Abstract
Purpose
The objective of this study was to determine whether the so-called “shift” or “drift” problem might occur when generic anti-epileptic drugs are interchanged, and thus to assess if generic anti-epileptic drugs are interchangeable and can be used in an efficacious and safe way on the basis of their bioequivalence to one and the same reference product.
Methods
The bioequivalence of topiramate and gabapentin generics was evaluated. For proper interstudy comparison, individual exposure data (AUC and Cmax) for each bioequivalence study present in the registration dossier was normalized based on the absolute exposure data of one of two innovators. The exposure-normalized plasma concentration curves of the generic product arms between studies were compared, providing indirect evidence of bioequivalence of the different generics. Additionally, comparisons were made for generic–generic as well as innovator–innovator exchange based on absolute exposure data from individual bioequivalence studies.
Results
In almost all cases, estimated 90% confidence intervals of the AUC and Cmax ratios for generic–generic interchange were within the routine 80–125% criterion. When absolute, non-corrected exposure data were used for this interstudy comparison, in a number of cases 90% confidence intervals outside the 80–125% criterion were found upon interchanging generics from two studies. However, a similar pattern of 90% confidence intervals outside the 80–125% criterion was observed for the comparison of innovator arms, despite the fact that the innovator was identical in all studies.
Conclusion
Our results strongly indicate that the so-called drifting problem upon generic–generic substitution does not result in important differences in exposure upon exchanging topiramate generics or gabapentin generics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Upon development and initial marketing authorization, new pharmaceutical formulations receive a 10-year period of data protection. Following the expiry of this protection period, various new generic agents using the innovator’s active substance appear on the market. For such generics, no new clinical trials are required, whereas authorization is in most cases granted solely on the proof of bioequivalence after a single-dose controlled trial comparing the generic with the innovator product. If proven to be bioequivalent, the generic product is considered therapeutically equivalent to the innovator formulation [1, 2]. Official regulations regarding generic products require tests to establish whether the pharmacokinetic parameters AUC and Cmax are within established limits compared with the same parameters of the innovator.
Under current regulations, the testing of the relative exposure of a generic against exposure obtained with other generics is not required. However, due to the unavailability of such data, a so-called “shift” or “drift” problem may occur when generics are interchanged, and is a reason for concern [3]. While generics are interchangeable with the innovator product, generics themselves may not be, thereby possibly causing loss of efficacy and/or toxicity. The problem may become relevant for certain drugs with a narrow therapeutic window, including anti-epileptic drugs. Seizure control may be lost or anti-epileptic plasma levels may be increased, causing toxicity, when such a drift would indeed occur if patients switch from one generic to another.
Published studies predict, through various simulation methods, the potential of “drift” between generic formulations. Results from these predictive analyses indicate that the drift problem proportionally increases with the number of generic formulations released and the deviation from unity of the actually observed 90% confidence intervals [3]. Other potential problems associated with switching between generic medications include the use of dissimilar excipients, the different appearance of the formulations, and different product names, the latter two factors possibly resulting in changes in patient adherence patterns [4, 5].
In epidemiological terms, epilepsy is a common chronic disorder affecting about 50 million people worldwide with 200,000 new cases every year, therefore representing a significant part of any country’s public healthcare program [6, 7]. The use of generics in epilepsy is subject to intensive debate. This debate relates to, for example, bioequivalence requirements, possible difference in variability in exposure and consequences of small differences, problems with medicine supply, and possible legal consequences when patients do not provide explicit permission for being switched to a generic medicine [8–12]. It is unclear what the consequences are in terms of adverse events, and thus the costs for society and the consequences for the individual patient [13].
Epilepsy is an area in which many generics are available. Recently, we discussed the available evidence that generic interchange of anti-epileptic drugs does indeed lead to significant differences in exposure [14]. It appeared that only on very rare occasions, a relevant reduced exposure was reported upon switching to a generic anti-epileptic drug [11]. Other reported data suggesting both upward and downward difference in exposure upon generic substitution were in fact only able to show difference in exposure when other factors besides generic exchange (e.g., different dose, exchange with a different type of formulation) were present [15]. A number of published surveys [16–18] as well as observational studies [19, 20] suggest a difference in epilepsy-related adverse events after switching from branded to generic anti-epileptics. However, these studies do not provide evidence for real differences in exposure, or for a causal relationship between generic substitution and, for example, the occurrence of seizures. It was therefore concluded that at present no evidence is available to support the hypothesis that differences in exposure are responsible for adverse events observed upon generic exchange of anti-epileptic drugs [14]. This conclusion is also apparent in a recent meta-analysis by Kesselheim et al. In this analysis, the apparent increase in seizures obtained purely from observational studies is proposed to be explained by undue worries from patients or physicians about the effectiveness of generic anti-epileptic drugs after a switch [21].
The present study focuses on the possible consequences of generic–generic exchange for relative exposure, and for that reason compares different topiramate (Topamax®) and gabapentin (Neurontin®) generics that are currently registered in The Netherlands in an attempt to quantify possible differences in bioavailability when exchanging different generics. Data were obtained from the generics’ registration files as available from the Dutch Medicines Evaluation Board (referred to as the Agency), and analyzed according to current methodological and statistical principles [14].
Materials and methods
Data collection
The data used in this study were obtained from bioequivalence studies submitted for the topiramate and gabapentin generic products that were registered in January 2008. All data were available and accessible through the Agency’s database.
Our interstudy comparison of topiramate exposure focused on the relative exposure obtained by administration of 200 mg, since in almost all cases, bioequivalence in the registration file was indeed demonstrated using a 200-mg dose. For gabapentin, exposure was compared at the 400-mg, 600-mg, and 800-mg levels. In all cases, registration of each individual strength was supported by a bioequivalence study at that strength.
In each bioequivalence trial, the same innovator product was used, i.e., Topamax® (Janssen-Cilag) for topiramate, and Neurontin® (Pfizer) for gabapentin. Actually measured plasma concentrations varied between different bioequivalence studies, a known phenomenon that is most likely due to differences in study design, e.g., different volunteers (with regard to, for example, age, ethnicity, weight, etc.), different sampling times, or different laboratories applying different analysis methodologies.
The 200-mg Topamax® mean AUCs in the included bioequivalence studies ranged from 125.3 to 158.6 μg*h/ml, and Cmax from 4.17 to 5.05 μg/ml. Likewise, Neurontin® mean AUCs ranged from 25.9 to 33.8 μg*h/ml, and Cmax from 1.00 to 1.15 μg/ml for the 400-mg strength, from 41.3 to 47.6 μg*h/ml and from 1.36 to 1.58 μg/ml for the 600-mg strength, and from 37.0 to 56.9 μg*h/ml and from 1.28 to 1.77 μg/ml for the 800-mg strength.
Normalization of the absolute exposure data
In order to ensure uniformity of the study-to-study comparison and avoid bias due to differences in actual plasma exposure between different bioequivalence source studies, the innovator arm in each bioequivalence study was used to correct for any absolute difference in the generic arms. For this purpose, the average of the mean AUC0-t and Cmax of all innovator arms was calculated and the study with the closest to average value was used as a reference standard. Subsequently, the absolute AUC and Cmax of each generic was corrected using the ratio of the reference standard vs the innovator AUC and Cmax of the bioequivalence study in question. By applying this correction, generic exposure was normalized, allowing more reliable interstudy comparisons. After this normalization, the 90% confidence intervals were estimated.
Estimation of the 90% confidence interval for the relative exposure
Ninety percent confidence intervals were calculated using standard methodology for calculating a confidence interval for a difference between means. Details of this methodology are shown in the supplementary material.
Results
Topiramate (Topamax®)
In January 2008, 13 different 200-mg generic topiramate products were registered in The Netherlands. Registration of a number of these generics was based on the same dossier, i.e., the approval of these 13 generic topiramate products was based on 7 different bioequivalence studies, each applying a 200-mg dose. A summary of the actual point estimates and 90% confidence intervals for the AUC and Cmax ratios of topiramate generics in all available bioequivalence studies is provided in Table 1. The mean point estimate for the AUC ratio for all evaluated studies was 101.16%, and 99.55% for the Cmax ratio. The mean absolute deviation of the point estimate from unity for all evaluated studies was 1.16% for the AUC ratio, and 3.52% for the Cmax ratio.
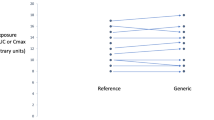
Seven available bioequivalence studies allowed for 21 generic–generic interstudy comparisons. Indirectly estimated point estimates and 90% confidence intervals for these comparisons using the exposure-normalized data are summarized in Table 2. Figure 1 graphically represents the 90% confidence intervals for the exposure-normalized data for the AUC0-t and Cmax ratio. For all topiramate generic–generic comparisons, 90% confidence intervals obtained using exposure-normalized AUC and Cmax values were within the 80–125% range. The mean point estimate for the AUC ratios was very close to 100%, i.e., 100.38%, and 96.62% for the Cmax ratio. The mean of the absolute deviation of the point estimate from unity for all evaluated studies was 2.21% for AUC, and 4.53% for Cmax.
Indirectly estimated 90% confidence intervals of the AUC and Cmax ratios using exposure-normalized AUC0-t (top) and Cmax (bottom) for topiramate generics. The comparisons are represented on the x-axis, e.g., G1/G2 indicates the comparison of generic product used in Study 1 with the generic used in Study 2. The green area indicates the bioequivalence acceptance limits of the 90% confidence interval range of 80–125%
Comparison of the absolute, not-normalized AUC and Cmax, both for the generic and the innovator Topamax®, yielded a number of 90% confidence intervals outside the 80–125% range for bioequivalence. However, in this case a very similar pattern of 90% confidence intervals was observed for the generic–generic and innovator–innovator exchange, despite the fact that the innovator Topamax® was identical in all studies (Figure shown in the Appendix).
Gabapentin (Neurontin®)
In The Netherlands, Neurontin is registered as 100-, 300- or 400-mg capsules, and as 600- or 800-mg tablets. Since gabapentin pharmacokinetics is nonlinear [22], separate bioequivalence studies for each strength have been submitted for the 400-, 600- and 800-mg strengths in support of generic applications. As was the case with topiramate generics, for gabapentin generics, a number of applications were based on the same dossier, i.e., registration of eight 400-mg strengths, five 600-mg strengths, and five 800-mg strengths was based on in total 4 bioequivalence studies per strength. Since per dose strength (i.e., 400, 600, and 800 mg) 4 bioequivalence studies were available, 6 comparisons per dose strength were possible.
Table 3 shows a summary of the actual 90% confidence intervals for gabapentin AUC and Cmax ratios in the studies submitted to the Agency in support of an application for a generic gabapentin formulation. The mean point estimates for the AUC ratios for all evaluated 400-, 600- and 800-mg studies were very close to 100%, i.e., 100.2%, 102.7%, and 100.2% respectively, and 100.6%, 103.6%, and 97.7% for the Cmax ratios respectively. The mean of the absolute deviation of the ratio from unity for all evaluated 400-mg, 600-mg, and 800-mg studies was 1.48%, 3.18%, and 3.55% for AUC respectively, and 2.63%, 5.03% and 4.20% for Cmax.
Point estimates and 90% confidence intervals per dose for each generic–generic interstudy comparison using exposure-normalized data are summarized in Fig. 2 and Table 4. In the vast majority of cases 90% confidence intervals were within the 80–125% margin for bioequivalence, except the Cmax ratio for G1/G4 with a lower end of the 90% confidence interval of 79.03%, the Cmax ratio for G5/G7 and G6/G7 with a high end of the 90% confidence interval of 125.45% and 125.75% respectively, and the AUC ratio for G10/G11, with a high end of the 90% confidence interval of 127.62%.
Indirectly estimated 90% confidence intervals of the AUC and Cmax ratios comparing the exposure-normalized AUC0-t (left figures) and Cmax (right figures) for gabapentin 400-mg, 600-mg, and 800-mg generics (top, middle, bottom). The comparisons are represented on the x-axis, e.g., G1/G2 indicates the comparison of the generic product used in Study 1 with the generic used in Study 2. The green area indicates the bioequivalence acceptance limits of the 90% confidence interval range of 80–125%
Comparison of the absolute, not-normalized AUC and Cmax yielded a number of 90% confidence intervals for many generic–generic comparisons outside the 80–125% range for bioequivalence. However, a similar pattern of 90% confidence intervals was noted with generic–generic exchange and innovator–innovator exchange (Figure shown in the Appendix).
Discussion
In this study, exposure data obtained from bioequivalence studies were used in order to indirectly estimate 90% confidence intervals for generic–generic substitution. It appeared that absolute plasma levels obtained with the innovator drugs, i.e., Topamax® and Neurontin®, varied markedly among different bioequivalence studies. This is a known phenomenon that is most likely due to differences in study design, e.g., different volunteers (with regard to, for example, age, ethnicity, weight, etc.), different sampling times, or different laboratories applying different analysis methodologies that are used in the individual bioequivalence studies. Still, this variation impedes direct interstudy comparison and calculation of the 90% confidence intervals for AUC and Cmax ratios. Indeed, a large variation in 90% confidence intervals was observed applying the absolute, uncorrected AUC and Cmax from different bioequivalence studies for calculation of the generic–generic 90% confidence intervals. However, a very comparable pattern of 90% confidence intervals was obtained when indirectly estimating 90% confidence intervals for innovator–innovator exchange (see Appendix for Figures). This finding appears paradoxical for the innovator, since in all bioequivalence studies the same innovator product was used, and therefore the 90% confidence intervals for the innovator–innovator exchange would theoretically be expected to be within the 80–125% criterion for bioequivalence. The fact that the 90% confidence intervals for innovator–innovator comparisons were variable in an analogous manner to that observed for generic–generic exchange indicated that this variation is not due to actual formulation-related differences in exposure, but was related to the above-mentioned interstudy differences.
Considering all the bioequivalence studies assessed in this interstudy comparison, the mean point estimates obtained for the topiramate or gabapentin AUC and Cmax ratios were very close to 100%. The absolute differences in mean bioavailability of the various generic topiramate and gabapentin formulations are relatively low, below 3.55% for AUC0-t and 5.03% for Cmax. These absolute deviations are very much in line with those observed in a recently published FDA examination, using data from more than 2,000 bioequivalence studies of the FDA. In the FDA study, the mean absolute difference in bioavailability in these 2,000 studies was 3.56% and 4.35% for AUC and Cmax respectively [23]. This outcome further strengthens the position that generics will yield comparable efficacy and safety to the innovator formulation [24]. Furthermore, these comparable results between the generics registered in The Netherlands and by the FDA respectively are to be expected, as requirements for generics in the USA and the EU are comparable.
Results obtained upon comparison of the exposure-normalized generic exposure data indicate that for both topiramate and gabapentin, exchanging between different generics in almost all cases results in 90% confidence intervals of the AUC and Cmax ratios within the routine 80–125% range. Only in 4 out of 36 cases did the gabapentin–gabapentin 90% confidence intervals outer margin marginally exceed the 80–125% acceptance range, i.e., by yielding margins for the Cmax ratio of 79.03%, 125.45%, and 125.75%, and 127.62% for the AUC ratio.
Although in isolated cases the estimated 90% confidence intervals upon generic–generic exchange were indeed outside the 80–125% margin that is accepted for generics, it should be considered that the estimated generic–generic 90% confidence intervals are wider than those obtained in a direct head-to-head comparison between generics. This is because the indirect comparison applied in this investigation not only includes intraindividual variability, but also interindividual variability (see the Data analysis section in the Appendix). Indeed, the width of the 90% confidence interval for the between group analysis was, on average, a factor 3 larger than the 90% confidence intervals based on the within-subjects comparisons. Therefore, our method can be considered a conservative approach. An advantage of this conservative method is that when this indirectly estimated 90% confidence interval meets the 80–125% requirement, it is very unlikely that an actual 90% confidence interval obtained with intrastudy analysis will not meet the 80–125% standard. On the other hand, the 80 or 125% border will be crossed more easily with this indirect comparison than would occur with analysis based on intrastudy evaluations.
An advantage of our interstudy comparison is that the comparison is based on actual rather than theoretical exposure data. Therefore, the data are expected to represent the actual variability in exposure. Limitations of this interstudy comparison are that the relative exposure was estimated via an interstudy comparison, and was based on historical data present in the registration files. Furthermore, only topiramate and gabapentin data have been used in this interstudy comparison, results may not be valid for other medicinal products. Further investigation into this matter is therefore warranted. Moreover, confirmation of these data may come from a multiple arm clinical crossover study comparing multiple generic products with a single innovator product. Both types of investigations have been initiated at the Agency.
Another limitation of our study is that although our data indicate that generic–generic interchangeability is not expected to yield significant differences in exposure on a population level, it cannot be excluded that on an individual basis, exposure is different between the innovator and the generic, e.g., because of different intraindividual variability for the innovator and generic drugs. In order to clarify this aspect, intraindividual variability should be determined, for which a replicate cross-over design study, comparing multiple administrations of the innovator and the generic product, is needed. However, although such replicate design data are not available, it was noted that in all individual bioequivalence studies in our database, both for topiramate and gabapentin, the overall variation of exposure in the individual studies was comparable for the innovator and the generic. Therefore, no clear indication of possible differences in intraindividual variation in exposure is apparent from the data.
Furthermore, in this study the 80–125% acceptance was applied. This criterion is in line with the currently used criterion for assessment of new applications for generic anti-epileptics. However, in the current debate, one issue is the question whether anti-epileptic drugs should be considered a narrow therapeutic index drug, since significant differences in adverse events after relatively small differences in exposure are expected [13, 25].
The current data search of the Agency’s database also highlighted the existence of pseudo-variability of generic AEDs on the Dutch market, and probably on other markets as well. Thirteen topiramate and 18 gabapentin generic products are available on the Dutch market, but the registration of many of them is based on the same bioequivalence study, and as such these generics are in fact identical products. Therefore, the number of marketed generics that are really different is less than expected, as many identical generics exist solely for economic reasons, i.e., dossiers are sold between companies and/or products are imported from different EU countries. In those cases, the generics exist with different packaging/appearance whereas the formulations are actually identical. Although this limits the real number of different generics, it is at present virtually impossible for pharmacists and prescribers to identify generics that are actually registered based on a similar/identical dossier.
Our results strongly indicate that the so-called drifting problem upon generic–generic substitution is limited in clinical practice, i.e., does not result in important differences in exposure upon exchanging topiramate generics or gabapentin generics. The data obtained from this comparison may relieve some concerns in the field related to generic–generic substitution, and are therefore considered valuable information in the discussion on the issues surrounding generic–generic substitution. However, it is acknowledged that this conclusion is based on a between-study comparison with a limited number of medicinal products, and further confirmation of these data may come from more extensive interstudy comparisons, as well as a replicate cross-over design study, or multiple arm clinical crossover study comparing multiple generic products with a single innovator product, the latter study allowing for a direct intrastudy comparison of the relative bioavailabilities of different generics.
Conclusion
Our results, based on indirect comparison of relative exposure, strongly indicate that the so-called drifting problem upon generic–generic substitution does not result in major differences in exposure for topiramate and gabapentin generics.
References
Krämer G, Biraben A, Carreno M, Guekht A, de Haan GJ, Jedrzejczak J, Josephs D, van Rijckevorsel K, Zaccara G (2007) Current approaches to the use of generic antiepileptic drugs. Epilepsy Behav 11:46–52. doi:10.1016/j.yebeh.2007.03.014
Versantvoort CHM, Maliepaard M, Lekkerkerker JFF (2008) Generics: what is the role of registration Authorities. Neth J Med 66:62–66
Anderson S, Hauck WW (1996) The transitivity of bioequivalence testing: potential for drift. Intl J Clin Pharm and Therap 34:369–374
Anonymous (1987) For and against generic prescribing. Drug Ther Bull 25:93–95
Besag FMC (2000) Is generic prescribing acceptable in epilepsy? Drug Saf 23:173–182
Commission on Epidemiology and Prognosis, International League Against Epilepsy (1993) Guidelines for epidemiologic studies on epilepsy. Commission on Epidemiology and Prognosis, International League Against Epilepsy. Epilepsia 34:592–596
WHO. Epilepsy fact sheet No999. World Health Organization (January 2009). Available at www.who.int/mediacentre/factsheets/fs999/en/index.html. Accessed 11 November 2010
Welty TE (2007) Pharmacy and generic substitution of antiepileptic drugs: missing in action? Ann Pharmacother 41:1065–1068. doi:10.1345/aph.1K076
Berg MJ (2007) What's the problem with generic antiepileptic drugs? A call to action. Neurology 68:1245–1246. doi:10.1212/01.wnl.0000262876.37269.8b
Heany DC, Sander JW (2007) Antiepileptic drugs: generic versus branded treatments. Lancet Neurol 6:465–468. doi:10.1016/S1474-4422(07)70105-9
Burkhardt RT, Leppik IE, Blesi K, Scott S, Gapany SR, Cloyd JC (2004) Lower phenytoin serum levels in persons switched from brand to generic phenytoin. Neurology 63:1494–1496
Makus KG, McCormick J (2007) Identification of adverse reactions that can occur on substitution of generic for branded lamotrigine in patients with epilepsy. Clin Ther 29:334–341. doi:10.1016/j.clinthera.2007.02.005
Crawford P, Feely M, Guberman A, Kramer G (2006) Are there potential problems with generic substitution of antiepileptic drugs? A review of issues. Seizure 15:165–176. doi:10.1016/j.seizure.2005.12.010
Maliepaard M, Hekster YA, Kappelle A, van Puijenbroek EP, Elferink AJ, Welink J, Gispen-de Wieden CC, Lekkerkerker JFF (2009) Requirements for generic anti-epileptic medicines: a regulatory perspective. J Neurol 256:1966–1971. doi:10.1007/s00415-009-5231-2
Berg MJ, Gross RA, Tomaszewski KJ, Zingaro WM, Haskins LS (2008) Generic substitution in the treatment of epilepsy: case evidence of breakthrough seizures. Neurology 71:525–530. doi:10.1212/01.wnl.0000319958.37502.8e
Krämer GJ, Steinhoff BJ, Feucht M, Pfäfflin M, May TW (2007) Experience with generic drugs in epilepsy patients: an electronic survey of members of the German, Austrian and Swiss branches of the ILAE. Epilepsia 48:609–611. doi:10.1111/j.1528-1167.2007.01084_1.x
Wilner AN (2004) Therapeutic equivalency of generic antiepileptic drugs: results of a survey. Epilepsy Behav 5:995–998. doi:10.1016/j.yebeh.2004.05.011
Berg MJ, Gross RA (2006) Physicians and patients perceive that generic drug substitution of anti-epileptic drugs can cause breakthrough seizures—results from a U.S. survey. First North American Regional Epilepsy Congress: 60th Annual Meeting of the American Epilepsy Society; 1–5 December 2006; San Diego, California. Abstract 2.105.
Andermann F, Duh MS, Gosselin A, Paradis PE (2007) Compulsory generic switching of antiepileptic drugs: high switchback rates to branded compounds compared with other drug classes. Epilepsia 48:464–469. doi:10.1111/j.1528-1167.2007.01007.x
Zachry WM III, Doan QD, Clewell JD, Smith BJ (2008) Case-control analysis of ambulance, emergency room, or inpatient hospital events for epilepsy and antiepileptic drug formulation changes. Epilepsia 50:493–500. doi:10.1111/j.1528-1167.2008.01703.x
Kesselheim AS, Stedman MR, Bubrick EJ, Gagne JJ, Misono AS, Lee JL, Brookhart MA, Avorn J, Shrank WH (2010) Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and meta-analysis. Drugs 70:605–621. doi:10.2165/10898530-000000000-00000
Stewart BH, Kugler AR, Thompson PR, Bockbrader HN (1993) A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm Res 10:276–281
Davit BM, Nwakama PE, Buehler GJ, Conner DP, Haidar SH, Patel DT, Yang Y, Yu LX, Woodcock J (2009) Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother 43:1583–1597. doi:10.1345/aph.1M141
Nightingale SL (1998) Therapeutic equivalence of generic drugs: letter to health practitioners, 1998. Available at: www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ucm073182.htm. Accessed 11 November 2010
Gidal BE (2009) Bioequivalence of antiepileptic drugs: how close is close enough? Curr Neurol Neurosci Rep 9:333–337
Acknowledgements
All authors, except Nikola Banishki, are appointed to the Dutch Medicines Evaluation Board (MEB-CBG). Nikola Banishki has been working as a student at this Agency. All authors had access to the application dossiers filed at the Regulatory Agency. The opinions of the authors expressed in this paper do not necessarily reflect those of the Dutch Medicines Evaluation Board MEB-CBG.
Conflict of interest
None of the authors has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix Fig. 1
(PDF 12 kb)
Appendix Fig. 2
(PDF 18 kb)
ESM 1
(DOC 27 kb)
Rights and permissions
About this article
Cite this article
Maliepaard, M., Banishki, N., Gispen-de Wied, C.C. et al. Interchangeability of generic anti-epileptic drugs: a quantitative analysis of topiramate and gabapentin. Eur J Clin Pharmacol 67, 1007–1016 (2011). https://doi.org/10.1007/s00228-011-1041-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1041-4