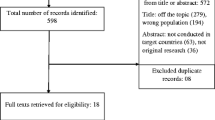
Abstract
The use of herbal medicines in pregnancy is common despite the lack of efficacy and safety data for these products. Pregnant women are often not aware that herbal preparations may be harmful in pregnancy and are often encouraged to use ‘natural’ products, such as herbal medicines, rather than pharmaceuticals, as herbal products are perceived as being safer. However, plant-based medicines can cause adverse reactions and can be highly toxic to pregnant women. Healthcare providers should establish, in non-judgmental and culturally appropriate ways, whether women are using herbal medicines, or other ‘natural’ or traditional medicines in pregnancy, and should report, or encourage reporting of, ongoing exposed pregnancies and adverse maternal and foetal events associated with use of such products to an appropriate reporting system, if available.
There is a need for evidence-based information regarding the use of most herbal medicines in pregnancy for both women and their healthcare providers. With the exception of certain ginger root extracts, there are no consistent data to support the use of any other herbal supplement during pregnancy and such products should not be recommended.
Current methods of research into the safety of herbal medicine use in pregnancy suffer from several methodological limitations, some of which can be overcome with novel methods of data collection. Collection of data directly from pregnant women allows the opportunity for a much more complete record of prescription, and non-prescription medicine use, including herbal medicines and other ‘natural’ health products, as well as the use of recreational drugs and alcohol use. Web- or app-based data collection can also be utilised in countries where national adverse reaction spontaneous reporting systems or registries are not available.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Safety and Pharmacovigilance of Herbal Medicines in Pregnancy
1.1 Therapeutic Drug Use in Pregnancy
Therapeutic drug use in pregnancy is common, with many women accessing non-prescription (‘over-the-counter’) medicines to treat pregnancy-related symptoms, including constipation, heartburn and urinary tract infections. Some pregnant women will also be prescribed medication to treat more serious conditions, such as depression, epilepsy, and asthma. It is estimated that about 80% of women take at least one non-prescription medicine or prescription medicine throughout the course of pregnancy [1].
Many physiological changes occur during the (up to) 40 weeks of pregnancy and have the ability to alter the absorption, distribution and elimination of conventional and herbal medicines taken by pregnant women. These changes include increased cardiac output, the rate of liver metabolism, plasma volume, glomerular filtration rate, and extent of fat stores, as well as changes in gastrointestinal function. There is a misconception that there is a placental barrier providing protection to the foetus, but almost all drugs are able to pass through the placenta freely.
Some therapeutic drugs are known to be teratogenic to the developing foetus, increasing the risk of several adverse pregnancy outcomes, including miscarriage, birth defects, low birth weight and neurodevelopmental delay. Early pregnancy, during the first trimester, is the most sensitive time for a teratogenic insult to occur, but the foetus is potentially at risk of teratogenic effects of maternal drug exposure for the full duration of pregnancy, and possibly even before conception. Up to 50% of pregnancies are unplanned, so exposure to medicines during conception and early pregnancy is not rare.
1.2 Herbal Medicine Use in Pregnancy
Pregnant women and their healthcare providers are often confused and/or concerned about the effects of taking therapeutic drugs in pregnancy. Safety data are often lacking, or conflicting, and women have historically been advised to avoid pharmaceutical drugs (conventional medicines) if possible because of the, often unknown, risks to the foetus. It is then perhaps unsurprising that some women may choose to use herbal medicines (or other ‘natural’ health products), believing that they are ‘natural’ and a ‘safer’ option during pregnancy, or in the preconception period, than are pharmaceutical medications [2]. Many other women in low- and middle-income countries often rely on herbal and other ‘traditional’ medicines as the only way to treat medical conditions and diseases [3].
1.3 Prevalence of Use of Herbal Medicines in Pregnancy
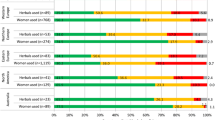
The prevalence of use of herbal medicines by women who are pregnant appears to be substantial. A multinational study, carried out in 23 countries, which included questionnaire data from 9459 women who were pregnant, or had a child under the age of one year, reported the prevalence of herbal medicine use during pregnancy to be 28.9% with most women using them to treat respiratory illness and nausea. The most commonly used herbs reported in this study were ginger, cranberry, valerian, and raspberry. The prevalence of use ranged from 9.4% to 82.3%, which may in part be explained by the use of different study methodologies and cultural and regional differences. Russia (69%), Poland (49.8%) and Australia (43.8%) reported the highest use of herbal medicines during pregnancy [4].
In developed countries, studies have shown that women who use herbal medicines in pregnancy are more likely to be middle-aged, have high levels of education and income, and be primiparous [5,6,7]. One study also reported that, among its sample, women who used herbal medicines were less likely to be smokers and more likely to be married [6].
2 Safety Concerns Associated with Use of Herbal Medicines During Pregnancy
2.1 Adulteration and Contamination of Herbal Medicines
The high risk of contamination or adulteration of herbal medicines with heavy metals, pesticides, and pharmaceutical medications [8, 9] is of particular concern with respect to use of these products by pregnant women.
The lack of regulation and ‘product standards’ for herbal medicines in some countries is problematic. Products may not be checked for the quantity or quality of active ingredients, for contaminants, may not provide patient information, and may not comply with internationally accepted pharmaceutical industry standards for assuring the quality of medicinal products. Consequently, products may be of poor and variable quality and have been found to contain high levels of bacterial pathogens [10], pesticides [11], and heavy metals [12]. In rare instances, even registered herbal medicines have been found to have serious quality problems; for example, in the UK, in 2016, St John’s wort tablets were found to contain toxic pyrrolizidine alkaloids above the threshold recommended by the Committee on Herbal Medicinal Products, a European expert body. The contamination was thought to be from accidental collection of local weeds during harvesting [13].
Reports of contaminated herbal medicines used in pregnancy have associated use of oral Ayurvedic medicines from India with adverse pregnancy outcomes, when taken by several pregnant women in the US and one in Australia [14, 15]. Six asymptomatic pregnant women (with blood lead concentrations between 16 and 64 μg/dL) used ten Ayurvedic products which were found to have a high lead content (as high as 2.4%), as well as traces of arsenic and mercury. Two of the women miscarried before 20 weeks’ gestation; both women had taken the product to promote fertility. It is unknown whether the pregnancy outcomes were related to reproductive issues or to the contaminated medicines [14]. Renal abnormalities (agenesis of one and absence of the other kidney), pulmonary hypoplasia and anhydramnios were reported in an infant exposed in utero to Ayurvedic medicines in the first trimester of pregnancy. Investigations showed very high maternal blood lead concentrations (67 μg/dL) and no genetic link to the abnormalities [15].
As pregnancy outcome data from women using contaminated medicines in pregnancy are scarce, it is difficult to establish causal associations. However, evidence from occupational or environmental exposure to heavy metals suggest that elevated concentrations of lead and arsenic in pregnant women have been associated with pregnancy loss, impaired intrauterine growth, and preterm labour [16,17,18,19,20,21,22]. Exposure to lead in pregnancy has also been associated with impaired postnatal neurodevelopment in the offspring [23,24,25].
Pregnant women should be made aware of the potential risks of contaminants and be advised not to use unregulated medicines prior to and during pregnancy.
2.2 Herbal Medicine Interactions with Conventional Medicines
Given that a large proportion of pregnant women use conventional medications, and the high prevalence of use of herbal medicines during pregnancy, a substantial number of pregnant women are likely to be using both conventional and herbal medicines, with the risk of potential herbal medicine-pharmaceutical drug interactions. A survey of 889 women in North East Scotland, which collected data on medicines use in pregnancy, identified several potential moderate to severe herb-drug interactions in around 12% of the study cohort. Using the Natural Medicines Comprehensive Database [26] to assess the potential for herbal and natural product interaction with prescribed medicines, the survey identified 34 potential herb-drug interactions among 23 participants. Ginger (Zingiber officinale) was noted to have the potential to cause interactions with concurrent prescription medicines, including one major interaction with nifedipine, and three moderate interactions with metformin, insulin, and aspirin. Ondansetron and chamomile (type not specified) were also reported as having the potential to cause a minor interaction [27].
St John’s wort (Hypericum perforatum L.) herb extract is well publicised as interacting with many conventional medicines: by inducing certain cytochrome P450 enzymes, St John’s wort extracts lower serum concentrations of certain medicines below the therapeutic range. Their use has been reported to reduce the efficacy of several antihypertensive, anticonvulsant, immunosuppressant, and antipsychotic medicines [28], which in pregnancy could have devastating effects, including death, for both the mother and baby.
It is advisable for pregnant women to consult with their doctor or pharmacist before they use herbal products in combination with prescribed or non-prescription medication. Authoritative information sources on herbal medicines interactions, written for healthcare professionals, are available [29].
2.3 Issues Relating to Formulations, Routes of Exposure, Dose, and Dosage
As with conventional medicines, the dose, duration of use, and route of administration of herbal preparations are important to consider where such products are used or considered for use in pregnancy. Culinary use of small quantities of herbs would not be expected to increase the risk of foetal harm, but use of high doses of herbal substances, concentrated extracts, and/or prolonged use should be avoided as general precautions.
Essential oils used in small amounts at low concentrations in commercially produced shampoo and soap products are not thought to be in quantities that would cause foetal harm when used appropriately. However, this knowledge is based on unpublished experience. Oral ingestion of essential oils confers maternal and, therefore, foetal toxicity and should be avoided in pregnancy.
Tinctures—alcohol extracts of herbal substances—should generally be avoided in pregnancy as alcohol is a known teratogen. However, inadvertent exposure to small quantities during pregnancy would not be considered to increase the risk of adverse pregnancy outcomes.
2.4 The Availability of Information on the Use of Herbal Medicines in Pregnancy
Online sources and books provide a wealth of information on herbal use for women who are, or intending to become, pregnant, but the majority of this is historical, empirical, and observational with little pharmacologic and animal safety data. Much of the information is of poor quality, often exaggerating the perceived benefits and trivialising, or ignoring, the potential harms. Reputable resources are available for health professionals to help provide some assessment of risk for exposed pregnancies [26, 29–30]; Medicines Complete [29, 30], and many countries commission Teratology Information Services to provide advice to pregnant women and/or their healthcare providers about drug and chemical exposures in pregnancy, including herbal medicines. Contact is usually via telephone but some services also provide online written information (Box 6.1). However, as with conventional medicines, providing a risk assessment can be difficult as there are substantial gaps in knowledge, data are often conflicting, dose and duration of use are poorly defined, and information is typically written in a generic fashion for a particular herbal substance rather than relating to a specific product.
Box 6.1 Authoritative Resources on Herbal Medicine Use in Pregnancy for Women and/or Their Healthcare Providers
If available, healthcare providers and women can contact their local Teratology Information Service for patient specific risk assessments where exposure to herbal medicine has occurred. https://www.entis-org.eu/centers. [31] https://mothertobaby.org/locations/. [32] Authoritative Information resources on the use of some herbal medicines in pregnancy: www.medicinesinpregnancy.org [33] http://naturaldatabase.therapeuticresearch.com/ [26] Williamson E, Driver S, Baxter K, Preston CL (eds), Stockley’s Herbal Medicines Interactions. [online] London: Pharmaceutical Press. http://www.medicinescomplete.com [29] Pharmaceutical Press Editorial. Herbal Medicines. [online] London: Pharmaceutical Press. http://www.medicinescomplete.com [30] |
3 Herbal Medicines Commonly Used to Treat Pregnancy-Related Conditions
Herbal medicines cited in the literature as most frequently being used during pregnancy vary between studies, and herbal substances are typically described only using common names, which are not precise, or not defined at all. Herbal medicines that are commonly reported across studies from Western countries include ginger root, ‘chamomile’ tea, cranberry, and echinacea [5, 7, 34,35,36]. The recommendations for using these herbals during pregnancy are conflicting. In all cases, further large pharmacoepidemiological studies are required to accurately assess the likelihood of adverse health outcomes for mothers and babies associated with use during pregnancy.
3.1 Ginger Root (Zingiber officinale Roscoe)
Ginger root (Zingiber officinale) has been studied in pregnancy for reducing nausea and vomiting, but with many of the studies only investigating efficacy, not foetal safety.
The available literature consists of 14 randomised controlled trials (RCTs) that include a total of 617 women who were exposed to ginger root preparations at fewer than 20 weeks’ gestation [37]. Prospective cohort studies contribute a further 1366 gestational ginger root exposures, with first trimester exposure confirmed in 593 of these women. A population-based, case-control study assessed associations between in utero ginger root exposure and specific congenital malformations in the infant. Ginger was consumed as either a powder, essence, extract or fresh and the dose ranged from 500 mg/d to 2.5 g/d. Data from four of the RCTs described above have also been meta-analysed. Collectively, these studies do not suggest an increase in risk of adverse foetal outcomes with exposure to ginger root preparations [37].
Two systematic reviews using overlapping data from 18 studies compared the antiemetic effects of ginger with placebo, vitamin B6, metoclopramide, and dimenhydrinate in pregnant women. Both reviews concluded ginger supplementation significantly relieved nausea compared with placebo, but there were no significant effects on vomiting [37, 38].
Several conventional pharmaceuticals can be recommended for use in pregnancy to treat symptoms of nausea and vomiting. Women with excessive nausea and vomiting (hyperemesis gravidarum) may need to seek hospital treatment for fluid and electrolyte replacement and treatment with a pharmaceutical antiemetic.
3.2 Chamomile (Chamaemelum nobile (L.) All)
The quality of data relating to the use of chamomile (Chamaemelum nobile) in pregnancy is poor, with the available cohort studies collecting exposure details retrospectively, after the participants’ pregnancies had ended. The types of chamomile preparations were not explicitly reported, described as an oral exposure most commonly by infusion in one study [39], and as oral or topical in another [40]. Common indications for use were anxiety, digestive problems, and stretch marks [40]. The data come from three Italian studies, which all compared pregnancies of daily consumers of chamomile to those of non-users, finding an association with use of chamomile and preterm labour [6, 39, 40] and threatened miscarriage [40]. In one study, all participants were classed as ‘healthy’, and in the other two, the analyses were part of larger studies, making it difficult to extrapolate the influence potential maternal risk factors may have had on the outcomes.
Due to the limited available data, it is recommended to avoid chamomile-containing products during pregnancy.
3.3 Cranberry (Vaccinium macrocarpon Aiton)
Cranberries are used for the treatment and prevention of urinary tract infections (UTIs), a common complaint in pregnancy due to hormonal changes. Research on the safety of cranberries during pregnancy is based on one cohort study (including women <16 weeks pregnant) and one randomised, placebo-controlled pilot study (most exposed in ‘early pregnancy’), showing no increased risk of maternal or foetal outcomes in >1000 pregnancies [41, 42] following the consumption of cranberry juice.
Data regarding the efficacy of cranberry for the prevention of UTIs are derived from studies using different cranberry preparations (juice, capsules, and tablets) and provide no clear consensus of clinical benefit [43]. Untreated UTIs can cause serious adverse outcomes, and conventional pharmaceutical treatment should not be withheld on account of pregnancy.
3.4 Echinacea (Echinacea spp.: E. angustifolia DC., E. pallida (Nutt.) Nutt., E. purpurea (L.) Moench)
Echinacea species are often used by patients to ‘strengthen the immune system’ and to treat colds and other upper respiratory tract infections, urinary tract infections, and slow-healing wounds.
Two studies have investigated the use of ‘echinacea’ preparations in pregnancy. One study explored pregnancy outcomes among 206 women who had used an echinacea preparation for 5 to 7 days. Most women used either E. angustifolia or E. purpurea (not further specified) as capsules/tablets (58%) or tincture (38%). The dose ranged from 250-1000 mg/d or 2-10 tincture drops. Of these, 112 women (54%) were exposed in the first trimester and 17 (8%) in all three trimesters. These women were compared with non-users of echinacea matched for maternal age, alcohol intake, and smoking status. No differences in the rates of gestational age, birth weight, foetal distress, or major congenital malformations were reported among the echinacea group, compared with the control group [44]. However, this study included only very small numbers of participants and does not provide conclusive evidence on the likelihood of adverse outcomes in the foetus following exposure to echinacea in utero.
The second study from the Norwegian Mother and Child cohort study reported 363 women using echinacea in pregnancy within a cohort of 68,522. Details regarding the dose, echinacea species, preparation type, or stage of pregnancy at exposure were not reported. Users of echinacea were older and less likely to smoke but did not have an increased risk of having a child with a malformation or an adverse pregnancy outcome when compared to non-users [45].
The European Medicines Agency (EMA) does not recommend the use of echinacea in pregnancy due to the limited safety data available [46].
3.5 Other Herbs with Known Adverse Effects in Pregnancy
Single studies have reported adverse pregnancy outcomes following the use of almond oil and liquorice (Glycyrrhiza glabra L.).
Regular users of almond oil (n = 123) had a significantly higher risk of a preterm delivery than non-users even when other risks factors for preterm birth, such as age, smoking, twin pregnancy, and recreational drug use, were controlled for [39]. Most of the women in this study applied almond oil to their abdomen to reduce the risk of stretch marks during their pregnancy.
Women who took up to 2104 mg/day of liquorice (Glycyrrhiza glabra) between the fourth day and 25th week of gestation had a marginally increased risk of stillbirth than those not exposed. Other adverse outcomes were not reported [47]. When comparing pregnancy outcomes in fourteen women who regularly use liquorice during pregnancy with 238 pregnancies in non-users, a higher frequency of threatening miscarriages (35.7%) and preterm labour (16.7%) was seen in the liquorice-exposed group [40].
Although most herbal medicines have not been formally investigated for their embryotoxicity, teratogenic, and abortifacient potential, many are not recommended for use during pregnancy due to the theoretical risks from their known constituents and pharmacological effects. Examples include black cohosh (Actaea racemosa L.), blue cohosh (Caulophyllum thalictroides (L.) Michx.), and motherwort (Leonurus cardiaca L.), all of which are not recommended as they have traditionally been used to stimulate menstruation or provoke abortion by acting on the smooth muscle of the uterus [48,49,50].
4 Safety Monitoring Systems for Medicines Used in Pregnancy
Safety information for pregnant women and their clinicians is frequently reliant on observational studies where data have been voluntarily reported by women who have often been inadvertently or unavoidably exposed to medicines in pregnancy. Study designs are usually cohort or case-control investigations. Difficulties arising with this type of data collection are due to, but not limited to, selection bias with regard to those pregnancies that are successfully followed up, small sample sizes, and inaccurate or missing data on exposures and dose, timings, duration and indication.
Many spontaneous reporting systems from national healthcare registries and marketing authorisation holders encourage reporting of suspected adverse reactions associated with prescribed and herbal medicines, which can provide useful data for safety signal detection. However, adverse event reporting can create bias, where adverse pregnancy outcomes are overrepresented with no data to determine the frequency of risk within an exposed population. Teratology Information Services (TIS) provide routine collection of data from pregnant women, or their healthcare providers, who contact the service for advice regarding maternal use of medicines. Although this method allows prospective data collection, methodological issues remain, including difficulties collecting adequate numbers of exposed pregnancies for rarely used medicines, and sample bias, as enquiries to TISs are often biased towards high-risk pregnancies. Further, not all countries have these voluntary reporting systems in place and those that do find it very labour intensive to track and follow up pregnancies to collect outcome data, and busy clinicians find the burden of reporting pregnancies too great.
4.1 Reporting of Adverse Reactions Associated with Herbal Medicine Use in Pregnancy
Between 2006 and 2014, the UK ‘Yellow Card Scheme’ (the national system for voluntary reporting of suspected adverse reactions associated with medicines, including herbal medicines) received around 60 reports involving herbal medicines each year; 40% of these were submitted by the general public (e.g. patients or their carers) rather than by health professionals [51]. The number of pregnancy-specific reports was not provided.
Healthcare providers are rarely informed by their patients about herbal medicine use in pregnancy [52], and health professionals may omit to ask their patients about this. Teratology Information Services are also not commonly asked for advice about the use of herbal medicines by either patients or their healthcare providers. The Berlin Teratology Information Service (Embryotox) reported that only 6% of enquiries to the service were regarding ‘alternative medications’ [53]. For the UK Teratology Information Service (UKTIS), which has been in operation and collecting surveillance data, including those concerning herbal medicines, since 1984, fewer than 1% of all enquiries made to the service are in relation to herbal products. Enquiries regarding the use of peppermint oil, St John’s wort and ‘senna’ are the most commonly recorded herbal medicine exposures in the database.
4.2 Safety Monitoring Systems for the Collection of Herbal Medicine Exposure Data in Pregnancy
In order to increase reporting of medicines in pregnancy in countries that may not have national reporting systems or registries in place, and to improve some of the issues around missing maternal and infant details, collection of exposure data on non-prescription medicines, and to reduce the burden on clinicians, web-based applications are being developed.
The UKTIS has created a bespoke, online, patient-oriented pregnancy recording system, BUMPS (Best Use of Medicines in Pregnancy), where women are invited to set up an account and input information regarding all exposures in pregnancy. There is a dedicated section to record use of herbal medicines, including the proprietary and ingredient names, dose, dose frequency, duration of use, route of administration, and stage of pregnancy of exposure (see Boxes 6.2a and 6.2b). The system is designed to be updated throughout pregnancy and to record the outcome(s) once the pregnancy has ended. Women are requested to report ongoing pregnancies in early pregnancy, preferably before any prenatal screening or knowledge of the pregnancy outcome has occurred. Although the data are analysed separately, the system also permits the reporting of previous pregnancies. Where a liveborn child is recorded in the system, neurodevelopmental milestones are requested annually from 6 months of age. Engagement with women in this way provides an opportunity to stay in contact throughout their child’s life to improve detection of longer-term outcomes, including childhood illnesses, and development milestones.
Box 6.2a Extracts from the Best Use of Medicines in Pregnancy (BUMPS) questionnaire: Medicines and Vaccines

Box 6.2b Extract from ‘Medicines Used in the 3 Months Before or During Pregnancy’ Questionnaire

Targeted promotion of the system is required, highlighting the opportunity and importance of women informing UKTIS of their use of herbal products in pregnancy. Only 1.5% of women who have reported to the system to date have reported these types of exposures. The most common products to be reported have been essential oils, used topically or accidentally ingested.
Other monitoring systems have been designed to provide easier ways to capture data. One such development is WEB-RADR [54]: recognising adverse drug reactions, a mobile application enabling patients and healthcare providers to report suspected adverse drug reactions and receive up-to-date information and news alerts. Country-specific mobile apps have been launched in three countries (the Netherlands, the UK, and Croatia), and a generic multi-country version, the Med Safety app, has been launched in Burkina Faso, Zambia, Armenia, Ghana, Ethiopia, Botswana, Cote d’Ivoire, and Uganda. WEB-RADR could be a useful tool to provide a way for herbal medicine adverse event reports to be captured from women who regularly use herbal medicines whilst pregnant, particularly where national reporting systems are not available. Pregnant women are the correct demographic to be targeted by social media widening the reach of these systems.
Despite novel methods of data collection, the fragmentation of this information is problematic. Many registries, databases and TISs exist internationally, often collecting small numbers of pregnancy outcomes for new medicines, or medicines that are not routinely prescribed to women of child-bearing age. In order to address how these data can be more effectively collated to provide timely signal detection, a five-year Innovative Medicines Initiative (IMI)-funded project, ConcePTION, is underway to establish a common data model and to explore the possibility of secure data-sharing platforms. Harmonisation of data collection is imperative to achieve timely responses to detect harms of medicines, including herbal medicines.
5 Conclusions
The available published data suggest herbal medicine use in pregnancy is associated with possible teratogenicity, risk from contaminated products, and the potential for interactions with conventional pharmaceuticals. However, these findings often come from poor quality studies and individual case reports making it difficult to provide any guidance on the safety of their use in pregnancy. A risk-benefit analysis for almost all scenarios would suggest there are potential risks and little evidence of any benefit. Women should therefore be advised to avoid the use of herbal products when they are pregnant or trying to conceive.
For pregnant women who continue to use herbal medicines despite the warnings, or because of the lack of evidence of harm, data collection is vital to improve the quantity and quality of information provided to women and their healthcare providers about the products they are using.
Reporting systems designed for conventional pharmaceutical medicines can be utilized and have been adapted to collect adverse event reports following herbal medicines use also. Online data systems in the form of pregnancy registries, databases and apps provide an opportunity to collect data from women internationally about the herbal products they use in pregnancy and their birth outcomes. It remains to be seen if women will be willing to use these systems in enough numbers that data can be collected on a large enough scale, to provide meaningful guidance on the use of individual herbal products. Education and promotion are the next steps to encourage reporting and providing robust evidence-based safety data for pregnant women in the future.
References
Lupattelli A, Spigset O, Twigg MJ et al (2014) Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open 4(2):e004365. https://doi.org/10.1136/bmjopen-2013-004365
Pallivalapila AR, Stewart D, Shetty A, Pande B, Singh R, McLay JS (2015) Use of complementary and alternative medicines during the third trimester. Obstet Gynecol 125(1):204–211. https://doi.org/10.1097/AOG.0000000000000596
Towns AM, van Andel T (2016) Wild plants, pregnancy, and the food-medicine continuum in the southern regions of Ghana and Benin. J Ethnopharmacol 179:375–382. https://doi.org/10.1016/j.jep.2016.01.005
Kennedy DA, Lupattelli A, Koren G, Nordeng H (2013) Herbal medicine use in pregnancy: results of a multinational study. BMC Complement Altern Med 13:355. https://doi.org/10.1186/1472-6882-13-355
Broussard CS, Louik C, Honein MA, Mitchell AA (2010) Herbal use before and during pregnancy. Am J Obstet Gynecol 202(5):443.e1–443.e6. https://doi.org/10.1016/j.ajog.2009.10.865
Trabace L, Tucci P, Ciuffreda L et al (2015) “Natural” relief of pregnancy-related symptoms and neonatal outcomes: above all do no harm. J Ethnopharmacol 174:396–402. https://doi.org/10.1016/j.jep.2015.08.046
Forster DA, Denning A, Wills G, Bolger M, McCarthy E (2006) Herbal medicine use during pregnancy in a group of Australian women. BMC Pregnancy Childbirth 6:21. https://doi.org/10.1186/1471-2393-6-21
Saper RB, Kales SN, Paquin J et al (2004) Heavy metal content of ayurvedic herbal medicine products. JAMA 292(23):2868–2873. https://doi.org/10.1001/jama.292.23.2868
Chan K (2003) Some aspects of toxic contaminants in herbal medicines. Chemosphere 52(9):1361–1371. https://doi.org/10.1016/S0045-6535(03)00471-5
de Sousa Lima CM, Fujishima MAT, de Paula LB, Mastroianni PC, de Sousa FFO, da Silva JO (2020) Microbial contamination in herbal medicines: a serious health hazard to elderly consumers. BMC Complement Med Ther 20(1):17. https://doi.org/10.1186/s12906-019-2723-1
Luo L, Dong L, Huang Q et al (2021) Detection and risk assessments of multi-pesticides in 1771 cultivated herbal medicines by LC/MS-MS and GC/MS-MS. Chemosphere 262:127477. https://doi.org/10.1016/j.chemosphere.2020.127477
Luo L, Wang B, Jiang J et al (2020) Heavy metal contaminant in herbal medicines: determination, comprehensive risk assessments, and solutions. Front Pharmacol 11:595335. https://doi.org/10.3389/fphar.2020.595335
Medicines and Healthcare products Regulatory Agency (2016) Press Release: Precautionary recall-six batches of St John’s Wort Tablets. https://www.gov.uk/government/news/precautionary-recall-six-batches-of-st-johns-wort-tablets. Accessed 1 Nov 2020
Center for Disease Control and Prevention (2012) Lead poisoning in pregnant women who used Ayurvedic medications from India – New York City, 2011–2012. Morb Mortal Wkly Rep 61(33):641–646
Wong A, Dargan P, Koutsogiannis Z, Sokol J, Ramkrishna J, Greene SL (2015) Chronic Ayurvedic medicine use associated with major and fatal congenital abnormalities. Med J Aust 203(11):443–444. https://doi.org/10.5694/mja15.00636
Quansah R, Armah FA, Essumang DK et al (2015) Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis. Environ Health Perspect 123(5):412–421. https://doi.org/10.1289/ehp.1307894
Wang H, Li J, Zhang X et al (2018) Maternal serum arsenic level during pregnancy is positively associated with adverse pregnant outcomes in a Chinese population. Toxicol Appl Pharmacol 356:114–119. https://doi.org/10.1016/j.taap.2018.07.030
Borja-Aburto VH, Hertz-Picciotto I, Rojas Lopez M, Farias P, Rios C, Blanco J (1999) Blood lead levels measured prospectively and risk of spontaneous abortion. Am J Epidemiol 150(6):590–597. https://doi.org/10.1093/oxfordjournals.aje.a010057
González-Cossío T, Peterson KE, Sanín LH et al (1997) Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics 100(5):856–862. https://doi.org/10.1542/peds.100.5.856
Nishioka E, Yokoyama K, Matsukawa T et al (2014) Evidence that birth weight is decreased by maternal lead levels below 5 μg/dl in male newborns. Reprod Toxicol 47:21–26. https://doi.org/10.1016/j.reprotox.2014.05.007
Jelliffe-Pawlowski LL, Miles SQ, Courtney JG, Materna B, Charlton V (2006) Effect of magnitude and timing of maternal pregnancy blood lead (Pb) levels on birth outcomes. J Perinatol 26(3):154–162. https://doi.org/10.1038/sj.jp.7211453
Rothenberg SJ, Schnaas L, Perroni E, Hernández RM, Martínez S, Hernández C (1999) Pre- and postnatal lead effect on head circumference: a case for critical periods. Neurotoxicol Teratol 21(1):1–11. https://doi.org/10.1016/s0892-0362(98)00034-8
Hu H, Téllez-Rojo MM, Bellinger D et al (2006) Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect 114(11):1730–1735. https://doi.org/10.1289/ehp.9067
Schnaas L, Rothenberg SJ, Flores M-F et al (2006) Reduced intellectual development in children with prenatal lead exposure. Environ Health Perspect 114(5):791–797. https://doi.org/10.1289/ehp.8552
Liu J, Chen Y, Gao D, Jing J, Hu Q (2014) Prenatal and postnatal lead exposure and cognitive development of infants followed over the first three years of life: a prospective birth study in the Pearl River Delta region, China. Neurotoxicology 44:326–334. https://doi.org/10.1016/j.neuro.2014.07.001
The Natural Medicines Comprehensive Database. http://naturalmedicines.therapeuticresearch.com. Accessed 1 Nov 2020
McLay JS, Izzati N, Pallivalapila AR et al (2017) Pregnancy, prescription medicines and the potential risk of herb-drug interactions: a cross-sectional survey. BMC Complement Altern Med 17(1):543. https://doi.org/10.1186/s12906-017-2052-1
British National Formulary. BMJ Group and Pharmaceutical Press. http://www.medicinescomplete.com. Accessed 1 Nov 2020
Williamson E, Driver S, Baxter K, Preston CL (eds) Stockley’s herbal medicines interactions. Pharmaceutical Press, London. https://about.medicinescomplete.com/publication/stockleys-herbal-medicines-interactions-2/
Pharmaceutical Press Editorial. Herbal Medicines. Pharmaceutical Press, London. https://about.medicinescomplete.com/publication/herbal-medicines/
European Network of Teratology Information Services (ENTIS). https://www.entis-org.eu/centers. Accessed 1 Nov 2020
MotherToBaby, a service of the Organisation of Teratology Information Specialists (OTIS), USA. https://mothertobaby.org/affiliates/. Accessed 1 Nov 2020
Best Use of Medicines in Pregnancy, a service of the UK Teratology Information Service (UKTIS). www.medicinesinpregnancy.org. Accessed 1 Nov 2020
Kennedy DA, Lupattelli A, Koren G, Nordeng H (2016) Safety classification of herbal medicines used in pregnancy in a multinational study. BMC Complement Altern Med 16:102. https://doi.org/10.1186/s12906-016-1079-z
Nordeng H, Bayne K, Havnen GC, Paulsen BS (2011) Use of herbal drugs during pregnancy among 600 Norwegian women in relation to concurrent use of conventional drugs and pregnancy outcome. Complement Ther Clin Pract 17(3):147–151. https://doi.org/10.1016/j.ctcp.2010.09.002
Holst L, Wright D, Haavik S, Nordeng H (2011) Safety and efficacy of herbal remedies in obstetrics-review and clinical implications. Midwifery 27(1):80–86. https://doi.org/10.1016/j.midw.2009.05.010
Stanisiere J, Mousset P-Y, Lafay S (2018) How safe is ginger rhizome for decreasing nausea and vomiting in women during early pregnancy? Foods 7(4):50. https://doi.org/10.3390/foods7040050
Hu Y, Amoah AN, Zhang H et al (2020) Effect of ginger in the treatment of nausea and vomiting compared with vitamin B6 and placebo during pregnancy: a meta-analysis. J Matern Neonatal Med 1–10. https://doi.org/10.1080/14767058.2020.1712714
Facchinetti F, Pedrielli G, Benoni G et al (2012) Herbal supplements in pregnancy: unexpected results from a multicentre study. Hum Reprod 27(11):3161–3167. https://doi.org/10.1093/humrep/des303
Cuzzolin L, Francini-Pesenti F, Verlato G, Joppi M, Baldelli P, Benoni G (2010) Use of herbal products among 392 Italian pregnant women: focus on pregnancy outcome. Pharmacoepidemiol Drug Saf 19(11):1151–1158. https://doi.org/10.1002/pds.2040
Heitmann K, Nordeng H, Holst L (2013) Pregnancy outcome after use of cranberry in pregnancy—the Norwegian Mother and Child Cohort Study. BMC Complement Altern Med 13:345. https://doi.org/10.1186/1472-6882-13-345
Wing DA, Rumney PJ, Preslicka CW, Chung JH (2008) Daily cranberry juice for the prevention of asymptomatic bacteriuria in pregnancy: a randomized, controlled pilot study. J Urol 180(4):1367–1372. https://doi.org/10.1016/j.juro.2008.06.016
de Rossi P, Cimerman S, Truzzi JC et al (2020) Joint report of SBI (Brazilian Society of Infectious Diseases), FEBRASGO (Brazilian Federation of Gynecology and Obstetrics Associations), SBU (Brazilian Society of Urology) and SBPC/ML (Brazilian Society of Clinical Pathology/Laboratory Medicine): recommendations for the clinical management of lower urinary tract infections in pregnant and non-pregnant women. Braz J Infect Dis 24(2):110–119. https://doi.org/10.1016/j.bjid.2020.04.002
Gallo M, Sarkar M, Au W et al (2000) Pregnancy outcome following gestational exposure to echinacea: a prospective controlled study. Arch Intern Med 160(20):3141–3143. https://doi.org/10.1001/archinte.160.20.3141
Heitmann K, Havnen GC, Holst L, Nordeng H (2016) Pregnancy outcomes after prenatal exposure to echinacea: the Norwegian Mother and Child Cohort Study. Eur J Clin Pharmacol 72(5):623–630. https://doi.org/10.1007/s00228-016-2021-5
European Medicines Agency (2015) European Union herbal monograph on Echinacea purpurea (L.) Moench, herba recens. https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-echinacea-purpurea-l-moench-herba-recens_en.pdf
Choi JS, Han JY, Ahn HK et al (2013) Fetal and neonatal outcomes in women reporting ingestion of licorice (Glycyrrhiza uralensis) during pregnancy. Planta Med 79(2):97–101. https://doi.org/10.1055/s-0032-1328102
Dugoua J-J, Perri D, Seely D, Mills E, Koren G (2008) Safety and efficacy of blue cohosh (Caulophyllum thalictroides) during pregnancy and lactation. Can J Clin Pharmacol 15(1):e66–e73
Dugoua J-J, Seely D, Perri D, Koren G, Mills E (2006) Safety and efficacy of black cohosh (Cimicifuga racemosa) during pregnancy and lactation. Can J Clin Pharmacol 13(3):e257–e261
Liu J, Peng C, Zhou Q-M, Guo L, Liu Z-H, Xiong L (2018) Alkaloids and flavonoid glycosides from the aerial parts of Leonurus japonicus and their opposite effects on uterine smooth muscle. Phytochemistry 145:128–136. https://doi.org/10.1016/j.phytochem.2017.11.003
Safety, regulation and herbal medicines: a review of the evidence. A report prepared by the UHMAC (HMAC) for the HM and PWG (2014) https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/545681/HMAC_-_HerbalsafetyOctober2014Final.pdf#:~:text=
Gardiner P, Jarrett K, Filippelli A, Pecci C, Mauch M, Jack B (2013) Herb use, vitamin use, and diet in low-income, postpartum women. J Midwifery Womens Health 58(2):150–157. https://doi.org/10.1111/j.1542-2011.2012.00240.x
Philipps W (2017) Herbal medicines in pregnancy – pharmacovigilance and risk communication. https://www.bfarm.de/SharedDocs/Downloads/DE/Service/Termine-und-Veranstaltungen/dialogveranstaltungen/dialog_2017/170914/15_Philipps.pdf?__blob=publicationFile&v=3. Accessed 1 Nov 2020
WEB-RADR: Recognising adverse drug reactions. https://web-radr.eu/. Accessed 1 Nov 2020
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Stephens, S. (2022). Safety and Pharmacovigilance of Herbal Medicines in Pregnancy. In: Barnes, J. (eds) Pharmacovigilance for Herbal and Traditional Medicines. Adis, Cham. https://doi.org/10.1007/978-3-031-07275-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-07275-8_6
Published:
Publisher Name: Adis, Cham
Print ISBN: 978-3-031-07273-4
Online ISBN: 978-3-031-07275-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)