Abstract
Introduction
Augmented renal clearance (ARC) defined as creatinine clearance (Clcr) above 130 mL/min/1.73m2 may lead to suboptimal antibacterial treatment. The aim of this study was to determine a strategy for meropenem administration to achieve both pharmacodynamic-pharmacokinetic (PK-PD) target (50%fT > MIC) and better clinical outcomes in patients with VAP and ARC.
Materials and methods
In this randomized clinical trial, patients with VAP and high risk for ARC were recruited. An 8-h urine collection was performed on the 1st, 3rd, and 5th days of study to measure Clcr. Included patients were divided into three groups: (1) 1 g meropenem, 3-h infusion, (2) 2 g meropenem, 3-h infusion, (3) 1 g meropenem, 6-h infusion. On the 2nd, 3rd, and 5th days of treatment, peak and trough blood samples were collected to undergo HPLC assay. MICs were assessed using microdilution method. Patients were also clinically monitored for 14 days.
Results
Forty-five patients were included. Group 3 showed significanty higher rate of patients achieving fT > MIC > 50% (100% for group 3 versus 40% for group 2 and 13% for group 1; p = 0.0001). Mean fT > MIC% was significantly higher in group 3 (78.77 ± 5.87 for group 3 versus 49.6 ± 7.38 for group 2 and 43.2 ± 7.98 for group 1; p = 0.0001). Statistical analysis showed no significant differences among groups regarding clinical improvement.
Conclusion
According to the findings of this trial, prolonged meropenem infusion is an appropriate strategy compared to dose elevation among ARC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ARC is a clinical phenomenon mostly detected among critically ill patients, defined as enhanced Clcr of greater than 130 mL/min/1.73m2 [1, 2]. Further investigations demonstrated a significant impact on treatment outcome in ARC patients due to suboptimal plasma levels of drugs, especially time-dependent antibiotics [3,4,5,6]. Therefore, new dosing strategies in order to cope with ARC effects are strongly needed [4, 7].
Ventilator-associated pneumonia (VAP) is a life-threatening infection in the ICU. The mortality of VAP reaches 30%, and the adequacy of the initial empirical treatment greatly influences the prognosis [8]. ARC may increase the risk of suboptimal treatment and mortality in VAP patients. Meropenem being an important agent in empiric therapy for VAP, and as a time-dependent antibiotic, is particularly at risk of over-filtration in ARC patients [9,10,11,12].
The aim of this study was to determine a proper dosing strategy for meropenem administration, in order to achieve fT > MIC > 50% and to evaluate clinical outcomes in critically ill patients with VAP and ARC.
Materials and methods
Study design
This single-center study was conducted as a randomized clinical trial in Loghman Hakim Medical Center, affiliated with Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran. Recruitment occurred from October 2019 to March 2021.
Study participants
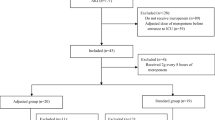
The patients were considered eligible for inclusion if they met all of the following criteria: (1) a definite or highly probable diagnosis of VAP according to CPISFootnote 1 (a score of more than 6 is considered high risk for pneumonia) [13], (2) high risk for developing ARC according to ARC score [14] or ARCTIC score [15], (3) receiving meropenem, (4) serum creatinine less than 1.3 mg/dL, (5) being at least 18 years old.
Patients were excluded (1) if measured Clcr was less than 130 mL/min/1.73m2 on the 1st, 3rd, and 5th days of study; (2) if they developed AKIFootnote 2; and (3) if meropenem administration was discontinued due to any reason.
The included patients were block-randomized into the three study groups: (1) receiving 1 g meropenem q8h during 3-h infusion, (2) receiving 2 g meropenem q8h during 3-h infusion, (3) receiving 1 g meropenem q8h during 6-h infusion. Time/concentration curves are shown in Figs. 1 and 2.
All patients initially received empiric antibiotic regimen for VAP including meropenem, vancomycin, and an aminoglycoside intravenously.
Study procedures
The patients diagnosed with VAP were included and assigned into one of the three study groups on the first day of suspicion of ARC based upon scoring systems [14, 15] (Table 1). On this day (day 1), meropenem was started as study protocol according to the intervention group that the patient was block-randomized into. At the same day, the patients underwent an 8-h urine collection for Clcr measurement in order to confirm ARC [4]. Clcr was measured using below equation [4]:
Clcr = [urine creatinine (μg/mL) × urine volume (mL)] / [serum reatinine (μg/mL) × T (min)].
Cutoff point for Clcr was 130 mL/min/1.73m2 based upon previous studies.
If ARC was not confirmed, the patient was excluded and meropenem administration was changed to the standard regimen. If ARC was confirmed, the patient persisted in the study and the protocol was continued for him. Blood sample (5 mL) collection took place on the next day (2nd day) from the arterial catheter of the patients. Peak blood samples were collected 30 min after the end of 3-h (groups 1 and 2) or 6-h (group 3) meropenem infusion. Trough blood samples were collected 30 min before the start of the next dose of meropenem infusion. Urine collection for ARC confirmation was repeated on the 3rd and the 5th days of study to ensure the persistence of ARC. Also on these days, blood samples were collected from the patients. Blood samples were immediately centrifuged for 10 min at 4000 rpm, and serum was separated and stored at − 80 °C for later analysis.
Meropenem assay
In order to analyze the serum samples, validated Agilent Infinity Lab High-Performance Liquid Chromatography (HPLC) system was used. Samples were separated on Agilent Infinity Lab 2.6 m C18 column (250 × 4.6 mm with 3.5-mm spherical particles). The mobile phase used for analysis was 10.53 mmol/L ammonium acetate:acetonitrile (91:9, v/v) (pH = 4). The mobile phase was delivered at a total flow rate of 1 mL/min. The UV detector was adjusted at 298 nm. C18 column temperature was maintained at 35 °C. The total run time was set for 10.0 min [16, 17].
Sample preparation involved plasma protein precipitation with acetonitrile and a wash step with dichloromethane. Acetaminophen was chosen as the internal standard due to structural and behavioral similarities to meropenem. Initially, 950 μL of serum was added to 50 μL of acetaminophen (800 μg/mL) following the addition of 1000 μL of acetonitrile. After shaking for 10 min by Heidolph vortex mixer and 10-min centrifugation by Hettich Micro 200 Centrifuge at 1000 g, a 1000 μL of supernatant was added to 1000 μL methylene chloride. After a 10-min shaking by vortex mixer and a 10-min centrifugation at 1000 g, 20 μL of the aliquot of the upper aqueous layer was injected into the C18 analytical column [11].
The assay was linear from 0.25 to 20 µg/mL with an imprecision and inaccuracy < 7% at high, medium, and low concentrations.
MIC assessment
MIC determination of strains isolated from tracheal aspiration cultures of the included patients was performed using broth microdilution method as described by the Clinical and Laboratory Standards Institute (CLSI) [18]. The 96-well microplate contained Mueller–Hinton broth with varying concentrations of meropenem (ranging from 100 to 0.25 μg/mL). The wells were inoculated with 50μL of suspended culture to inoculum density of 105 CFU/mL per well. The microplate was incubated at 37° for 24 h. Then it was visually evaluated and MIC was recorded as the lowest meropenem concentration, where no turbidity (visible growth) was observed.
PK-PD parameter analysis and clinical monitoring
Peak and trough serum concentrations from samples collected on the 2nd, 3rd, and 5th days of study were measured through HPLC analysis. Then PK parameters consisted of clearance (Cl), elimination constant (K), and half-life (T1/2) were calculated for each patient. Assuming one compartmental first-order kinetic, the time within the dosing interval where the concentration is maintained above MIC was calculated (fT > MIC).
PK-PD target attainment was evaluated through calculation of fT > MIC%. Each patient was also monitored for clinical outcomes for 14 days following the start of antibacterial treatment. Clinical improvement factors included the days it took for fever resolution, secretion decrease, leukocytosis resolution, and also the duration of intubation, hospitalization in the ICU, and mortality rate.
Statistical analysis
The statistical analysis was performed using the statistical software package IBM-SPSS statistics version 26.0 (IBM Corp., New York, NY, USA). Qualitative variables were analyzed by chi-square method and presented as frequencies and percentages. Continuous variables with non-normal distribution were analyzed by Kruskal–Wallis method and expressed as mean ± standard deviation and median values with interquartile ranges. Post-hoc analysis as Fisher’s test was performed for significantly non-equal variances between groups. A P value < 0.05 was considered statistically significant.
Results
In this study, 195 patients were included. One hundred seventeen patients were excluded, because measured Clcr did not meet the cutoff point for ARC. Twenty-one cases expired during the first 5 days of treatment (7 cases in group 1 – 9 cases in group 2 – 5 cases in group 3). In 12 cases, urine was not properly collected. Forty-five patients were block-randomized into the three study groups. Demographic characteristics and the clinical factors are shown in Table 2. MIC results are illustrated in Table 3. Statistical analysis proved the variances to be normally distributed (P value > 0.05), except for peak and trough concentrations. PK factors are shown in Table 4.
Patients were monitored clinically for treatment outcomes for 14 days. Clinical factors are illustrated in Table 5. Statistical analysis showed no significant differences among groups regarding clinical improvement (P value > 0.05) although fT > MIC% proved to be significantly different among groups (P value < 0.05) (Table 5). Post-hoc analysis revealed group 3 (1 g meropenem q8h infused over 6 h) to show significantly higher levels of fT > MIC% compared to group 2 (p = 0.0001) and group 1 (p = 0.0001). Mean fT > MIC % rates were significantly higher in group 3 (78.77 ± 5.87 for group 3 versus 49.6 ± 7.38 for group 2 and 43.2 ± 7.98 for group 1; p = 0.0001). Group 3 also showed significantly higher rate of patients achieving fT > MIC > 50% (100% for group 3 versus 40% (6/15) for group 2 and 13% (2/15) for group 1; p = 0.0001). A comparison between the three groups regarding fT > MIC% is illustrated in Fig. 3.
Discussion
In this randomized clinical trial on 45 ARC patients receiving meropenem, fT > MIC% and clinical factors were evaluated between three groups. This survey demonstrated the superiority of prolonged (6 h) infusion of meropenem in order to achieve fT > MIC > 50%.
Previous studies mainly suggested two strategies to overcome ARC—(1) dose elevation to 6 g meropenem per day [11] and (2) infusion prolongation to 3–6 h [19]—however, the investigations were mostly observational and the results showed obvious inconsistency. Previous studies also proved the advantage of prolonged infusion of beta-lactams among critically ill patients regarding fT > MIC%, which is similar to our conclusion, except for ARC identification [20,21,22,23]. However, no robust prospective controlled study investigating prolonged infusion of beta-lactams among ARC-identified population exists.
Previous studies mostly revealed that higher beta-lactam concentrations alone cannot significantly influence their efficacy. Based on numerous in vitro and in vivo experimental data, it is the duration of effective exposure that is more important for these time-dependent antibiotics [24].
The patients were also monitored for clinical improvement for 14 days following VAP diagnosis. The comparison of all parameters among the three groups proved no significant differences. A possible explanation is the difference between plasma concentrations of antibiotic versus tissue penetration. Free concentrations in plasma are often viewed as an acceptable approximation for free concentrations at the site of infection, but this is not always the case [25, 26]. Tissue penetration of meropenem in respiratory tract (expressed as percentage of tissue vs. plasma concentration) is reported in literature as 40% in the lung. This relatively low concentration in pulmonary tissue might explain why attaining fT > MIC > 50% might not necessarily improve clinical outcomes [27]. Another explanation is the small study population which is inadequate to properly reflect the influence of dosing approaches. Therefore, further robust prospective investigations evaluating clinical outcomes among larger populations are suggested.
Conclusion
According to the findings of this trial, prolonged meropenem infusion is an appropriate strategy compared to dose elevation among ARC patients.
Notes
Clinical Pulmonary Infection Score.
Acute kidney injury.
References
Mahmoud S, Shen C (2017) Augmented renal clearance in critical illness: an important consideration in drug dosing. Pharmaceutics 9(3):E36
Sime FB, Udy AA, Roberts JA (2015) Augmented renal clearance in critically ill patients: etiology, definition and implications for beta-lactam dose optimization. Curr Opin Pharmacol 24:1–6
Udy AA, Roberts JA, Lipman J (2013) Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med 39(12):2070–2082
Hobbs AL et al (2015) Implications of augmented renal clearance on drug dosing in critically ill patients: a focus on antibiotics. Pharmacotherapy 35(11):1063–1075
Carrie C et al (2018) Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of β-lactams administered by continuous infusion: a prospective observational study. Int J Antimicrob Agents 51(3):443–449
Aa U et al (2011) Sub-therapeutic initial-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest 10
Heffernan AJ et al (2018) How to optimize antibiotic pharmacokinetic/pharmacodynamics for Gram-negative infections in critically ill patients. Curr Opin Infect Dis 31(6):555–565
Mahmood SN, Shorr AF (2021) Issues in antibiotic therapy for hospital-acquired and ventilator-associated pneumonia: emerging concepts to improve outcomes. Expert opinion on pharmacotherapy pp. 1–7
Carlier M et al (2013) Meropenem and piperacillin/tazobactam prescribing in critically ill patients: does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit Care 17(3):1–9
Minichmayr IK et al (2018) Development of a dosing nomogram for continuous-infusion meropenem in critically ill patients based on a validated population pharmacokinetic model. J Antimicrob Chemother 73(5):1330–1339
Tamatsukuri T et al (2018) The exploration of population pharmacokinetic model for meropenem in augmented renal clearance and investigation of optimum setting of dose. J Infect Chemother 24(10):834–840
Tröger U et al (2012) Decreased meropenem levels in Intensive Care Unit patients with augmented renal clearance: benefit of therapeutic drug monitoring. Int J Antimicrob Agents 40(4):370–372
Pugin J et al (1991) Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic blind bronchoalveolar lavage fluid. American Review of Respiratory Disease 143(5_pt_1):1121–1129
Udy AA et al (2013) Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: identifying at-risk patients. Crit Care 17(1):1–9
Barletta JF et al (2017) Identifying augmented renal clearance in trauma patients: validation of the augmented renal clearance in trauma intensive care scoring system. J Trauma Acute Care Surg 82(4):665–671
D’Cunha R et al (2018) Quantification of cefepime, meropenem, piperacillin, and tazobactam in human plasma using a sensitive and robust liquid chromatography-tandem mass spectrometry method, part 1: assay development and validation. Antimicrob Agents Chemother 62(9):e00859-e918
Ibrahim F et al (2020) Analytical methods for the determination of certain antibiotics used in critically ill patients. Pharm Res 2(1):99–117
Abbey TC, Deak E (2019) What’s New from the CLSI Subcommittee on Antimicrobial Susceptibility Testing M100. Clin Microbiol Newsl 41(23):203–209
Udy AA et al (2017) Association between augmented renal clearance and clinical outcomes in patients receiving β-lactam antibiotic therapy by continuous or intermittent infusion: a nested cohort study of the BLING-II randomised, placebo-controlled, clinical trial. Int J Antimicrob Agents 49(5):624–630
Roberts JA et al (2014) DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58(8):1072–1083
Abdul-Aziz MH et al (2016) Is prolonged infusion of piperacillin/tazobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort. J Antimicrob Chemother 71(1):196–207
Yu Z et al (2018) Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: A meta-analysis. PloS One 13(7):e0201667
Luo J et al (2019) Prolonged versus Intermittent Infusion of Antibiotics in Acute and Severe Infections: A Meta-analysis. Arch Iran Med 22(10):612–626
Drusano G (2007) Pharmacokinetics and pharmacodynamics of antimicrobials. Clin Infect Dis 45(Supplement_1):S89-S95
Lodise T et al (2011) Penetration of meropenem into epithelial lining fluid of patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 55(4):1606–1610
Rodvold KA, George JM, Yoo L (2011) Penetration of anti-infective agents into pulmonary epithelial lining fluid. Clin Pharmacokinet 50(10):637–664
Craig WA (1997) The pharmacology of meropenem, a new carbapenem antibiotic.Clin Infect Dis 24(Supplement_2):S266-S275
Funding
HPLC was funded by Tarbiat Modares University.
Author information
Authors and Affiliations
Contributions
S. Razzazzadeh carried out sampling and calculations. I. Alavi Darazam evaluated clinical criteria. M. Hajiesmaeili provided administrative support for the clinical process. J. Salamzadeh performed statistical analysis. A. Mahboubi performed MIC assessments. E. Sadeghnezhad contributed to meropenem HPLC assay. Z. Sahraei conceived the original idea and the trial designing and was in charge of overall direction. All authors contributed to the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Institutional ethics approval was provided according to local protocols with the ethics committee code of IR.SBMU.PHARMACY.REC.1398.095. The trial was registered at the Iranian Registry of Clinical Trials with the registration number of IRCT20130917014693N12.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Razzazzadeh, S., Darazam, I.A., Hajiesmaeili, M. et al. Investigation of pharmacokinetic and clinical outcomes of various meropenem regimens in patients with ventilator-associated pneumonia and augmented renal clearance. Eur J Clin Pharmacol 78, 823–829 (2022). https://doi.org/10.1007/s00228-022-03291-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03291-5