Abstract
Purpose of Review
The purpose of the review is to explore the reasons for low penetration of biosimilars in the United States (US) and to compare and contrast the US and European experiences.
Recent Findings
A biosimilar is a biologic that is highly similar to and with no clinically meaningful differences from an existing Food and Drug Administration (FDA)-approved reference product, where the reference product is the biologic that was originally approved by the FDA. The Biologics Price Competition and Innovation Act of 2009 (BPCIA) created an abbreviated approval pathway (351(k)) for biosimilars to encourage their development and created provisions for interchangeability. Since then, 19 biosimilars have been approved and 7 have been marketed via this pathway; however, the market penetration and utilization of biosimilars in the US has been low. For example, infliximab-dyyb (Inflectra®), while approved in 2016, has captured less than 5% of the US infliximab market share. The European Union (EU) established a biosimilar pathway in 2005 with their first biosimilar approved in 2006. The biosimilar approval pathway in the EU and US are similar in that their goal is to prove biosimilarity. De novo studies to establish safety and efficacy of the biosimilar are not needed. In contrast, as part of the approval in the EU, a transition study in which patients are switched from a reference to a biosimilar is required. Individual countries are left to decide interchangeability. From a clinical perspective, the main concerns regarding biosimilars have been about switching patients from reference to biosimilars especially in clinically stable patients, a bias towards associating more adverse effects with biosimilars, and the differentiation of biosimilars used for chronic versus short-term conditions with objective efficacy measures. Finally, the idea that a biosimilar’s approval can be based on extrapolation from other indications has also led to reluctance in using them. Such clinical conundrums were likely also faced in the EU; however, its economic milieu has helped to increase adoption over the last decade. These techniques include price competition by using all available biosimilars, price regulation by national authorities, and incentives for prescribers to use biosimilars. While recognizing differences in the payer system, some or all of these techniques may help increase adoption in the US where individualized contracting and significant rebates offered by the reference products’ manufacturers have made switching to the biosimilar financially unfavorable.
Summary
A pathway for accelerated biosimilar approval was developed in the US in 2009. Nonetheless, biosimilar penetration to the market remains low compared to the EU due to unique issues in the US, including the rebates provided by manufacturer of the reference biologic. While being sensitive to differences in payer structure between the US and countries in EU, it is possible to adopt some of their techniques.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biosimilars are relatively new to the US and also have been slow to penetrate the market. Biosimilars have been available in EU for over a decade and are widely prescribed, administered, and accepted [1]. Our aim is to describe the difference from a regulatory and clinical perspective and to shed light on how US clinicians may be encouraged to increase utilization of biosimilars in routine practice.
Background
A biosimilar is a non-patented biologic that is highly similar to and with no clinically meaningful differences from an existing FDA-approved, patented reference product, where the reference product is the biologic that was originally approved [2,3]. Two key components of the biosimilar definition are “highly similar” and “no clinically meaningful differences.” “Highly similar” refers to a high level of similarity between the structure and function of the biosimilar and its reference biologic where minor differences are acceptable. In fact, a reference drug may itself have gone through some structural changes in the years since its approval, including those caused by alterations in the manufacturing process [4]. Manufacturers of biosimilars are expected to submit relevant data, including pharmacokinetic, pharmacodynamic, and immunogenicity studies, to the FDA to prove similarity. The FDA then establishes that the level of similarity between biosimilar candidate and reference biologic is high enough to warrant market authorization [3,4]. With regard to the second key component, “no clinically meaningful differences,” a licensed biosimilar should not produce any clinically meaningful differences in its safety and efficacy from the reference product. Manufacturers prove high similarity and lack of clinically meaningful differences via pharmacokinetic, pharmacodynamic, and immunogenicity studies. Thus, de novo phase III clinical trials, which are essential to the New Drug Application pathway, are optional for biosimilars [3]. Further, biosimilars do not need to demonstrate clinical efficacy and safety for every indication as the reference. Thus, the FDA may approve the biosimilar for some indications by theory of extrapolation, whereby information derived from one indication is used to support use for another indication [9].
Approval Pathways
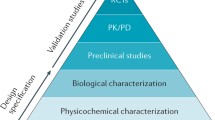
The Biologics Price Competition and Innovation act of 2009 (BPCIA) created an abbreviated approval pathway (351(k)) for biosimilars (Fig. 1). The BPCIA provided requirements to prove biosimilarity, established a 4-year and 12-year exclusivity for the initial reference biologic before any biosimilar application will be submitted or approved, respectively, introduced interchangeability requirements, and delineated a process to facilitate resolution of patent disputes between biosimilar and reference drug manufacturers [5]. An application submitted through the 351(k) pathway includes analytical, animal, and clinical studies; however, the FDA may waive the need for any one of these component(s) [6]. There are a few alternate ways that biosimilars or biosimilar-like products have been approved prior to the development of the 351(k) pathway. In some cases, a reference biologic was not available in which case a 505(b) [2] pathway was utilized, which is a hybrid between the abbreviated and new drug application processes. Examples of drugs approved via this pathway are insulin (Baslagar®) and human growth hormone (Omnitrope®) and are referred to as follow-on biologics.
One key goal of the BCPIA was to define interchangeability [7•]. If considered interchangeable, the biosimilar would be listed as such in the Purple Book. The Purple Book is a list of all biologics, including biosimilars, and provides exclusivity and interchangeability information when available [8]. Nonetheless, the FDA has not yet deemed any biosimilar interchangeable to its reference biologic and thus, none are included in the Purple Book. A draft form of the guidance on interchangeability, released in 2017, faced pushback from several medical societies [9,10]. The final guidance for industry regarding interchangeability was released in May 2019. A dedicated switching study or an integrated switching study is recommended to establish interchangeability. In a dedicated switch study, there is a lead-in period of treatment with the reference biologic followed by randomization into two arms: a non-switching arm (patients who continue treatment with reference) and a switching arm (patients who change their treatment to a biosimilar). An integrated switch study follows the same mechanism as a dedicated switch study (i.e., randomization to a switching and non-switching arm) but instead of a lead-in phase, the original PK/PD study that was used to determine biosimilarity is used (Fig. 2) [11].
Other recent laws have encouraged development and approval of biosimilars at a national level. In the recently enacted CURES Act of 2018, the biosimilar’s manufacturer can bring a lawsuit against the reference drug’s manufacturer if the latter refuses to provide sufficient samples for the biosimilar manufacturer to perform appropriate testing to determine biosimilarity. Further, the FDA can approve a secondary Risk Evaluation and Mitigation Strategy (REMS), a drug safety program, for a biosimilar if the reference and biosimilar manufacturers are unable to agree on a single rule [12].
While some federal work supporting biosimilars has been on-going as described above, states have concurrently been working on legislation regarding biosimilars. Currently, 41 states have enacted legislation involving biosimilars in varying capacities. Of the remaining nine states, four have pending laws and five have no anticipated legislation in 2019. One issue, however, is that due to the lack of interchangeability guidance at the federal level, the ability for automatic substitution at the pharmacy level has been hindered. For example, in Florida, the law allows pharmacies to substitute a biosimilar for a reference product if the FDA has determined the biologic is interchangeable and the prescriber has not “expressed a preference against substitution.” [13] This exemplifies the states’ reliance of interchangeability guidance from the FDA.
There were 19 biosimilars approved in the US since 2006 of which 7 have launched (Table 1). Of those that have launched, market share has been low (e.g., less than 5% for infliximab-dyyb (Inflectra®)). In contrast, during same timeframe (2006–2018), more than 40 biosimilars were approved in the EU. This can be explained by the EU having established a regulatory framework for biosimilar approval well before the US coupled with higher volume of applications and approvals of biosimilars in the EU.
European Experience With Biosimilars
A pathway for biosimilar authorization in EU has been established in 2005. In 2006, two specific guidelines on quality, clinical, and nonclinical issues were released [14]. In fact, EU was the first region to create a regulatory approval process for biosimilars. A contrast to the US experience, as of May 2018, there were 43 biosimilars approved in EU versus 9 in the US. The first biosimilar in the EU was approved in 2006 while the first biosimilar in the US was not approved until 2015 [15].
In the European Union (EU) model, the Committee for Medicinal Products for Human Use (CHMP) provides the initial assessment on a biosimilar application. After an initial assessment, the European Medicines Agency (EMA) approves the medication. The FDA adopted most of the components required for submission to the EMA, though some differences exist. One difference is that in the FDA pathway, there is requirement for a transition study in which patients who are on the reference biologic are switched to the biosimilar (i.e., phase IV or post-marketing study) to ensure there is no safety concerns in the pre- and post-switch groups. Second, the EMA has not and does not plan on creating a definition of interchangeability such that the individual countries determine interchangeability [14]. Since the ability to substitute is not based on any premise developed by the EMA, the use of automatic substitution should in theory be easier to adopt. Nonetheless, the stance on interchangeability from country to country has been mixed. Only two countries (Poland and Estonia) allow automatic substitution and another three (France, Lithuania, Netherlands) have laws for restricted automatic substitution. Thirteen countries have ruled against automatic substitution altogether [16].
Additional Evidence for the Clinician
While the more streamlined and less-detailed framework may be one of the explanations why biosimilars have been adopted more widely in the EU, it is likely not the only reason as evidenced by the mixed adoption of interchangeability policies among the countries. Thus, it may be prudent to next investigate the clinical issues which could help or hinder adoption of biosimilars in a market. One issue is the differentiation between treatment naïve (new start) vs. treatment-experienced (switch) patients (Fig. 3). Many prescribers are comfortable with prescribing a biosimilar in patients who have not been exposed to either the reference biologic or biosimilar. Concerns arise for those patients who may be stable on a reference drug. This view has been evident in discussions within health systems and in public forums. For example, physicians from several prominent organizations including the Coalition of State Rheumatology Organizations, American Society of Clinical Oncology, and American Academy of Dermatology Association have expressed concerns about the effect of switching between reference biologic or biosimilar in patients. Issues impacting patient safety include disease worsening, loss of response, flares, antibody development, and risk of more adverse events than with current therapy [9].
To alleviate concerns about switching, several “switching studies” have been conducted to assess efficacy and safety in patients who are switched from the reference to the biosimilar. For example, for an infliximab biosimilar, 28 switching studies have been published [17•]. In one of the largest phase IV, randomized switching studies, patients were randomized to either continue reference drug or switch to infliximab biosimilar with the primary endpoint of disease worsening after 1 year. Of the 482 patients, disease worsening occurred in 26% (n = 53) of the reference group and 30% (n = 61) of the biosimilar group (per-protocol set; adjusted treatment difference − 4.4%, 95% CI − 12.7 to 3.9). Adverse events were also similar between groups (reference vs. biosimilar: serious, 10% vs. 31%; overall, 70% vs. 68%; discontinuation, 4% vs. 3%) [18••].
Despite several such switching studies, prescribers continue to be reluctant to use biosimilars in patients that have stabilized on the reference biologic. Three reasons can be considered. One reason may be a bias towards associating biosimilars with more adverse effects than the reference. A recently published article in the Journal of Managed Care and Specialty Pharmacy explored this topic. Thirty-one switch studies with over 3000 patients were reviewed for adalimumab, bevacizumab, etanercept, and infliximab and their biosimilars. The discontinuation rate was higher in those studies where prescribers and patients were aware of their treatment (14.3% (range, 0.0–33.3) in non-blinded vs. 6.95% (range, 5.2–11.0) in blinded studies). Specifically, discontinuation rate due to adverse events were higher in the non-blinded studies (5.6%) vs. the blinded studies (3.1%). While the study could not prove causation, it did suggest there could be some bias among patients and prescribers when they are aware that they are receiving a biosimilar [17•]. The bias to associate adverse effects with biosimilars over their reference biologic may play a role in both the US and EU.
A second reason is the difference between biosimilars used in the short term with an objective, measurable endpoint versus those that do not meet this requirement. For example, the effect of filgrastim, which is used for supportive care in oncology patients, can be measured by an increase in hematological parameters after which the drug is discontinued. On the other hand, response to infliximab is more subjective, assessed by “overall treatment response.” Further, the duration of therapy for infliximab is longer than filgrastim, thus increasing the chance of antibody formation and thus loss of response. This is despite controlled trials indicating immunogenicity (i.e., antibody formation) is not a concern [7•].
A third reason is concerns over extrapolation of indication. For example, infliximab-dyyb, the first biosimilar to infliximab, was studied in rheumatology indications but not for gastrointestinal (GI) use (Crohn’s disease, ulcerative colitis). Some have argued that the pathophysiology for some GI indications is different than rheumatology and thus, extrapolation should not equate to assurance of sound evidence of adequate safety and efficacy. Concerns regarding extrapolation were also faced in EU, and medical professional societies voiced concern as such [9].
Biosimilar Economics
Beyond the clinical considerations that hinder biosimilar adoption are the economic incentives. While the biosimilar is often discounted from the reference product at ~ 20–30% the wholesale acquisition cost, individual contracts often dictate which product is ultimately adopted. Within such contracts, manufacturers of reference products often provide significant discounts and rebates in response to launch of biosimilars, making a switch to the biosimilar financially unfavorable. Thus, institutions and payors feel compelled to remain using the reference product [19].
It is likely then that biosimilars were met with the same resistance from a clinical perspective as described above. How then were these concerns alleviated and/or what reasons compelled a wider adoption rate in EU? It may not be that any specific measures were undertaken to alleviate the clinical concerns described above. Rather, several key observations may help shed light on why they have been more successful in the EU than the US. The first is price competition. Adding more similar products to the market creates price competition, thus driving down prices of all the available products, even if the market share of the biosimilar remains low relative to the reference. In the US, there has been a tendency to choose a preferred product within a class, thus decreasing competition in the market [20]. Second, pricing of biosimilars is regulated by national authorities for ambulatory patients in EU. Two mechanisms within pricing regulations are to ensure the biosimilar price falls within a certain discount threshold relative to reference products and the use of maximum prices. In the US, prices are set by the manufacturer and are subject to clandestine and complex contracts between the manufacturers and payers [7•]. Third, there are more incentives to encourage prescribing of biosimilars which could also be incorporated into pricing and reimbursement. For example, a prescriber may be required to use a biosimilar in 15% of their patients by the payer. This increases the prescriber’s experience with the biosimilar, making them more likely to prescribe a biosimilar in the future [21]. Additional policies to encourage prescribing of biosimilars include transparent pricing and penalties for excessive use of the reference product [22]. In the US, such strategies have not been adopted.
Conclusion
In conclusion, there are several similarities and differences between the EU and US regulations around biosimilars. On the other hand, the clinical concerns have likely been similar between the two regions. It appears then that other factors such as market competition, pricing, and incentives have been critical to the success of biosimilars in EU.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Aideed H. The European biosimilars landscape: what to expect in the year ahead. Available at: ://www.biosimilardevelopment.com/doc/the-European-biosimilars-landscape-what-to-expect-in-the-year-ahead-0001 Accessed October 6, 2018.
Information for consumers (biosimilars). U.S. Food and Drug Administration. August 27, 2015. Available at: http://www.fda.gov/drugs/developmentapprovalprocess/ howdrugsaredevelopedandapproved/approvalapplications/therapEuropeticbiologicapplications/biosimilars/ucm241718.htm. Accessed October 18, 2017.
Biosimilar and interchangeable products. U.S Food and Drug Administration. October 23, 2017. Available at: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapEuropeticBiologicApplications/Biosimilars/ucm580419.htm#biosimilar. Accessed October 2, 2018.
Mehr S, Zimmerman MP. Is a biologic produced 15 years ago a biosimilar of itself today? Am Health Drug Benefits. 2016 Dec;9(9):515–8.
Biologics Price Competition and Innovation Act of 2009. Biologics Resource Center. 2017. Available at: https://www.biosimilarsresourcecenter.org/laws-regulations/federal-legislation-implementing-biosimilars-pathway/biologics-price-competition-and-innovation-act-of-2009/. Accessed October 3. 2018.
Christl, L. FDA’s overview of the regulatory guidance for the development and approval of biosimilar products in the US. U.S. Food and Drug Administration. Available at: https://www.fda.gov/downloads/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapEuropeticbiologicapplications/biosimilars/ucm428732.pdf . Accessed October 4, 2018
• Peterson J, Budlong H, Affeldt T, Skiermont K, Kyllo G, Heaton A. Biosimilar Products in the Modern U.S. Health Care and Regulatory Landscape. JMCP. 2017;23(12):1255–9. Provides tangible recommendations to increase biosimilar uptake.
Purple Book: lists of licensed biological products with reference product exclusivity and biosimilarity or interchangeability evaluations. U.S. Food and Drug Administration. October 1, 2018. Accessed September 30, 2018.
Syrop J. Physicians express concern about biosimilar interchnageability to FDA. June 30, 2017. Available at: https://www.centerforbiosimilars.com/news/physicians-express-concerns-about-biosimilar-interchangeability-to-fda. Accessed September 29, 2018
Levy S. AMCP calls on FDA to finalize guidance on biosimilar interchangeability. Drug Store News. Available at: https://www.drugstorenews.com/pharmacy/amcp-calls-on-fda-to-finalize-guidance-on-biosimilar-interchangeability/. Accessed September 27, 2018.
Considerations for demonstrating interchangeability with reference product: guidance for industry. US Food and Drug Administration. May 2019.
Davio K. Five things to know about the CREATES act. Managed Markets Network. Available at: https://www.ajmc.com/newsroom/5-things-to-know-about-the-creates-act. Accessed October 5, 2018
State Biosimilars and Interchangeable Laws, Legislation, and Regulations. Biosimilar resource center. June 2017. Available at: https://www.biosimilarsresourcecenter.org/map/. Accessed October 5. 2018
Schiesti M, Zabranksy M, Sorgel F. Ten years of biosimilars in Europe: development and evolution of the regulatory pathways. Drug Des Develop Therap. 2017;11:1509–14.
Harston A and Storaska A. How the U.S. compares to Europe on biosimilar approvals and products in the pipeline. May 1, 2018. Available at : http://www.biosimilarsip.com/2018/05/02/how-the-u-s-compares-to-Europe-on-biosimilar-approvals-and-products-in-the-pipeline/. Accessed October 5, 2018.
Biosimilar substitution in Europe. Generics and biosimilar initiative. Available at: http://www.gabionline.net/Reports/Biosimilar-substitution-in-Europe). Accessed October 5. 2018.
• Odinet J, Day CE, Cruz JL, et al. The Biosimilar Nocebo effect? A systematic review of double-blinded versus open-label studies. JMCP. 2018;24(10):952–9. Provides a novel term known as “nocebo” effect that may be referenced in future materials.
•• Jørgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomized, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304–16. Commonly cited large randomized switching study among several indications which showed no differences in efficacy and safety outcomes among several indications.
Quereshi ZP, Nagal S, et al. Biosimilar filgrastim use in the United States vs the European Union and Japan-why does it lag behind and what can be done? Jama Oncol. 2018. Published online December;3:2018. https://doi.org/10.1001/jamaoncol.2018.5636.
The impact of biosimilar competition in Europe. Quintiles MS. May 2017. Available at: https://www.medicinesforEurope.com/wp-content/uploads/2017/05/IMS-Biosimilar-2017_V9.pdf. Accessed October 5, 2018.
Moorkens E, Vulto AG, Huys I, Dylst P, Godman B, KEuropeerleber S, et al. Policies for biosimilar uptake in Europe: an overview. PLoS One. 2017 Dec 28;12(12):e0190147.
IMS Institute for Healthcare Informatics. Assessing biologic uptake and competition in European markets. http://www.imhealth.com/files/web/IMS%20InstituteHealtcare%20Briefs/Assessing_Biosimilar_uptake_and_competition_in_European markets.pdf. Published October 2014. Accessed December 10, 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pharmacology Care
Rights and permissions
About this article
Cite this article
Vakil, N., Fanikos, J. Regulatory and Clinical Perspective on Biosimilars: a Comparison of the US and European Experiences. Curr Emerg Hosp Med Rep 7, 111–117 (2019). https://doi.org/10.1007/s40138-019-00185-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-019-00185-2