Abstract
Purpose
The objective of the study was to examine the safety of ginger use during pregnancy on congenital malformations and selected pregnancy outcomes.
Methods
The Norwegian Mother and Child Cohort study, a large population-based cohort, provided the data used in this study. Our study population consisted of 68,522 women. Data on ginger use and socio-demographic factors were retrieved from three self-administered questionnaires completed by the women during weeks 17 and 30 of the pregnancy and when their child was 6 months old. Data on pregnancy outcomes were provided by the Medical Birth Registry of Norway.
Results
Among the 68,522 women in the study, 1,020 (1.5 %) women reported using ginger during pregnancy. The use of ginger during pregnancy was not associated with any increased risk of congenital malformations. No increased risk for stillbirth/perinatal death, preterm birth, low birth weight, or low Apgar score was detected for the women exposed to ginger during pregnancy compared to women who had not been exposed.
Conclusion
Use of ginger during pregnancy does not seem to increase the risk of congenital malformations, stillbirth/perinatal death, preterm birth, low birth weight, or low Apgar score. This finding is clinically important for health care professionals giving advice to pregnant women with NPV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ginger rhizome (Zingiber officinale Roscoe) has been used since ancient times for various conditions, including different types of nausea [1]. Various potentially active components have been identified [2, 3]. The mechanism of action has not been established fully, though it is attributed to the active components’ ability to affect serotonin and muscarinic receptors in the gastrointestinal tract [4]. The components in ginger may also have antiemetic actions via the central nervous system [3].
Ginger is currently included in the pharmacopoeias of many Western countries [1]. Ginger root can be used in fresh or dried form, or prepared as a tea. Furthermore, ginger is one of the most commonly used herbs during pregnancy in Western countries, preferentially against nausea and vomiting during pregnancy (NVP) [5]. Several of the latest published studies (from 2005 onwards) report usage rates of higher than 10 % [6–9]. Up to 80 % of pregnant women experience NVP [10], which impacts a woman’s life and potentially results in reduced quality of life, negative socio-economic consequences, and even elective termination of pregnancy, implying the importance of treatments to alleviate the symptoms [10, 11]. Because NVP is experienced primarily during the first trimester when organogenesis occurs, teratogen effects are a concern. This possibility has led to caution in prescribing and taking medications to treat this illness. Consequently, many women try complementary and alternative treatments instead, such as acupressure, acupuncture, and ginger [10].
Several studies have examined the effectiveness of ginger in relieving NVP [12–20]. Ginger has been reported to be more effective than placebo [12, 13, 16, 17, 21]. Ginger has also been shown to be equally or more effective than vitamin B6 [14, 15, 18, 20] and dimenhydrinate [19]. Nine of the already mentioned randomized controlled trials (RCTs) [12–16, 18–21] were included in a Cochrane review [10]. After evaluating the methodology of the studies and pooling selected results, the Cochrane review concluded that “ginger may be helpful to women”, but that “the evidence of effectiveness was limited, and not consistent” [10]. The doses most commonly used were between 1,000 mg and 1,500 mg per day. Reported adverse reactions were mild, mostly mild gastrointestinal effects, drowsiness, and headache. There were no reports of negative pregnancy outcomes.
Despite the widespread use of ginger, evidence-based data on the safety of use during pregnancy is limited. This may be due to the fact that pharmacovigilance is not compulsory for herbal remedies and because many consider natural remedies as safe in general. However, it is well recognised that absence of evidence is not the same as evidence of absence. Most textbooks classify ginger as relatively safe to use during pregnancy based on a lack of reports of negative pregnancy outcomes, but recommend caution due to limited data [22–24]. However, women with a history of miscarriage, vaginal bleeding, or clotting disorder are advised by others to avoid ginger during pregnancy [25]. Furthermore, ginger is reported to be contraindicated close to labour because of an increased risk of post-partum haemorrhage [26]. These concerns are probably due to ginger’s ability to inhibit thromboxane synthetase and, consequently, platelet aggregation in vitro [27], though conflicting evidence exists regarding the consequences of this property in vivo [28–30]. In addition, theoretical concerns related to the developing embryo have been related to ginger’s effect on receptor binding of testosterone possibly affecting sex steroid differentiation of the fetal brain [31]. In the German Commission E Monographs, ginger is contraindicated for use in morning sickness, though in the expanded edition the authors argue against this contraindication [1].
To date, only one study has investigated the safety of ginger use during pregnancy in particular [32]. The cohort study examined the safety of ginger use in 187 women and reported no adverse pregnancy outcomes (major malformations, live birth, birth weight, and gestational age) due to ginger intake during early pregnancy. However, because of the small sample size, the study had low statistical power. Thus, uncertainty still remains regarding the safety of ginger use during pregnancy.
To increase the existing body of evidence regarding safety, we conducted a study focusing on the safety of ginger use during pregnancy based on data from a large population-based cohort. The primary aim of the study was to investigate whether exposure to ginger was associated with an increased risk of congenital malformations. The secondary aim was to investigate the effects of ginger use on vaginal bleeding, stillbirth/perinatal death, birth weight, preterm birth, and Apgar score.
Materials and methods
Data from the Norwegian Mother and Child Cohort Study (MoBa) and records from the Medical Birth Registry of Norway (MBRN) provided the data used in this study. MoBa is a population-based prospective cohort study conducted by the Norwegian Institute of Public Health and including more than 100,000 pregnancies [33]. The women received an informed consent form and the first questionnaire by post with an appointment for a routine ultrasound examination during week 17–18 of their pregnancy [34]. The target population was women who gave birth in Norway during the recruitment period (1999–2008). Recruitment began in the county of Hordaland in western Norway in 1999 and expanded gradually. From 2005, the study was nation-wide with 50 of the 52 hospitals in Norway participating. Only facilities with more than 100 births per year were targeted. The last birth in the cohort occurred in June 2009. In an assessment of the MoBa study in 2009, the participation rate was 43.8 % [34]. Information from MoBa was retrieved from three self-administered questionnaires [35–37]. The first and second questionnaires were completed during pregnancy weeks 13–17 and 30, and provide a wide range of information regarding socio-demographic characteristics, outcomes of previous pregnancies, medical history, maternal health, lifestyle habits, drug exposure, and other exposures [34–36]. The third questionnaire was distributed when the child was 6 months old and includes information on the last pregnancy period (from week 30 of pregnancy) [34, 37]. Among the women who agreed to participate in MoBa, the response rate was 95 % for the first questionnaire, 92 % for the second questionnaire, and 87 % for the third questionnaire [34].
MBRN was established in 1967 and is based on compulsory notification of every birth or late abortion in Norway [38]. Information on pregnancy, delivery, and the health of the neonate is included in the registry. MBRN provides data on all live births, still births, and induced abortions after gestational week 12 (after week 16 up to 2002) [39].
Data from MoBa is linked to the MBRN via the woman’s personal identification number, which is assigned to people registered in the National Population Register as residents of Norway. MoBa was approved by the Regional Committee for Ethics in Medical Research, Region South, and the Norwegian Data Inspectorate.
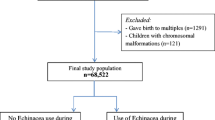
The MoBa quality assured data file (version 4) released for research was used in the current study. This file included 72,934 women who delivered between 1999 and 2006. The women included in the current study had both a record in MBRN and answered the first questionnaire (n = 69,930). Women who gave birth to multiples (n = 1291) or who gave birth to children with chromosomal malformations (n = 121) were excluded. Thus, the final study population consisted of 68,522 pregnant women and their newborn children, corresponding to 94.0 % of the original data file. Among these women, 92.5 % had answered the second questionnaire and 87.3 % had answered the third questionnaire.
Exposure variable
Information on ginger use came from the three MoBa questionnaires [35–37]. Several indications were specifically named in each questionnaire, including NVP. For each indication, the woman could specify several products and exposure windows; in the first questionnaire: 6 months before pregnancy, gestational weeks 0–4, 5–8, 9–12, and 13+ (until completion of the first questionnaire); in the second questionnaire: five exposure weeks could be specified, 13–16, 17–20, 21–24, 25–28, 29+ (until completion of the second questionnaire); and in the third questionnaire: last part of pregnancy, 0–3 months after birth and 4–6 months after birth. In order to enhance reporting of supplements and herbal remedies, the women were specifically asked in all three questionnaires to give the complete name(s) of all vitamins and dietary supplements they had used, including alternative/herbal remedies and diet products. In this question, timing of use was not requested. The authors reviewed all herbal products for ginger as an ingredient.
Exposure was classified as use of ginger during pregnancy (total), use of ginger during the first trimester, or use of ginger during the second and/or third trimester.
Outcome variables
Information on outcomes was retrieved from the MBRN. Diagnoses were based on the International Classification of Diseases, 10th Revision (ICD-10) [40]. All birth defects diagnosed by paediatricians and/or geneticists in the first week after birth or while the infants were in the hospital during their first year of life are included in the MBRN records. Malformations were defined as any birth defect registered in the MBRN. Malformations were classified as major according to the International Clearinghouse for Birth Defects definition. Cardiovascular malformations included all malformations classified with ICD-10 code Q20-28 [41]. The following outcome variables were included in the study: all malformations, major malformations, cardiac malformations, stillbirth/perinatal death, low birth weight (<2,500 g), preterm birth (<37 weeks), and Apgar score <7 at 5 minutes after birth.
Outcome variables related to maternal vaginal bleeding were retrieved from the MoBa questionnaires: hospitalization due to bleeding and vaginal bleeding. The amount and timing of bleeding was also reported. All outcomes were dichotomized yes/no.
Potentially confounding factors
The following socio-demographic factors and lifestyle variables were included in the analysis: maternal age (≤24 years, 25–29 years, 30–34 years, ≥35 years), parity [0 previous live births, ≥1 previous live birth(s)], education (primary, secondary, tertiary – short, tertiary – long), marital status (married or cohabitating, not), pre-pregnancy body mass index (BMI) (underweight, normal weight, overweight, obese), physical activity (never, less than once a week, 1–2 times weekly, 3 or more times weekly), smoking at the end of pregnancy (no, sometimes, daily), any folic acid use (no; yes, before or during; yes, before and during), previous miscarriages or stillbirths (yes, no), year of delivery (1999–2002, 2003–2006), and infant sex (boy, girl).
Maternal nausea was characterized as nausea during the first part of pregnancy (reported in the first questionnaire), nausea during the second part of pregnancy (reported in the second questionnaire), nausea during the first and second part of pregnancy (reported in both the first and second questionnaire), nausea and vomiting during the first and second part of pregnancy (reported in both the first and second questionnaire), NVP in previous pregnancies, or hospitalization during pregnancy due to prolonged nausea and vomiting. Sick leave was included. These variables were dichotomized yes/no.
Statistical analysis
Pearson’s chi-square test was used to identify associations between maternal characteristics and the use of ginger, or between variables listed in Table 2 and the use of ginger. A p-value <0.05 was considered significant. Univariate and multivariate logistic regression analysis were used to obtain crude and adjusted odds ratios (ORs), respectively, to estimate the risk of malformations and selected pregnancy outcomes associated with ginger exposure during pregnancy. ORs are presented with 95 % confidence intervals (CIs). All statistically or clinically significant variables listed in Table 1 or Table 2 were considered possible confounding factors. The backward deletion strategy described by Rothman et al. [42] was used to select the different variables included in the potential confounder set. In addition, certain potential confounders were included in the set based on theoretically possible influences [42]. The following confounder set was used when estimating the risk for malformations and preterm birth: maternal age, parity, pre-pregnancy BMI, level of education, maternal smoking at the end of pregnancy, any maternal folic acid use, nausea and vomiting during first and second part of pregnancy, previous miscarriages or stillbirths, year of delivery, and infant sex. The same confounder set was used to estimate the risk for the remaining selected pregnancy outcomes, with the addition of the length of gestation. Possible high inter-correlations among the independent variables were checked for using multiple regression analysis and making sure the tolerance values for collinearity statistics were adequate (<0.1). Post-hoc power analyses were conducted to estimate the statistical power of our data for each of the outcomes studied [43].
All statistical analyses were performed using Predictive Analytics software PSAW version 18 for Windows (SPSS, Chicago, IL, USA).
Results
Among the 68,522 women included in the study, 1,020 women (1.5 %) reported using ginger during pregnancy. Of the women who reported ginger use in relation to timing, we found that 466 women (45.7 %) used ginger during the first trimester. NVP was the most frequently reported indication for the use of ginger (655 of 1,020, 63.8 %). A total of 50,912 (74.3 %) women reported having experienced NVP. Of these women, 671 (1.3 %) used herbs to relieve the symptoms and 4,280 (8.4 %) used medications. Only 40 women (3.9 %) reported the use of ginger against other indications [influenza/cold (n = 18), pelvic distortion due to pregnancy (n = 5), reflux (n = 9), other non-specified illness (n = 8)].
Women who reported using ginger during pregnancy were more likely to have a higher level of education (tertiary – short or tertiary – long), to be non-smokers, and to have used folic acid before and during or only during pregnancy (Table 1). These women were also more likely to have experienced any NVP, to have been hospitalized during pregnancy due to prolonged NVP, to have had NVP during a previous pregnancy, to have been on sick leave during pregnancy, and to have given birth during the period 2003–2006 compared to the women who did not use ginger (Table 2). Of the women who used ginger, 15 % also used medications against NVP, compared to only 5 % of the women who did not use ginger (Table 2). The medication most often used against NVP in addition to ginger was antihistamines (9.8 %), most commonly meclizine.
Among women who used ginger during pregnancy, a higher percentage experienced vaginal bleeding after week 17 compared to controls (7.8 % vs. 5.8 %, p = 0.007). The association remained significant when adjusting for maternal age, parity, pre-pregnancy BMI, maternal smoking, maternal folic acid use, NVP during both the first and second part of pregnancy, previous miscarriages or stillbirths, and physical activity (adjusted OR 1.4, 95 % CI 1.0–1.7, p = 0.02). However, when the analyses were restricted to vaginal bleeding more than spotting, neither crude nor adjusted ORs revealed a significant association (crude OR 1.1, 95 % CI 0.8–1.7; adjusted OR 1.2, 95 % CI 0.8–1.9). Sub-analyses according to the timing of hospitalization did not reveal any association between the use of ginger, bleeding, and hospitalization timing.
Use of ginger during the first trimester of pregnancy or at any time during pregnancy did not increase the risk of malformations in general, major malformations, or cardiac malformations (Table 3). Neither the crude ORs nor the adjusted ORs revealed significant associations between exposure to ginger and malformations. In addition, no significant associations between the use of ginger during pregnancy and risk of stillbirth/perinatal death, low birth weight, preterm birth, or low Apgar score were found in univariate analyses (Table 4). These results did not change after adjusting for potentially confounding factors, including NVP.
Discussion
Exposure to ginger during pregnancy was not associated with an increased risk of congenital malformations, stillbirth/perinatal death, low birth weight, preterm birth, or low Apgar score. To the best of our knowledge, this is the largest population-based cohort study to date investigating pregnancy outcomes after the use of ginger. In addition, we were able to investigate several pregnancy outcomes giving a broader safety profile than studies with a single investigated outcome. These findings are in accordance with prior studies and long-term traditional use in which no negative effects on pregnancy outcome have been reported [10, 32]. Because of the widespread use of ginger during pregnancy, these findings are clinically important. Health care personnel have to base their advice on evidence-based knowledge, and although RCTs provide evidence of effect against NVP, health care personnel cannot recommend such use before evidence of safety during pregnancy is documented.
Interestingly, however not statistically significant, seven of eight outcomes depicted in Table 4 had ORs below 1 indicating a possible positive effect of ginger. This may be due to a protective effect of NVP as suggested by previous studies [10].Though we adjusted for maternal NVP we may not fully have overcome confounding by indication.
The small increased risk of vaginal bleeding among the women who used ginger during pregnancy may be due to chance, the underlying ailment or due to use of ginger. As ginger may inhibit thromboxane synthetase, an increased risk of bleeding is theoretically plausible. Studies on this property in humans, however, report conflicting results [27–30]. It is reassuring that when the analyses were restricted to more severe vaginal bleeding, no association was found. Nevertheless, risk of bleeding during pregnancy is something that should be investigated in depth with respect to dosage.
Significantly more of the ginger-using women also used conventional medications for NVP compared to the non-users, indicating that the use of ginger is, not instead of, but complementary to the use of conventional medication. This finding is in agreement with the findings of Nordeng et al. [9] and may be explained by the severity of symptoms and/or willingness to medicate in general.
The high prevalence of NVP in the present study is in accordance with earlier published findings [10]. More surprisingly is the low prevalence of treatment of NVP; only 1.3 % and 8.4 % of the woman with NVP reported treating it with herbs or medications, respectively. The association of ginger with both sick leave and hospitalization may indicate that only the women with the most persistent NVP try treatment.
Of note, the women who used ginger were more educated and smoked to a lesser degree than women who did not use ginger. This observation is in agreement with studies characterizing the users of herbal medicines and complementary and alternative medicine (CAM) during pregnancy in general [6, 7, 44, 45]. Furthermore, the association between ginger and folic acid use is in agreement with earlier published results in which the use of herbs during pregnancy was associated with the use of multivitamins [44]. More women who gave birth in the period from 2003 to 2006 used ginger compared to women who delivered during the period 1999–2002. This finding reflects the frequent use of CAM in the general population during recent years in many Western countries [46–48].
This study has several methodological strengths. The prospective design of the MoBa study enables avoiding a risk of recall bias. Because of the detailed nature of the questionnaires, important information on a number of potentially confounding factors was available, and the Medical Birth Registry gave access to several important outcome variables. Validation studies of the accuracy of the MBRN have been conducted, reporting satisfactory ascertainment [49, 50]. Yet, the possibility of under-reporting minor malformations, especially among early stillbirths, cannot be ruled out.
The current study also has some limitations that need to be addressed. Firstly, because of a low response rate in MoBa, selection bias may have occurred. However, Nilsen et al. reported that, even though several prevalence estimates were shown to be biased in MoBa, estimates of exposure-outcome associations were reliable [51]. Secondly, the MoBa study is based on self-reported use, and as such the reporting might not be complete. However, because of the longitudinal study design, exposure reporting is not affected by the outcome of the pregnancy; the women reported using ginger in the first two questionnaires before the outcome of the pregnancy was known. Thirdly, information on dosage and administration was not available and the timing of exposure could only be recorded when ginger was used for the specific given indications. Finally, even though this is the largest study identified to date studying the safety of ginger use during pregnancy, the number of cases with malformations was low. Power analyses revealed that the statistical power of our data was sufficient to rule out two-fold or greater increases in the risk of outcomes that occurred more frequently than 2 % in the study population. For more rare outcomes, such as cardiac malformations, low Apgar score, and stillbirth/perinatal death, the statistical power was 60 %, 72 %, and 50 %, respectively.
In conclusion, no associations were found between the use of ginger and malformations. This finding is reassuring and supports previous findings. In addition, the results do not suggest that the use of ginger during pregnancy increases the risk for any of the following pregnancy outcomes: stillbirth/perinatal death, low birth weight, preterm birth, and low Apgar score. However, an association was found between ginger use and vaginal bleeding including spotting after pregnancy week 17. Even though this association was no longer significant when the analyses were restricted to more severe bleeding incidents, this finding should be explored in later studies, taking into account the dosage and administration form of ginger, before any firm conclusions can be drawn. This information will be helpful to pregnant women and health care professionals involved in pregnancy care when considering treatment for NVP.
References
Blumenthal M, Goldberg A, Brinkmann J (Eds.) (2000) Herbal Medicine. Expanded Comission E Monographs. Integrative Medicine, Newton
Natural medicines comprehensive database (2012) Ginger monograph. Natural medicines comprehensive database. http://naturaldatabase.therapeuticresearch.com/home.aspx?cs=&s=ND. Accessed 16 March 2012
Lumb AB (1993) Mechanism of antiemetic effect of ginger. Anaesth 48:1118
Pertz HH, Lehmann J, Roth-Ehrang R, Elz S (2011) Effects of Ginger Constituents on the Gastrointestinal Tract: role of Cholinergic M(3) and Serotonergic 5-HT(3) and 5-HT(4) Receptors. Planta Med 77:973–978. doi:10.1055/s-0030-1270747
Adams J, Lui CW, Sibbritt D, Broom A, Wardle J, Homer C, Beck S (2009) Women's use of complementary and alternative medicine during pregnancy: a critical review of the literature. Birth 36:237–245. doi:10.1111/j.1523-536X.2009.00328.x
Forster DA, Denning A, Wills G, Bolger M, McCarthy E (2006) Herbal medicine use during pregnancy in a group of Australian women. BMC Pregnancy Childbirth 6:21. doi:10.1186/1471-2393-6-21
Holst L, Wright D, Haavik S, Nordeng H (2009) The use and the user of herbal remedies during pregnancy. J Altern Complement Med 15:787–792. doi:10.1089/acm.2008.0467
Nordeng H, Havnen GC (2005) Impact of socio-demographic factors, knowledge and attitude on the use of herbal drugs in pregnancy. Acta Obstet Gynecol Scand 84:26–33. doi:10.1111/j.0001-6349.2005.00648.x
Nordeng H, Bayne K, Havnen GC, Paulsen BS (2011) Use of herbal drugs during pregnancy among 600 Norwegian women in relation to concurrent use of conventional drugs and pregnancy outcome. Complement Ther Clin Pract 17:147–151. doi:10.1016/j.ctcp. 2010.09.002
Matthews A, Dowswell T, Haas DM, Doyle M, O'Mathuna DP (2010) Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev 9:CD007575. doi:10.1002/14651858.CD007575.pub2
Mazzotta P, Stewart DE, Koren G, Magee LA (2001) Factors associated with elective termination of pregnancy among Canadian and American women with nausea and vomiting of pregnancy. J Psychosom Obstet Gynaecol 22:7–12
Vutyavanich T, Kraisarin T, Ruangsri R (2001) Ginger for nausea and vomiting in pregnancy: randomized, double-masked, placebo-controlled trial. Obstet Gynecol 97:577–582. doi:10.1016/S0029-7844(00)01228-X
Keating A, Chez RA (2002) Ginger syrup as an antiemetic in early pregnancy. Altern Ther Health Med 8:89–91
Sripramote M, Lekhyananda N (2003) A randomized comparison of ginger and vitamin B6 in the treatment of nausea and vomiting of pregnancy. J Med Assoc Thai 86:846–853
Smith C, Crowther C, Willson K, Hotham N, McMillian V (2004) A randomized controlled trial of ginger to treat nausea and vomiting in pregnancy. Obstet Gynecol 103:639–645. doi:10.1097/01.AOG.0000118307.19798.ec
Willetts KE, Ekangaki A, Eden JA (2003) Effect of a ginger extract on pregnancy-induced nausea: a randomised controlled trial. Aust N Z J Obstet Gynaecol 43:139–144
Fischer-Rasmussen W, Kjaer SK, Dahl C, Asping U (1991) Ginger treatment of hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol 38:19–24
Ensiyeh J, Sakineh MA (2008) Comparing ginger and vitamin B6 for the treatment of nausea and vomiting in pregnancy: a randomised controlled trial. Midwifery. doi: 10.1016/j.midw.2007.10.013
Pongrojpaw D, Somprasit C, Chanthasenanont A (2007) A randomized comparison of ginger and dimenhydrinate in the treatment of nausea and vomiting in pregnancy. J Med Assoc Thai 90:1703–1709
Chittumma P, Kaewkiattikun K, Wiriyasiriwach B (2007) Comparison of the effectiveness of ginger and vitamin B6 for treatment of nausea and vomiting in early pregnancy: a randomized double-blind controlled trial. J Med Assoc Thai 90:15–20
Ozgoli G, Goli M, Simbar M (2009) Effects of ginger capsules on pregnancy, nausea, and vomiting. J Altern Complement Med 15:243–246. doi:10.1089/acm.2008.0406
Goldstein L, Berkovitch M (2007) Antiemetics. In: Schaefer C, Peters P, Miller R (eds) Drugs during pregnancy and lactation, 2nd edn. Academic Press, London, pp 82–83
Mills E, Dugoua JJ, Perri D, Koren G (2006) Herbal Medicines in Pregnancy and Lactation. An evidence-Based Approached. Taylor & Francis Group, London and New York
Briggs GG, Freeman RK, Yaffe SJ (2008) Drugs in Pregnancy and Lactation,8th edn. Lippinncott Williams & Wilkins, Philadelphia
Tiran D (2012) Ginger to reduce nausea and vomiting during pregnancy: Evidence of effectiveness is not the same as proof of safety. Complement Ther Clin Pract 18:22–25. doi:10.1016/j.ctcp. 2011.08.007
Westfall RE (2004) Use of anti-emetic herbs in pregnancy: women's choices, and the question of safety and efficacy. Complement Ther Nurs Midwifery 10:30–36. doi:10.1016/S1353-6117(03)00057-X
Srivastava KC (1984) Effects of Aqueous Extracts of Onion, Garlic and Ginger on Platelet-Aggregation and Metabolism of Arachidonic-Acid in the Blood Vascular System - Invitro Study. Prosta Leukotr Med 13:227–235
Bordia A, Verma SK, Srivastava KC (1997) Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids 56:379–384
Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, Day RO, McLachlan AJ (2005) Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol 59:425–432. doi:10.1111/j.1365-2125.2005.02322.x
Srivastava KC (1989) Effect of onion and ginger consumption on platelet thromboxane production in humans. Prostaglandins Leukotrienes Essent Fatty Acids 35:183–185
Backon J (1991) Ginger in preventing nausea and vomiting of pregnancy; a caveat due to its thromboxane synthetase activity and effect on testosterone binding. Eur J Obstet Gynecol Reprod Biol 42:163–164
Portnoi G, Chng LA, Karimi-Tabesh L, Koren G, Tan MP, Einarson A (2003) Prospective comparative study of the safety and effectiveness of ginger for the treatment of nausea and vomiting in pregnancy. Am J Obstet Gynecol 189:1374–1377. doi:10.1067/S0002-9378(03)00649-5
Norwegian Institute of Public Health (2010) The Norwegian Mother and Child Cohort Study. Norwegian Institute of Public Health. http://www.fhi.no/eway/default.aspx?pid=238&trg=MainArea_5811&MainArea_5811=5903:0:15,4329:1:0:0:::0:0. Accessed 02 February 2012
Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C (2006) Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 35:1146–1150. doi:10.1093/ije/dyl170
Norwegian Institute of Public Health The Nowegian Mother and Child Cohort Study. Questionnaire 1. Norwegian Institute of Public Health. http://www.fhi.no/dokumenter/1f32a49514.pdf. Accessed 01 March 2012
Norwegian Institute of Public Health The Nowegian Mother and Child Cohort Study. Questionnaire 3. Norwegian Institute of Public Health. http://www.fhi.no/dokumenter/7b6b32b0cd.pdf. Accessed 01 March 2012
Norwegian Institute of Public Health The Nowegian Mother and Child Cohort Study. Questionnaire 4. Norwegian Institute of Public Health. http://www.fhi.no/dokumenter/9ecca1c459.pdf. Accessed 01 March 2012
Irgens LM (2000) The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand 79:435–439
Norwegian Institute of Public Health (2012) Medical Birth Registry of Norway. Norwegian Institute of Public Health. http://www.fhi.no/eway/default.aspx?pid=238&trg=MainArea_5811&MainArea_5811=5895:0:15,3320:1:0:0:::0:0&MainLeft_5895=5825:73560::1:5896:4:::0:0. Accessed 27 February 2012
World Health Organization (2010) International statistical classification of disease and related health problems, 10th revision. World Health Organization. http://apps.who.int/classifications/icd10/browse/2010/en. Accessed 27 February 2012
Norwegian Institute of Public Health (2011) Definisjonsrapporter for variabler i Medisinsk fødselsregister (in Norwegian) In English: Definition report for variables in the Medical Birth Registry of Norway. Norwegian Institute of Public Health. http://www.fhi.no/dokumenter/8105c63e8e.pdf. Accessed 01 March 2012
Rothman KJ, Greenland S, Lash TL (2008) Modern Epidemiology, 3rd edn. Lippincott Williams & Wilkins, Philadelphia
DSS Research (2012) Researcher's Tool Kit. Statistical Power Calculator. Two Sample Tests Using Percentage Values. DSS Research. http://www.dssresearch.com/toolkit/spcalc/power_p2.asp. Accessed 29 February 2012
Moussally K, Oraichi D, Berard A (2009) Herbal products use during pregnancy: prevalence and predictors. Pharmacoepidemiol Drug Saf 18:454–461. doi:10.1002/pds.1731
Refuerzo JS, Blackwell SC, Sokol RJ, Lajeunesse L, Firchau K, Kruger M, Sorokin Y (2005) Use of over-the-counter medications and herbal remedies in pregnancy. Am J Perinatol 22:321–324. doi:10.1055/s-2005-873235
Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC (1998) Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 280:1569–1575. doi:10.1001/jama.280.18.1569
Tindle HA, Davis RB, Phillips RS, Eisenberg DM (2005) Trends in use of complementary and alternative medicine by US adults: 1997-2002. Altern Ther Health Med 11:42–49
Hanssen B, Grimsgaard S, Launso L, Fonnebo V, Falkenberg T, Rasmussen NK (2005) Use of complementary and alternative medicine in the Scandinavian countries. Scand J Prim Health Care 23:57–62. doi:10.1080/02813430510018419
Melve KK, Lie RT, Skjaerven R, Van Der Hagen CB, Gradek GA, Jonsrud C, Braathen GJ, Irgens LM (2008) Registration of Down syndrome in the Medical Birth Registry of Norway: validity and time trends. Acta Obstet Gynecol Scand 87:824–830. doi:10.1080/00016340802217184
Kubon C, Sivertsen A, Vindenes HA, Abyholm F, Wilcox A, Lie RT (2007) Completeness of registration of oral clefts in a medical birth registry: a population-based study. Acta Obstet Gynecol Scand 86:1453–1457. doi:10.1080/08037050701645090
Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, Alsaker ER, Haug K, Daltveit AK, Magnus P (2009) Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol 23:597–608. doi:10.1111/j.1365-3016.2009.01062.x
Acknowledgments
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no NO-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant no. 151918/S10). We are grateful to all of the participants and their families for taking part in this study. We thank Gro C Havnen and Ingebjørg Sandøy Rødahl for their help identifying and classifying the herbal products.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heitmann, K., Nordeng, H. & Holst, L. Safety of ginger use in pregnancy: results from a large population-based cohort study. Eur J Clin Pharmacol 69, 269–277 (2013). https://doi.org/10.1007/s00228-012-1331-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1331-5