Abstract
Purpose
A rapidly increasing use of biological drugs has led to substantial costs. Shift to biosimilars enables considerable reduction of these costs without jeopardizing the treatment of patients, but most countries have extensive possibilities of untapped cost-savings. The aim of this study was to describe the Danish quick and near-complete implementation of the two first TNF inhibitor biosimilars (infliximab and etanercept).
Methods
We shed light on the considerations and experiences made during the implementation, and present key figures from the implementation.
Results
The infliximab biosimilar constituted 90.6% of the total amount of infliximab four months following patent expiration of the biooriginator. Similar results were seen for etanercept biosimilar. Substantial cost reductions were experienced in the way that e.g. the infliximab-shift reduced cost by two thirds.
Conclusion
We believe that a thorough preparation and an organizational setting supporting the implementation is crucial for the successful implementation. This same implementation model will be used for future biosimilars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biologics and biosimilars
The definition of biological medicines (biologics) varies, but the European Medicines Agency (EMA) defines them as “contain[ing] active substances from a biological source, such as living cells or organisms” [1]. In general, newer biologics are expensive and are responsible for a substantial part of the increasing expenditure on pharmaceutical drugs worldwide. This has been highlighted by a recent market watch that placed eight biologics among the ten best-selling drugs globally in 2017 [2]. The high level of global expenditure on biologics means that the potential for cost savings following patent expiration is substantial. Usually, at least one generic drug is branded following the expiration of a drug patent. Biologics are manufactured as biological copies, rather than by chemical synthesis and so biosimilars are not true generics. There is no consensus definition of a biosimilar, but the EMA defines it as a drug that is “highly similar” to the reference medicine (biooriginator) [1]. In many countries, great efforts are made to reduce drug expenditures by increasing the use of biosimilars, but their implementation has tended to be slow, probably in the main due to a theoretical uncertainty about the efficacy and safety of biosimilars among patients and healthcare professionals. For those patients who are already taking the biooriginator there is a specific concern that the non-medical shift from biooriginator to biosimilar could cause the development of drug antibodies that could potentially weaken the effect of the treatment and increase the risk of adverse drug reactions. The slow implementation results in a loss of opportunities to make economic savings. Here we present a Danish model for fast and near-complete implementation of biosimilars [3].
According to the market watch, the two biological anti-inflammatory drugs, Remicade and Enbrel, the biooriginators of infliximab and etanercept, were among the ten best-selling drugs globally in 2017. Remicade was the sixth best-selling drug, with sales of 5.8 billion USD, and Enbrel was the eighth, with sales of 5.4 billion USD [2]. Biosimilars of both drugs have been branded globally. In Denmark, a complete shift to biosimilars was undertaken, including non-medical shifts. Thus, patients receiving effective treatment with the biooriginator were shifted to a biosimilar entirely for economic reasons. The shifts resulted in considerable cost savings despite increasing drug consumption.
The Danish setting
Denmark, in northern Europe, is an EU member with 5.8 million inhabitants. In Denmark, health services are paid through taxation, and biological anti-inflammatory drugs are provided free of charge to patients in hospital-based out-patient clinics [4]. Increasing use of expensive biological anti-inflammatory drugs has placed a heavy demand on hospital drug budgets. Public healthcare services are provided by the five politically independent regions of the nation, who are responsible for running the hospitals. Purchase of medicines for hospital use is centralized in Denmark. Amgros is the regional authorities’ pharmaceutical procurement service, i.e., the body that bulk-purchases the drugs, handles national tendering procedures, and supplies the drugs to regional hospital pharmacies. Amgros cooperates with the Danish Medicines Council (DMC). At the time biosimilars were introduced, Amgros cooperated with the predecessor of the DMC, the Council for the Use of Expensive Hospital Medicines (RADS). DMC and RADS have appraised expensive drugs covering several therapeutic areas for in-hospital use and have provided national guidelines. All five regions are represented on both councils, and have agreed to implement the recommendations, although each region organizes its own implementation. The five Regional Drug and Therapeutics Committees are key players in this regional implementation.
Initiatives for implementing biosimilars
As a matter of principle, RADS decided in May 2014 that any drug assessed by the EMA as a biosimilar could be used by Danish patients. This included all drug-naïve patients, both new patients and those switching from another drug due to lack of effect or adverse events, and patients currently receiving the biooriginator. When the biosimilars of infliximab and etanercept were approved for use in Denmark, RADS arranged discussions between committee members and members of the respective clinical societies for dermatology, gastroenterology and rheumatology. The continuing pressure on healthcare budgets was expected to force a change in attitudes towards biosimilars. However, the clinicians were not familiar with biosimilars and were concerned about their safety and efficacy. These concerns included the core issue of extrapolation in biosimilars, such as when to accept safety and efficacy data from one indication and apply it to another, or when to apply the data from one population to another. The main topics of discussion at these meetings were efficacy, safety, immunogenicity, interchangeability, the traceability of the drug, and how to monitor efficacy. The meetings reached a consensus on the use of biosimilar infliximab and etanercept involving the close monitoring of usage, efficacy, and adverse reactions. Furthermore, the Danish Medicines Agency (DMA) was urged to produce informative written material on biosimilars for healthcare professionals and patients [5].
RADS decided to undertake a fast and complete shift to biosimilars in the hope of reducing the costs of hospital drugs without affecting the quality of patient treatment. A national biosimilar task force reporting to RADS was subsequently established with the purpose of 1) exploring potential practical problems in the hospitals/departments, 2) preparing the implementation, 3) having continuous discussions with the drug suppliers, 4) conducting meetings with clinicians, administrators, and patient organizations, 5) assessing the need for providing educational materials.
Even before the decision was taken regarding the implementation of biosimilars, Amgros was negotiating with potential companies. This was important to speed up the agreement of contracts and to ensure a stable drug supply as soon as the decision was made.
Several initiatives were adopted to assess the possible negative effects of the shift to biosimilars, including mandatory registration of batch numbers when treating patients and when reporting potential adverse events. The very extensive Danish clinical quality databases and national registries were used to address these matters.
Patient outcomes following the implementation
Research undertaken in patients with rheumatoid arthritis found no difference in disease activity one year after the non-medical shift from Remicade to the biosimilar Remsima [6]. In another group of patients with rheumatoid arthritis, no relationship was found between development of anti-drug antibodies and treatment withdrawal [7]. Furthermore, the DMA paid particular attention to new signals in reported potential adverse drug reactions, and declared in a newsletter in August 2016 that the reported potential adverse drug reactions gave no reason to suspect any differences between the biooriginators and biosimilars of infliximab and etanercept [8]. These findings are in agreement with an opinion paper, published in 2017, listing theoretical risks and actual experiences of the interchangeability of biosimilars. The authors concluded that biosimilars approved in the EU do not differ from their corresponding biooriginators with respect to their safety and efficacy [9].
Methods
Sales of infliximab and etanercept in Danish community pharmacies are negligible. Data on net purchases of infliximab and etanercept by hospitals were examined by using the database of hospital drug sales by Amgros and the Danish hospital pharmacies. Data were recorded daily, but we aggregated them to monthly periods. Furthermore, data are presented as the sum of all hospitals’ purchases. Sub-analyses were conducted to evaluate potential differences in the biosimilar proportion of drug purchases between regions and specialties, but these revealed no noticeable differences. From now on, we will refer to the sale as consumption; they are equivalent due to the small quantities stored in each department. Data on the implementation of infliximab and etanercept in various European countries were received from IQVIA MIDAS.
Results
Drug consumption
A shift for infliximab from Remicade to the biosimilar Remsima was implemented following the patent expiration on 13 February 2015. Fig. 1a presents Danish hospitals’ monthly consumption in Defined Daily Doses (DDD) of infliximab, grouped by brand name. The first sale of Remsima to a hospital department took place on 27 March 2015. By April 2015, Remsima constituted 18.9% of the total infliximab in DDD and the percentage rose rapidly over subsequent months, reaching 90.6% in July 2015. On average, Remsima accounted for 97.6% of consumption in 2016. As seen in Fig. 1a, there was an increasing consumption of infliximab following the shift to Remsima. Later, Inflectra became cheapest and the shift from Remsima to Inflectra was even faster. Inflectra was first sold on 21 September 2017, and by October 2017 it constituted 90.3% of total infliximab sales in DDD, the figure rising to 98.5% in November 2017. There was no difference in the pattern of implementation between the Danish regions, despite each of them organizing it independently, (Supplementary material).
The shift from Enbrel to Benepali commenced with its first sale to a hospital department on 5 April 2016. Fig. 2a shows the monthly consumption in DDD of etanercept, grouped by brand name. Benepali constituted 15.3% of total etanercept consumption in April 2016, and rose considerably in the subsequent months (64.7% in May 2016, 85.4% in June 2016). In 2017, Benepali accounted for 84.2% of total etanercept consumption. Benepali does not cover all the indications of Enbrel, so 100% consumption of biosimilars was not possible.
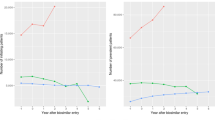
When comparing proportions of biosimilar consumption, it is important to take the date of patent expiration into account, since this differs between countries. As shown in Fig. 3a, the Danish implementation of infliximab was extremely rapid. At the time of the implementation of etanercept, several other countries had improved their implementation, but it still took Norway approximately 1 year to achieve a similar proportion of consumption of the biosimilar (Fig. 3b). The UK had a slower implementation of etanercept than Denmark and Norway but reached 75% implementation 2 years after the introduction of its biosimilars. Other countries had a less than 25% implementation of etanercept biosimilars after 1 year.
Drug costs
The total monthly drug costs of infliximab and etanercept are illustrated in Fig. 1b and Fig. 2b. The cost of infliximab was reduced by approximately two-thirds when changing from Remicade to Remsima, equivalent to a cost saving of 200 million DKK (approximately 24 million GBP) in 2015, which was the year Remsima was introduced [10].
Discussion
Like generic drugs, implementation of biosimilars aims solely at reducing drug costs. There are many reasons for the very rapid and complete shift from biooriginators to biosimilars. We believe one of the most important of these was the way the in-hospital drug sector is organized in Denmark. Through the Regional Drug and Therapeutics Committees, the discussions were held with local clinicians, the shift was followed closely by the hospital pharmacies, and the biosimilar was automatically delivered to the ward, unless reasons for not doing so were specifically stated. In some regions, the biooriginator could only be prescribed if the physician had an explicit reason for not using the biosimilar, but the regions had slightly different ways of managing the shift. The clinicians were probably primarily motivated by the threat of job cuts if drug budgets were exceeded. The direct consequences of overspending differ by region, but in general at least some of the overspending needs to be payed by either the department or the hospital. Conducting a non-medical shift requires staff time to inform patients, since they may be worried about changing from a well-tolerated and effective drug to a new, “unknown” medication. We believe the substantial cost reductions outweigh these considerations. However, it is important to ensure that the reductions in drug costs are not wiped out by increases in other costs. A study of patients with rheumatoid arthritis concluded that there was no change in the use of outpatient healthcare resources in the 6 months following patients’ non-medical shift to a biosimilar compared with the previous 6 months [11]. Further studies among all patient groups assessing all potential related increases in cost are warranted. Furthermore, studies accessing the prescriber’s opinions on the described shifts (or upcoming shifts) could give valuable knowledge leading to better involvement of clinicians and improved implementation strategies in the future. Other interesting research areas would be implementation of biosimilars where the new (and cheaper) drug differs from biooriginator with regard to administration path. This has already been the case for trastuzumab in oncology, where biooriginator Herceptin could be given both intravenously or as a subcutaneous injection but the biosimilars are only for intravenous use.
The Danish structure, with its national tendering, probably contributed to the substantial drug discounts obtained. It is not known whether corresponding cost savings can be achieved in future introductions of biosimilars, but a recent press release regarding the upcoming Danish shift to adalimumab biosimilar exceeds expectations with cost-reductions of 87% [12]. Achieving savings should always be a goal, since all unnecessary costs indirectly represent sacrifices borne by all other Danish patients.
Recommendations
Compared with shifts in other countries, the Danish implementation was rapid and almost complete. We believe thorough preparation and resolute implementation, in conjunction with provision of comprehensive information to patients, are keys to a successful non-medical shift to biosimilars. We plan to use the same method of implementation for future biosimilars. However, new and unresolved challenges lie ahead, for instance, whether patients could keep switching between different brand names (continuous interchangeability).
References
European Medicines Agency - Overview - Biosimilar medicines. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/general/general_content_001832.jsp. Accessed 6 Aug 2018
Urquhart L (2018) Market watch: top drugs and companies by sales in 2017. Nat Rev Drug Discov 17:232. https://doi.org/10.1038/nrd.2018.42
Aronson JK, Goldacre B, Ferner RE (2018) Prescribing biosimilars. BMJ 362:k3141. https://doi.org/10.1136/bmj.k3141
Danish Regions Vederlagsfri udlevering af medicin,. http://www.regioner.dk/sundhed/medicin/vederlagsfri-udlevering-af-medicin. Accessed 26 Jun 2017
RADS anbefaling vedrørende brug af biosimilært infliximab og etanercept. http://rads.dk/media/3488/rads-notat-om-anvendelsen-af-biosimilaere-juni-2016.pdf. Accessed 23 Jul 2018
Glintborg B, Sørensen IJ, Loft AG, Lindegaard H, Linauskas A, Hendricks O, Hansen IMJ, Jensen DV, Manilo N, Espesen J, Klarlund M, Grydehøj J, Dieperink SS, Kristensen S, Olsen JS, Nordin H, Chrysidis S, Dalsgaard Pedersen D, Sørensen MV, Andersen LS, Grøn KL, Krogh NS, Pedersen L, Hetland ML, all departments of rheumatology in Denmark (2017) A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis 76:1426–1431. https://doi.org/10.1136/annrheumdis-2016-210742
Glintborg B, Kringelbach T, Bolstad N, Warren DJ, Eng G, Sørensen IJ, Loft AG, Hendricks O, Hansen IMJ, Linauskas A, Nordin H, Kristensen S, Lindegaard H, Jensen DV, Goll GL, Høgdall E, Gehin J, Enevold C, Nielsen CH, Krogh NS, Johansen JS, Hetland ML (2018) Drug concentrations and anti-drug antibodies during treatment with biosimilar infliximab (CT-P13) in routine care. Scand J Rheumatol 47:418–421. https://doi.org/10.1080/03009742.2017.1376110
Danish Medicines Agency Nyt Om Bivirkninger. Nr. 7, årgang 7, august 2016. https://laegemiddelstyrelsen.dk/da/nyheder/nyhedsbrevet-nyt-om-bivirkninger/nyt-om-bivirkninger-august-2016/~/media/DE9B53A7B08846E186A210B97381675E.ashx. Accessed 9 Aug 2018
Kurki P, van Aerts L, Wolff-Holz E, Giezen T, Skibeli V, Weise M (2017) Interchangeability of Biosimilars: a European perspective. BioDrugs Auckl 31:83–91. https://doi.org/10.1007/s40259-017-0210-0
AMGROS Markedsovervågning 3. kvartal 2016. https://www.amgros.dk/viden-og-analyser/rapporteringer/markedsovervaagning/. Accessed 10 Sep 2018
Glintborg B, Sørensen J, Hetland ML (2018) Does a mandatory non-medical switch from originator to biosimilar infliximab lead to increased use of outpatient healthcare resources? A register-based study in patients with inflammatory arthritis. RMD Open 4:e000710. https://doi.org/10.1136/rmdopen-2018-000710
(2018) Regioner kan spare 335 mio. kr. om året på gigtmedicin. In: Dagens Pharma. https://dagenspharma.dk/regioner-kan-335-mio-kr-om-aaret-paa-gigtmedicin/. Accessed 19 Nov 2018
Author information
Authors and Affiliations
Contributions
TBJ and HRC initiated this analysis. All authors work with rational pharmacotherapy in Denmark and cover all five Danish regions as well as the regional authorities’ pharmaceutical procurement service. DB, EAS, BKP, SEA, MMHC, LN, and HRC were key figures during the shift to biosimilars of infliximab and etanercept. TBJ, DB and HRC planned the analysis. TBJ analysed the data. All authors have critically revised the manuscript and approved the final version for publication. HRC stands as guarantor of the article. The corresponding author attests that all listed authors meet authorship criteria and that no other people meeting the criteria have been omitted.
Corresponding author
Additional information
Pharmacoeconomics
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(SVG 193 kb)
Rights and permissions
About this article
Cite this article
Jensen, T.B., Bartels, D., Sædder, E.A. et al. The Danish model for the quick and safe implementation of infliximab and etanercept biosimilars. Eur J Clin Pharmacol 76, 35–40 (2020). https://doi.org/10.1007/s00228-019-02765-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02765-3