nano-fluids in industrial and commercial aspects, sedimentation and instability of nanoparticles have been an indispensable challenge. Nano-fluids are colloidal suspensions, which are comprised of nano-sized solid particles dispersed in a conventional heat transfer liquid with poor thermal conductivity such as: water or ethylene glycol. This type of coolant was introduced by Choi [1] in Argonne National Laboratory (ANL). Thermal features and stability of nano-fluids have been a hotly debated topic during the last two decades due to their promising application in thermal engineering [2–4]. Usually, for preparing the nano-fluids, the two-step method is preferred such that the desired mass of nanoparticle is well-dispersed into the weighted base fluid, while it is agitated in a clean flask [5]. This is a cost-effective and economical method when compared to the single-step method. However, after preparation, treatments should be applied to the nano-fluids, which are regarded as interesting subjects for heat transfer researchers.
Instability of nano-fluids can be defined as the gradual sedimentation (or scale formation) of nanoparticles simultaneously with agglomerating or clustering of nanoparticles inside the base fluid. Many efforts have been made to determine the influence of different parameters on the stability of nano-fluids. It is generally believed that nanoparticles can form aggregations due to Van der Waals forces. Therefore, physical or chemical treatments can have significant impact on stability of nano-fluids [6, 7]. As an evidence for such efforts, Witharana et al. [8] established experiments to quantify the influence of different base fluids on the stability of ZnO, Al2O3 and TiO2 nanoparticles and concluded that polyethylene glycol is the best medium for dispersing of nanoparticles. Lin et al. [9] studied the thermal properties and stability of Al2O3 nanoparticles dispersed in water. They used QF-STK190 as a dispersant and showed that the best stability can be seen in nano-fluids prepared with dispersant. There are other works in the literature, which have been summarized in Table 1.
Based on Table 1, several stabilizing techniques can be found in the literature, which comes as follows.
1.1 The electrostatic stabilization
In this technique, nano-fluids can be stabilized by controlling the chemical characteristics of solution such as pH and concentration of existing ions. In this method, Zeta potential can be a gold parameter in determining the isoelectric point (IEP) and assessing the optimum pH, in which the zeta potential of solution should be far from (smaller or higher than) the isoelectric point. Noticeably, in IEP, zeta potential is minimum (approximately zero) and the least stability of nano-fluids can be seen in this point [8].
1.2 The steric stabilization
In this case, the properties of surface are the key factors for stabilizing the nano-fluids. These parameters can be controlled by adding surfactants or dispersants such that coalescence (cohesive behavior of particles) significantly decreases and subsequently, reduces the tendency of particles to be attached to the surface [17, 18].
1.3 The depletion stabilization
In this case, a new free polymer is added into the base fluid, which changes the intermolecular forces between nanoparticles. Since particles with lower size have higher surface energies, these types of nano-fluids have higher potential to form the aggregations and subsequently have higher tendency to be settled on the surface as fouling layer [8, 19].
1.4 Ultrasonic agitations
These techniques can lead to reduction of agglomeration by cracking down the clusters into the smaller pieces. Such methods strongly depend on the nominal power, frequency of sonication and sonication time [8, 19–23]. In the present work, as a continuation of our previous works [24–30], influences of addition of surfactant, sonication and stirring on stability of CuO–water, CuO–ethylene glycol and CuO–water/ethylene glycol nano-fluids were experimentally investigated and briefly discussed.
2 Experimental details
2.1 Materials
As mentioned in previous section, for preparing the nano-fluids, the two-step method was employed. Dry hydrophilic spherical CuO nanoparticles were purchased from PlasmaChem GmbH, Germany. The thermo-physical properties of nanoparticles have been presented in Table 2. As a base fluid, deionized water and ethylene glycol and also their 50 % volumetric mixture were provided from Sarir Chemical Company (SCC) and Sigma Aldrich respectively. Thermo-physical properties of ethylene glycol have also been represented in Table 3.
2.2 Initial preparation of nano-fluids
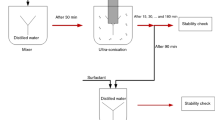
Using the two-step method, different processes were carried out for preparing stable nano-fluids. These processes summarily include: Initially, desired mass of nanoparticles was added into the weighted base fluid, while it was agitated in a clean flask. The magnetic stirrer was employed to agitate the nanoparticle inside the base fluid with average speed of 450 rpm. In order to ensure about the morphology, size, and purity of nanoparticles, X ray diffraction and particle count size and scanning electron microscopic image, experiments were performed. Results of these tests can be seen in Figs. 1, 2 and 3 respectively.
Noticeably, ultrasonic device was employed to crack the agglomerations and clusters into smaller pieces (UP-400S, Hielscher), followed by pH control and stirring. In order to manipulate the pH of nano-fluids, buffer solutions of NaOH and HCl 0.1M were implemented. For measuring the zeta potential and particle size, DLS analyzer was used (Malvern Zen-3600).
For investigating the influence of surfactant on stability of nano-fluids, three different types of surfactants namely: SDS, PVP and Triton X-100 were added at 0.1 % of general volume of nano-fluid (purchased from Merck Darmstadt, Germany). Specifications of surfactants have been given in Table 4.
3 Results and discussions
In this section, influence of different parameters such as sonication time, adding the surfactant, stirring and pH control on the stability of nano-fluids have been discussed.
3.1 Time-settlement experiments
After CuO nanoparticles were dispersed in different base fluids, post treatments were applied to the nano-fluids to enhance their stability. Table 5 represents the results of time-settlement experiments after post-treatments.
According to Table 5, ethylene glycol was the best medium for dispersing the CuO nanoparticles. Therefore, experiments were conducted to investigate the influence of pH control, sonication and stirring techniques on the stability of CuO/EG nano-fluids.
3.2 Influence of pH on stability of nano-fluids
In a nano-suspension, dispersed nanoparticles can attract or repel other particles. These interactions depend on the distance between nanoparticles and the total interface energy that is the sum of the van der Waals attraction forces and the electro-static repulsion forces. This relationship can be interpreted using DLVO theory [31]. When nanoparticles are uniformly dispersed within the base fluid, have high surface charge repulsive force. Such forces can prevent the nanoparticles from formation of agglomeration. There is a reference point, which is called the IEP. In this point, the zeta potential of nano-fluid is equivalent to zero and nano-fluid is in its most instability condition. As the pH of nano-fluids departs from the IEP of nanoparticles, the colloidal particles are more stable. The more stable nano-fluid is, the higher thermal conductivity nano-fluids have [31]. Results of time-settlement experiment demonstrated that pH control has a strong influence on the stability of nano-fluids. When pH of nano-fluid is adjusted, the height of sedimentation layer of particles inside the vessel is minimized and agglomeration inside the bulk of nano-fluid is not seen. Figure 4 demonstrates the stability of CuO/water nano-fluids after 45 days of preparation using pH control.
The similar behaviors were seen for ethylene glycol (EG) and water/EG based nano-fluids. However, values of pH adjustment were different to those of adjusted for deionized water (see Table 5).
3.3 Influence of dispersion time and stirrer speed on stability of nano-fluids
In the present work, magnetic stirrer was implemented to disperse the nanoparticles inside the base fluid. Experiments indicated that the second effective parameter on the stability of nano-fluids is stirring speed. Results showed that, when nanoparticles were dispersed in the base fluid at higher stirring speed, height of sedimentation layer and agglomeration significantly decreases. Similar trends for CuO–EG nano-fluid and CuO–water/EG nano-fluids was seen. The main reason of this phenomenon can be interpreted as by increasing the speed of stirring, more nanoparticles can be contributed to the dispersing process. As a result, lower quantity of nanoparticles can settle down at the bottom of the vessel. Because agitation in bulk of fluid can be intensified at higher stirring speed. Similarly, dispersing time had a significant impact on stability and height of sedimentation layer of nanoparticles such that with increasing the dispersing time, height of sedimentation layer significantly decreased for all nano-fluids and concentrations. Figure 5 represents the influence of dispersion time and stirring speed, \(\omega ,\) on the height of sedimentation layer of nanoparticles. Noticeably, pH was adjusted without sonication technique and visual observations was carried out after 2 months of stabilization.
3.4 Influence of sonication time on stability of nano-fluids
Sonication is one of the favorable techniques for stabilizing the nano-fluids, which has widely been used for breaking the agglomerations. In this work, influence of sonication time and frequency on the stability of nano-fluids was experimentally studied. Results showed that with increasing the sonication time up to 60 min, height of sedimentation layer decreased, while increasing the sonication time interestingly caused the height of sedimentation to be increased. Figure 6 shows the influence of sonication time on the sedimentation height of CuO/water nanoparticles at different concentrations. Similarly, for CuO/EG and CuO–water/EG, the same behaviors were seen such that the optimum sonication time for CuO/EG and CuO–water/EG is 75 and 90 min respectively. When ultrasonic waves are applied to the nano-fluid, cavitation phenomenon occurs (due to the degradation of molecules), which leads the microbubbles to be formed inside the nano-fluids. The formed bubbles can collapse due to the bubble–bubble interaction and consequently, micro-streams are formed, which cause the agitation in bulk of nano-fluid. Also, due to the bubble collapsing phenomenon, local energy releases, which influences on the temperature of nano-fluids. Thus, the released energy and the micro-streams, both can have negative influence on the stability of nano-fluids. When sonication time increases more than 60 min, released energy and micro-streams can be intensified, as a result, stability of nano-fluids is more decreased [32]. Therefore, height of sedimentation layer increases again. So there is an optimum sonication time, which should be carefully considered.
3.5 Influence of surfactants on stability of nano-fluids
As mentioned, the best medium for dispersing the CuO nanoparticles was found to be ethylene glycol. Thus, studies were conducted to determine the suitable surfactant for stabilizing the CuO/ethylene glycol nano-fluids. The specifications of surfactants are given in Table 4. The formulation procedure was as follows:
The desired volume of surfactant was measured based on the general volume of nano-fluid. Using stirrer, the measured surfactant was uniformly mixed with the nano-fluids. Once again, time-settlement experiments were implemented to investigate the influence of surfactant. The procedure was carried out for each concentration of CuO nanoparticles. Table 6 represents the results of study on the stability of CuO/EG nano-fluids at different concentrations of nano-fluid. As can be seen in Table 6, the best stability was registered for SDS at volumetric concentration of 1 %. The volume of test nano-fluids was 400 mL. Therefore, the most stable nano-fluid can be prepared using ethylene glycol as base fluid and SDS as suitable surfactant. The best condition, in which the highest stability of nano-fluid (at wt% = 0.4) can be achieved, is given in Table 7.
3.6 Theoretical perspective to CuO nano-fluids stabilization
According to Stokes’ law the sedimentation velocity, V in a colloid can be expressed as follows:
where rate of sedimentation decreases with decreasing particle radius, R, gravity, g, density difference between the particle and the liquid, \((\rho_{p} - \rho_{l} )\) and increasing base liquid viscosity, μ. These are key parameters for a stable nano-fluid. This formula was applied to the nano-fluids under this study and results were plotted in Fig. 7. According to the obtained results, sedimentation velocity of particle for EG-based nano-fluid is very smaller in comparison with other base fluids. Thus, it can be concluded that EG-based nano-fluids were more stable rather than other test nano-fluids.
4 Conclusions
CuO nanoparticles were experimentally dispersed in different base fluids including water, ethylene glycol and water/ethylene glycol 50:50 (by vol%). Following conclusions have been made:
-
According to the time-settlement experiments, by increasing the stirring speed, nano-fluids were more stable. However, in case of using surfactant, at higher stirring speed, a foam layer was formed inside the vessel.
-
In terms of sonication, results showed that with increasing the sonication time, stability enhanced and then suppressed. In fact, there is an optimum point for sonication time which should carefully be considered.
-
Results of this research showed that SDS is the suitable surfactant in comparison with other additives, which stabilizes the nano-fluids up to 75 days at maximum mass concentration of nanoparticles.
-
Sedimentation velocity of nano-fluids was estimated by Stokes law. Results showed that ethylene glycol is better medium in comparison with water and water/EG, since sedimentation velocity for EG-based nano-fluids was considerably lower than other nano-fluid (approximately equivalent to zero). This conclusion agrees well with the experimental results.
-
Experiments showed that CuO/EG nano-fluids at any dilute concentration of CuO nanoparticles (0.1–0.4 %), pH = 10.1 and 75 min of sonication can be stabilized up to 75 days without any sedimentation and agglomeration.
All in all, CuO/ethylene glycol nano-fluid was found to have better stability behavior in comparison with other test nano-fluids.
References
Choi SUS (1995) Enhancing thermal conductivity of fluids with nanoparticles. In: Developments and applications of non-newtonian flows, ASME FED 231/MD66, pp 99–103
Lee S, Choi SUS, Li S, Eastman JA (1999) Measuring thermal conductivity of fluids containing oxide nanoparticles. J Heat Transf Trans ASME 121:280–289
Wang X, Xu X, Choi SUS (1999) Thermal conductivity of nanoparticle–fluid mixture. J Thermo Phys Heat Transf 13:474–480
Zafarani-Moattar MT, Majdan-Cegincara R (2013) Investigation on stability and rheological properties of nano-fluid of ZnO nanoparticles dispersed in poly (ethylene glycol). Fluid Phase Equilib 354:102–108
Sarafraz MM, Hormozi F (2014) Scale formation and subcooled flow boiling heat transfer of CuO–water nano-fluid inside the vertical annulus. Exp Thermal Fluid Sci 52:205–214
Saidur R, Leong KY, Mohammad HA (2011) A review on applications and challenges of nano-fluids. Renew Sustain Energy Rev 15:1646–1668
Peyghambarzadeh SM, Hashemabadi SH, Naraki M, Vermahmoudi Y (2013) Experimental study of overall heat transfer coefficient in the application of dilute nano-fluids in the car radiator. Appl Therm Eng 52:8–16
Witharana S, Palabiyik I, Musina Z, Ding Y (2013) Stability of glycol nano-fluids—the theory and experiment. Powder Technol 239:72–77
Lin C, Wang JC, Chen TC (2011) Analysis of suspension and heat transfer characteristics of Al2O3 nano-fluids prepared through ultrasonic vibration. Appl Energy 88:4527–4533
Das SK, Putra N, Thiesen P, Roetzel W (2003) Temperature dependence of thermal conductivity enhancement for nano-fluids. J Heat Transf 125:8
Asirvatham LG, Vishal N, Gangatharan SK, Lal DM (2009) Experimental study on forced convective heat transfer with low volume fraction of CuO/water nano-fluid. Energies 2(1):97–119
Hwang Y, Lee JK, Lee CH, Jung YM, Cheong SI, Lee CG, Ku BC, Jang SP (2007) Stability and thermal conductivity characteristics of nano-fluids. Thermochim Acta 455(1–2):70–74
Lee D, Kim J-W, Kim BG (2006) A new parameter to control heat transport in nano-fluids: surface charge state of the particle in suspension. J Phys Chem 110:4323–4328
Li XF, Zhu DS, Wang XJ, Wang N, Gao JW, Li H (2008) Thermal conductivity enhancement dependent pH and chemical surfactant for Cu–H2O nano-fluids. Thermochim Acta 469(1–2):98–103
Hwang YJ, Ahn YC, Shin HS, Lee CG, Kim GT, Park HS, Lee JK (2006) Investigation on characteristics of thermal conductivity enhancement of nano-fluids. Curr Appl Phys 6(6):1068–1071
Sarafraz MM, Hormozi F (2014) Convective boiling and particulate fouling of stabilized CuO–ethylene glycol nano-fluids inside the annular heat exchanger. Int Commun Heat Mass Transf. doi:10.1016/j.icheatmasstransfer.2014.02.019
Wen DS, Ding YL (2005) Formulation of nano-fluids for natural convective heat transfer applications. Int J Heat Fluid Flow 26:855–864
Zhu D, Li X, Wang N, Wang X, Gao J, Li H (2009) Dispersion behavior and thermal conductivity characteristics of Al2O3–H2O nano-fluids. Curr Appl Phys 9:131–139
Li X, Zhu D, Wang X (2007) Evaluation on dispersion behavior of the aqueous copper nano-suspensions. J Colloids Interface Sci 310:456–463
Wang JX, Zhu HT, Zhang CY, Tang YM, Ren B, Yin YS (2007) Preparation and thermal conductivity of suspensions of graphite nanoparticles. Carbon 45:226
Pak BC, Cho Y (1998) Hydrodynamic and heat transfer studies of dispersed fluids with sub-micronmetallic oxide particles. Exp Heat Transf 11:151–170
Witharana S, Hodges C, Xu D, Lai X, Ding Y (2012) Aggregation and settling in aqueous polydisperse alumina nanoparticle suspensions. J Nanopart Res 14:851
Palabiyik I, Musina Z, Witharana S, Ding Y (2011) Dispersion stability and thermal conductivity of propylene glycol-based nano-fluids. J Nanopart Res 13:5049–5055
Sarafraz MM, Hormozi F (2014) Convective boiling and particulate fouling of stabilized CuO–ethylene glycol nanofluids inside the annular heat exchanger. Int Commun Heat Mass Transf 53:116–123
Sarafraz MM, Pyghambarzadeh SM (2012) Nucleate pool boiling heat transfer to Al2O3–water and TiO2–water nano-fluids on horizontal smooth tubes with dissimilar homogeneous materials. Chem Biochem Eng Q 26(3):199–206
Sarafraz MM, Peyghambarzadeh SM (2013) Experimental study on sub-cooled flow boiling heat transfer to water–diethylene glycol mixtures as a coolant inside a vertical annulus. Exp Therm Fluid Sci 50:154–162
Sarafraz MM, Hormozi F (2014) Scale formation and sub-cooled flow boiling heat transfer of CuO–water nano-fluid inside the vertical annulus. Exp Therm Fluid Sci 52:205–214
Sarafraz MM, Hormozi F (2015) Pool boiling heat transfer to dilute copper oxide aqueous nanofluids. Int J Therm Sci 90:224–237
Farrokhpay S (2009) A review of polymeric dispersant stabilization of titania pigment. Adv Colloid Interface Sci 151:24–32
Chang H, Jwo C, Fan P, Pai S (2007) Process optimization and material properties for nano-fluid manufacturing. Int J Adv Manuf Technol 34(3):300–306
Ghadimi A, Saidur R, Metselaar HSC (2011) A review of nano-fluid stability properties and characterization in stationary conditions. Int J Heat Mass Transf 54:4051–4068
Wu TY, Guo N, Teh CY, Hay JXW (2013) Advances in ultrasound technology for environmental remediation, chapter 2. In: Hay JXW (ed) Theory and fundamentals of ultrasound. Springer, Germany
Acknowledgments
Authors of this work tend to dedicate this article to Imam Mahdi and appreciate Semnan University for their financial supports. Also special appreciation is dedicated to MERC (Material and Energy Research Center for sharing their scientific equipment).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamalgharibi, M., Hormozi, F., Zamzamian, S.A.H. et al. Experimental studies on the stability of CuO nanoparticles dispersed in different base fluids: influence of stirring, sonication and surface active agents. Heat Mass Transfer 52, 55–62 (2016). https://doi.org/10.1007/s00231-015-1618-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-015-1618-z