Abstract
Background
Spinal-cord injury (SCI) is a leading cause of neuropathic pain (NP). Current pharmaceutical treatments for NP in SCI patients are not effective. Two promising options are gabapentin (GP) and pregabalin (PB). Their predominant mechanism of action is believed to be the inhibition of calcium currents, leading in turn to reduced neurotransmitter release and attenuation of postsynaptic excitability. This could explain much of their efficacy in the treatment of both seizure disorders and pain syndromes. However, evidence for their efficacy in attenuating NP of SCI is still controversial.
Objective
To efficiently integrate valid information and provide a basis for rational decision making, through determining PB and GP efficacy in treating NP in SCI.
Methods
Literature was systematically reviewed. Medline, Embase, CINAHL and Cochrane Database were searched using search terms ‘gabapentin’, ‘pregabalin’, ‘neurontin’, ‘lyrica’, ‘neuropathic pain’ and ‘spinal-cord injury’. Studies were assessed independently by two authors.
Results
Five studies were eligible for inclusion. Two of them studied PB and three GP. Both GP and PB appear to be efficacious for NP in SCI. A clear comparison between the two drugs could not be performed. The literature data suggest that PB is more efficacious than GP in many important variables for NP in SCI, although PB use is followed by more side effects than GP. PB reduced Visual Analogue Score (VAS) in both studies (P < 0.001 and P = 0.016). On the other hand, for GP a maximum dosage of 3,600 mg/day reduced VAS score (P = 0.000), whereas a maximum dosage of 1,200 mg/day failed to do so.
Conclusion
There is a lack of studies comparing GP and PB in treating NP in SCI. This systematic review indicates the possible efficacy of PB and GP in NP of SCI. Recommendations for future research to inform clinical practice should include cost-effectiveness studies and dose-response analysis in order to determine the schema employed and the duration of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropathic pain (NP) is pain initiated or caused by a primary lesion or dysfunction in the nervous system [1]. It has heterogeneous causes such as spinal-cord injury (SCI), stroke, multiple sclerosis, diabetes mellitus and neoplasia [2]. NP is believed to be mediated by multiple mechanisms. It has been suggested that abnormal spinothalamic function with altered sensitivity to temperature and pinprick, neuronal hyperexcitability, excessive firing of pain-mediating nerve cells and insufficient segmental and non-segmental inhibitory circuits are involved [3–5]. The final result is an abnormal pain perception leading to clinical symptoms such as burning, stabbing, and stinging and pain that is similar in quality to electric shock [2].
SCI is one of the leading causes of NP. It is estimated that NP at or below the level of the injury occurs in up to 40% of patients with SCI [6]. It is an intractable type of pain and its prevalence differs, depending on the time since the injury and on the localisation of the pain in relation to the level of the injury [6, 7]. It causes emotional and physical discomfort and is associated with depressive symptoms leading to greater pain intensity [8]. Thus, search for effective treatment options is of a high priority.
Various pharmaceutical treatments have been proposed, such as opioids, anti-depressants, anti-convulsants, baclofen, non-opioid analgesics, alfa-adrenergic agonists and ketamine. However, efficacy is still not satisfactory and the use of many of these agents is often limited by significant side effects [9].
Two promising treatment options are gabapentin and pregabalin. Gabapentin (Neurontin) is an anti-convulsant drug that has become a treatment of choice to manage NP. There are data indicating the efficacy of gabapentin for postherpetic neuralgia [10], diabetic neuropathy [11], cancer pain and other chronic pain states. Preclinical studies in rat models with SCI indicated that gabapentin reduced allodynia [12]. Many clinical studies have been conducted to determine the efficacy of gabapentin on NP in SCI, including retrospective studies [13], uncontrolled open-label trials [14] and randomised controlled trials [15, 16]. However the data are still controversial and some researchers question gabapentin’s role in attenuating NP of SCI [13, 15]. Gabapentin is proven to be well tolerated and results in few side effects and lack of toxicity on any specific organ [9–11, 15–18].
Pregabalin (Lyrica) is also an anti-convulsant that has great bioavailability and safety profile and limited drug interactions. Recently, pregabalin was shown to be effective in randomised clinical trials for post-herpetic neuralgia [19–22] and diabetic peripheral neuropathy [23]. Preclinical data suggest that it reduces neurotransmitter release in hyperexcited neurons [24–26]. Two clinical studies indicate pregabalin efficacy for NP of SCI [27, 28]. A recent review also supports this efficacy, but fails to systematically review the literature and include a comparison with gabapentin [29].
Gabapentin and pregabalin are structurally related compounds. Both drugs are derivatives of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), with gabapentin originally designed as a GABAmimetic agent that could freely cross the blood–brain barrier. Gabapentin recently has received considerable attention as a potential analgesic for neuropathic pain [30]. Pregabalin can be considered as a successor to gabapentin, at least in terms of its basic chemical structure and therapeutic profile. Multiple modest cellular effects have been proposed for gabapentin and pregabalin, including modest actions on the GABAergic system [31] and on voltage-gated potassium channels [32], but a single mechanism of action is believed to predominate their pharmacology and to explain much of their efficacy in the treatment of both seizure disorders and pain syndromes: inhibition of calcium currents via high-voltage-activated channels containing the a2d-1 subunit, leading in turn to reduced neurotransmitter release and attenuation of post-synaptic excitability. This mechanism has consistently been observed at therapeutically relevant concentrations in pre-clinical studies of gabapentin and pregabalin [33, 34].
In order to efficiently integrate valid information and provide a basis for rational decision-making, the literature was systematically reviewed to determine the efficacy of pregabalin and gabapentin in the treatment of NP in SCI.
Methods
Search strategy
We systematically searched MEDLINE and EMBASE from 1980 to January 2008, as well as CINAHL and the Cochrane Controlled Trials Register (CCTR). In order to identify relevant studies, we used as search terms ‘gabapentin’, ‘pregabalin’, ‘neurontin’, ‘lyrica’, ‘neuropathic pain’ and ‘spinal-cord injury’. The reference lists of selected studies and review articles were reviewed for additional citations. No language and publication status restrictions were applied.
Study selection
Studies were assessed independently by two authors (EA, DK) who also independently extracted the data. Eligibility was determined by reading each abstract identified by the search and conclusions were reached by consensus. A study was eligible for inclusion in this review if it was a randomised controlled trial following certain eligibility criteria. Cohort studies, case reports, case series, observational studies and experimental models were excluded. Eligibility criteria were as follows:
-
1.
Population
The studies should have enrolled male and female patients over the age of 18 with SCI suffering from neuropathic pain at or below the level of injury.
-
2.
Intervention
We included studies that compared pregabalin and gabapentin at a licensed therapeutic dose with vehicle or another active treatment.
-
3.
Outcome
The outcomes under analysis were all variables determining efficacy, such as reduction in pain scores and pain relief and improvement in health status and quality of life.
Results
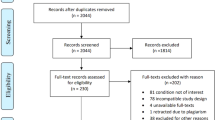
Out of 17 studies retrieved, 5 were eligible for inclusion in our review (see Table 1) [15, 16, 27, 28, 35]. Two of them studied pregabalin and three gabapentin. The three studying gabapentin had a crossover design [15, 16, 35]. Most of the excluded studies were reviews, open-label studies and case series.
Pregabalin for NP in SCI
An outcome assessment for the most important variables for pregabalin efficacy is presented in Table 2. The article by Vranken and colleagues indicates that there was a statistically significant decrease in mean pain score at endpoint for pregabalin treatment (VAS score) [28]. The pregabalin group also showed a statistically significant improvement for the Euro Quality of Life-5 Dimensions (EQ-5D) utility score and Euro Quality of Life-5 Dimensions Visual Analogue Scale (EQ-5D VAS) score compared with the placebo group, suggesting an overall increase in health status [28]. They also report that of the eight domains of the Short-Form Health Survey Questionnaire 36 (SF36) pregabalin treatment led to a significant improvement in the bodily pain domain. On the other hand, the PDI (assessment of disability) outcome did not differ between both groups at the end of the trial [28].
Siddall and colleagues also indicate that pregabalin was superior to placebo on the endpoint mean pain score [27]. The mean reduction from baseline to endpoint on each of the five Short-Form McGill Pain Questionnaire (SF-MPQ) scales and the mean reduction from baseline to endpoint in the sleep interference score was greater in the pregabalin group compared to the placebo [27]. Finally, the mean reduction from baseline to endpoint in the Hospital Anxiety and Depression Scale (HADS) anxiety score was greater in the pregabalin group than in the placebo, but there was no significant difference in the HADS depression score [27].
Overall, a great problem of both studies was study design, as all patients were permitted to remain on existing pain therapies except the ones receiving gabapentin, who were required to discontinue treatment. As Kruszewski and colleagues point out, this intentionally produced a gabapentin withdrawal state [36].
Gabapentin for NP in SCI
An outcome assessment for the most important variables for gabapentin efficacy is presented in Table 2. Levendoglu and collaborators indicate that gabapentin reduced the intensity as well as the frequency of pain [16]. Gabapentin showed efficacy by positively influencing Neuropathic Pain Scale (NPS), Lattinen Test (LQ) and Visual Analogue Scale (VAS) and relieved all neuropathic pain descriptors except the itchy, dull, sensitive, and cold types [16]. It also improved quality of life, since it positively influenced all LQ parameters including subjective pain intensity, pain frequency, disability due to pain and sleep quality. All patients completed this study.
One the other hand, Tai and colleagues indicate that gabapentin had some beneficial effects only on certain types of neuropathic pain and that there was a significant decrease in ‘unpleasant feeling’ but a non-significant decrease in ‘pain intensity’ and ‘burning sensation’ [15]. No significant difference was found among other pain descriptors during the gabapentin and placebo treatment [15]. This study, however, had a very small sample size (seven patients), a low maximum dosage of gabapentin (1,800 mg/day), poor outcome measure (only NPS) and generally poor study design.
Rintala and his team evaluated both amitriptyline and gabapentin [35]. They report that for pain intensity (VAS score) there was no significant difference between gabapentin and diphenhydramine therapy, and that in the amitriptyline group, pain intensity was significantly lower than in the gabapentin or diphenhydramine groups [35]. They also indicate that for patients with high baseline Centre for Epidemiologic Studies Depression Scale-Short Form score (CESD-SF), amitriptyline was more effective than diphenhydramine, and there was a non-significant trend suggesting that amitriptyline may be more effective than gabapentin [35]. Gabapentin was no more effective than diphenhydramine. They also report that there was no significant difference among the medications for those with lower CESD-SF scores [28]. Although this study was well designed, it has various limitations, as Rintala et al. point out [35]. Only 22 patients completed all three phases, an active placebo was used, and most importantly, a low maximum dosage of gabapentin was administered [35]. Rintala et al. used a maximum dosage of 1,200 mg/day [35], whereas Levendoglu et al. [16] administered a maximum dose of 3,600 mg/day, a factor that may explain the difference in gabapentin efficacy. It is worth noting that with a maximum dosage of 3,600 mg/day, each individual side effect did not differ significantly between gabapentin and placebo group with the most common side effect being weakness [35]. Therefore, in our opinion, the dose used by Rintala et al. was not sufficient. Another limitation of Rintala’s study was that all measures were based on self-reports.
In conclusion, the above-mentioned data indicate that maximum dosage and dose-response relationship for gabapentin efficacy in NP of SCI are of great importance. The data also indicate that gabapentin, when used at a maximum dosage of 3,600 mg/day with non-rapid dose escalation (4-week titration period), as Levendoglu et al. [16] suggest, is quite efficacious and with minimal side effects in treating NP of SCI. Thus, the schema employed by Levendoglu et al. could be a proposed treatment as well as a drug schema to be used in future studies evaluating gabapentin. On the other hand, the lower maximum gabapentin dosages with more rapid dose escalation used by the other two studies [15, 35] clearly are not efficacious and should be abandoned as treatment options.
Side effects
Safety and tolerability are issues of great importance when treating NP in SCI because of the chronicity of the condition and the already heavily affected health status of SCI patients.
Gabapentin was generally well tolerated with mild side effects. Levendoglu et al. [16] indicate that each individual side effect did not differ significantly between gabapentin and placebo group with the most common side effect being weakness. Tai et al. [15] also came to the same conclusion. Lastly, Rintala et al. [35] reported that the only side effect that differed significantly from the placebo group was nausea. However, one must keep in mind that this was a triple crossover clinical trial and that diphenhydramine was used as an active placebo [35], a fact that could have obscured possible significant differences regarding the side effects of gabapentin and amitriptyline. Furthermore, withdrawal because of possible side effects occurred five times during the gabapentin phase. Another important fact is that increased spasticity was reported significantly less often during gabapentin therapy than with the other two medications [35]. This evidence is quite important and will be further evaluated in the ‘Discussion’ section.
On the other hand, pregabalin shows a different safety/tolerability profile. Vranken et al. reported that the most frequent adverse effects were those related to the central nervous system such as dizziness, decreased intellectual performance, somnolence and nausea [28]. They also indicated that the effects were mild or moderate in intensity and that their incidence did not differ significantly between treatment groups [28]. However, this trial lasted only for 4 weeks and included a small number of patients (11 pregabalin, 10 placebo). Siddall et al. reported that side effects were generally mild or moderate in intensity and that somnolence and dizziness were the two most common adverse events [27]. They also reported that somnolence resulted in the withdrawal of four patients from pregabalin and none from placebo. Edema and clinically significant weight gain were also reported [27]. More serious side effects were present and reported in 19% of the pregabalin group [27]. What is interesting is that one patient had a withdrawal reaction manifesting as spasticity with impaired coordination. Overall, the group discontinuing treatment due to pregabalin was 62% larger than those discontinuing treatment due to placebo [27]. One also must keep in mind that in both pregabalin studies patients were allowed to remain on existing pain therapy.
Pregabalin vs. gabapentin
A clear comparison between gabapentin and pregabalin cannot be performed. There is not a study directly comparing them. The above-mentioned data suggest that pregabalin is more efficacious than gabapentin in many important variables for NP in SCI. However, methodological errors in all studies make this statement less than conclusive. On the other hand, it is clear that pregabalin use is followed by more side effects than gabapentin, some of them also quite serious. Dosing may play an important role. Dose-response relationship and cost effectiveness are not well established yet.
Discussion
There is a lack of studies, especially randomised controlled trials, comparing gabapentin and pregabalin in treating NP in SCI. The published studies are heterogeneous, use different measure scales, and most importantly, a number of them were poorly designed, especially for the evaluation of gabapentin. Furthermore, the fact that all patients in pregabalin studies were permitted to remain on existing pain therapies except the ones taking gabapentin is a great drawback that can not be ignored. The above evidence-based evaluation indicates the possible efficacy of both pregabalin and gabapentin in NP of SCI.
As far as safety and tolerability are concerned, gabapentin seems to be advantageous. Although poor design and heterogeneity are obstacles in comparing both drugs, serious side effects appeared only in pregabalin treatment. Overall, both drugs had few side effects compared to other treatment options for NP in SCI, such as tricyclic anti-depressants and opioids [37]. Opioids, although a well-established treatment, have side effects such as analgesic tolerance, withdrawal reactions after discontinuation and possibility of addiction that cannot be ignored. [38]. Amitriptyline, a tricyclic anti-depressant, is another agent that has been associated with significant analgesia in different animal models. Although amitriptyline has been a drug of choice for treating pain in people with SCI, only a few studies have described the effect of amitriptyline on chronic pain syndromes in the SCI population. These include two randomised controlled trials [35, 39]. Cardenas and collaborators conducted the first randomised controlled trial of the effectiveness of amitriptyline in relieving pain in patients with SCI and concluded that amitriptyline was not efficacious in relieving pain or improving the quality of life of participants with SCI. The results of that study differed from those of the study of amitriptyline by Rintala et al. who found that amitriptyline was relatively cost effective and more effective than gabapentin in relieving neuropathic pain at or below the level of injury in participants with SCI who have considerable depressive symptomatology. The pain, however, was not completely eliminated, even in those participants for whom amitriptyline was an effective anti-depressant therapy. Furthermore, amitriptyline has considerable side effects, some of which can be serious, particularly in the SCI population (strong anti-cholinergic activity, cardiovascular effects, lowering of the epileptic seizure threshold).
Spasticity is a factor of SCI that is quite important. Data suggest that gabapentin may be effective in controlling some features of spasticity in patients with SCI [40, 41]. Increased spasticity was reported significantly less often during gabapentin therapy than with the other two medications [35]. On the other hand, pregabalin does not seem to exhibit this feature. What is interesting is that in a pregabalin trial, one patient had a withdrawal reaction manifesting as spasticity with impaired coordination [27].
Dosage and schema employed seem to be of great importance, especially for gabapentin. Levendoglu et al. administered a maximum dose of 3,600 mg/day with more efficacy and non-significant side effects, compared to the other two studies, which used 1,800 and 1,200 mg/day respectively. So it appears that quantification of dose-response relationship for efficacy and adverse effects is of great importance.
Future studies with larger sample sizes and possibly higher dosages of GP may help further determine the efficacy of gabapentin and pregabalin in the treatment of SCI-related neuropathic pain. Since pregabalin and gabapentin are still expensive, cost-effectiveness studies should be performed too. Furthermore, individual symptoms of neuropathic pain (including allodynia, burning pain, shooting pain and hyperalgesia) were not scored following pregabalin treatment. In our opinion, a symptom-based analysis should be performed for gabapentin and pregabalin because these specific symptoms may respond differently to treatment.
In this review, it was not possible to draw any conclusions regarding a dose–response effect of pregabalin and gabapentin in central neuropathic pain. However, it is quite clear that recommendations for future research to inform clinical practice should include dose-response analysis, in order to determine the schema employed, the duration of treatment or the method of assessing improvement.
References
Mellegers MA, Furlan AD, Mailis A (2001) Gabapentin for neuropathic pain: systematic review of controlled and uncontrolled literature. Clin J Pain 17:284–295
Jensen TS, Gottrup H, Sindrup SH, Bach FW (2001) The clinical picture of neuropathic pain. Eur J Pharmacol 429:1–11
Belgrade MJ (1999) Following the clues to neuropathic pain. Postgrad Med 106:127–140
Finnerup NB, Jensen TS (2004) Spinal cord injury pain—mechanisms and treatment. Eur J Neurol 11:73–82
Nicholson BD (2004) Evaluation and treatment of central pain syndromes. Neurology 62:30–36
Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ (2003) A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103:249–257
Siddall PJ, Taylor DA, Cousins MJ (1997) Classification of pain following spinal cord injury. Spinal Cord 35:69–75
Cairns DM, Adkins RH, Scott MD (1996) Pain and depression in acute traumatic spinal cord injury: origins of chronic problematic pain? Arch Phys Med Rehabil 77:329–335
Warms CA, Turner JA, Marshall MH, Cardenas CC (2002) Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin J Pain 18:154–163
Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L (1998) Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA 280:1837–1842
Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, LaMoreaux L, Garofalo E (1998) Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 280:1831–1836
Hao JX, Xu XJ, Urban L, Wiesenfeld-Hallin Z (2000) Repeated administration of systemic gabapentin alleviates allodynia-like behaviors in spinally injured rats. Neurosci Lett 280:211–214
To TP, Lim TC, Hill ST, Frauman AG, Cooper N, Kirsa SW, Brown DJ (2002) Gabapentin for neuropathic pain following spinal cord injury. Spinal Cord 40:282–285
Attal N, Brasseur L, Parker F, Chauvin M, Bouhassira D (1998) Effects of gabapentin on the different components of peripheral and central neuropathic pain syndromes: a pilot study. Eur Neurol 40:191–200
Tai Q, Kirshblum S, Chen B, Millis S, Johnston M, DeLisa JA (2002) Gabapentin in the treatment of neuropathic pain after spinal cord injury: a prospective, randomized, double-blind, crossover trial. J Spinal Cord Med 25:100–105
Levendoglu F, Ogun CO, Ozerbil O, Ogun TC, Ugurlu H (2004) Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine 29:743–751
Tomson T, Johannessen SI (2000) Therapeutic monitoring of the new antiepileptic drugs. Eur J Clin Pharmacol 55:697–705
Iorio ML, Moretti U, Colcera S, Magro L, Meneghelli I, Motola D, Rivolta AL, Salvo F, Velo GP (2007) Use and safety profile of antiepileptic drugs in Italy. Eur J Clin Pharmacol 63:409–15
Lesser H, Sharma U, LaMoreaux L, Poole RM (2004) Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology 63:2104–2110
Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE (2005) Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 6:253–260
Rosenstock J, Tuchman M, LaMoreaux L, Sharma U (2004) Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 110:628–638
Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M (2005) Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed dose regimens. Pain 115:254–263
Dworkin RH, Corbin AE, Young JP Jr, Sharma U, LaMoreaux L, Bockbrader H, Garofalo EA, Poole RM (2003) Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 60:1274–1283
Dooley DJ, Mieske CA, Borosky SA (2000) Inhibition of K(+)-evoked glutamate release from rat neocortical and hippocampal slices by gabapentin. Neurosci Lett 280:107–110
Dooley DJ, Donovan CM, Pugsley TA (2000) Stimulus-dependent nodulation of [3H] norepinephrine release from rat neocortical slices by gabapentin and pregabalin. J Pharmacol Exp Ther 295:1086–1093
Maneuf YP, Hughes J, McKnight AT (2001) Gabapentin inhibits the substance P–facilitated K(+)-evoked release of [3H]glutamate from rat caudal trigeminal nucleus slices. Pain 93:191–196
Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK (2006) Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology 67:1792–1800
Vranken JH, Dijkgraaf MG, Kruis MR, van der Vegt MH, Hollmann MW, Heesen M (2007) Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain [Epub ahead of print]. doi:10.1016/j.pain.2007.06.033
Gray P (2007) Pregabalin in the management of central neuropathic pain. Expert Opin Pharmacother 8:3035–3041
Rose MA, Kam PC (2002) Gabapentin: pharmacology and its use in pain management. Anaesthesia 57:451–462
Errante LD, Williamson A, Spencer DD, Petroff OAC (2002) Gabapentin and vigabatrin increase GABA in the human neocortical slice. Epilepsy Res 49:203–210
McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH (2004) A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurons from neonatal rats. BMC Pharmacol 4:14
Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, Gothert M (2002) Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology 42:229–236
Taylor CP (2004) The biology and pharmacology of a2-d proteins. CNS Drug Rev 10:183–188
Rintala DH, Holmes SA, Courtade D, Fiess RN, Tastard LV, Loubser PG (2007) Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch Phys Med Rehabil 88:1547–1560
Kruszewski SP, Shane JA (2007) Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology 68:2158–2159
Watson CP (2000) The treatment of neuropathic pain: antidepressants and opioids. Clin J Pain 16:49–55
Katz N, Benoit C (2005) Opioids for neuropathic pain. Curr Pain Headache Rep 9:153–60
Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD (2002) Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain 96:365–373
Gruenthal M, Mueller M, Olson WL, Priebe MM, Sherwood AM, Olson WH (1997) Gabapentin for the treatment of spasticity in patients with spinal cord injury. Spinal Cord 35:686–689
Priebe MM, Sherwood AM, Graves DE, Mueller M, Olson WH (1997) Effectiveness of gabapentin in controlling spasticity: a quantitative study. Spinal Cord 35:171–175
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tzellos, T.G., Papazisis, G., Amaniti, E. et al. Efficacy of pregabalin and gabapentin for neuropathic pain in spinal-cord injury: an evidence-based evaluation of the literature. Eur J Clin Pharmacol 64, 851–858 (2008). https://doi.org/10.1007/s00228-008-0523-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0523-5