Abstract
Objective
To analyse and discuss the use and the safety profile of individual antiepileptic drugs (AEDs) in Italy.
Methods
The AED safety data referred to the period January 1988–June 2005 and were obtained from the database of the Italian Interregional Group of Pharmacovigilance (GIF). This database collects all spontaneous reports of suspected adverse drug reactions (ADRs) from six Italian regions which are the main contributors to the Italian spontaneous reporting system. Individual AED consumption data (defined daily dose/1,000 inhabitants per day) in the GIF area and in the whole of Italy referred to the period January 2003-June 2005 and were derived from drug sales data (Institute for Medical Statistics Health).
Results
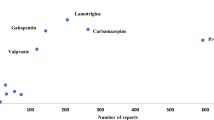
Phenobarbital was the most frequently used AED in the GIF area (4.26 DDD/1,000 inhabitants per day) followed by carbamazepine (1.97), valproic acid (1.33) and gabapentin (1.10). AED consumption in the whole of Italy showed a similar pattern. Gabapentin was the most frequently used AED among newer AEDs. In the GIF database 37,906 reports (up to June 2005) were present; 666 of them (1.76%) were associated with at least one AED (Anatomical Therapeutic Chemical code N03A). The AED with the highest number of reports was carbamazepine (208 reports) followed by phenobarbital (98), gabapentin (80), phenytoin (56), valproic acid (55), lamotrigine (51), oxcarbazepine (43) and vigabatrin (35). Use and toxicity profile were evaluated only for AEDs associated with at least 30 reports. Skin reactions were the most frequently reported ADRs, followed by haematological, general condition, hepatic, neurological and gastrointestinal adverse reactions. Phenobarbital, lamotrigine, carbamazepine and phenytoin had the highest percentage of skin reactions (69, 67, 60 and 54%, respectively). Many haematological reactions were reported for each AED; the highest percentage was related to valproic acid (25%). Vigabatrin was associated with the highest percentage of reactions related to hearing, vision and other senses (97%). Phenytoin and valproic acid had the highest percentage of hepatic reactions (30 and 20%), whereas gabapentin of nervous system, psychiatric, gastrointestinal and urinary reactions (26, 21, 21 and 14%, respectively) and phenobarbital of musculoskeletal reactions (13%).
Conclusions
In Italy antiepileptic drug therapy appears to be still dominated by traditional drugs. Our analysis showed a different safety profile related to each AED. Some of the drug-adverse reaction associations discussed are not included in the Italian drug leaflets or have not been reported before in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is one of the most common serious neurological disorders worldwide; in developed countries the incidence ranges from 30 to 50 per 100,000 individuals per year [1]. In Italy the incidence of epilepsy has been estimated at about 40 per 100,000 inhabitants per year [2].
Antiepileptic drugs (AEDs) are among the most commonly prescribed centrally active agents [3]. In Italy AEDs have been recently ranked as the second most prescribed and costly drug category among drugs acting on the central nervous system [4]. This is due also to the fact that some AEDs are widely used to treat conditions other than epilepsy, including migraine, neuropathic pain, bipolar disorder, anxiety and many other disorders [3].
Until the early 1990s six major compounds (phenobarbital, primidone, phenytoin, ethosuximide, carbamazepine and valproate) were available for the treatment of epilepsy. However, these drugs have pharmacokinetic limitations, teratogenic potential and a negative effect on cognitive functions that impairs the quality of patients lives and limits the use of these drugs in some patients. In addition 20–30% of patients are refractory to these drugs [5]. The development of new AEDs (felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, vigabatrin and zonisamide) has greatly expanded treatment options. Each of the available AEDs differs from the others in terms of pharmacological properties, efficacy spectrum, safety profile, potential interactions and cost [3, 6]. The adverse effects of AEDs are common, may have a considerable impact on patients quality of life, may be potentially life-threatening and contribute to treatment failure in up to 40% of patients [7].
In different studies, the proportion of patients with side effects from AED therapy ranges from less than 10 to over 70% depending on ascertainment methods, characteristics of the patients, AED dosage and duration of follow-up [8]. The tolerability profiles of AEDs differ substantially from one drug to another and it is not straightforward to establish which drug has the best one. However, the safety profile is often a determining factor in drug selection because efficacy rates shown by most AEDs are similar [7]. Clinical trials have provided inconclusive information to evaluate the comparative risk-benefit ratio. In addition, there is a lack of systematic pharmacoepidemiological studies investigating adverse drug reactions (ADRs) to AEDs, which makes it difficult to accurately assess the incidence of anticonvulsant-related ADRs [9, 10]. Moreover, clinical exposure to some of the newer drugs is still relatively limited and experience shows that it may take many years for important adverse effects to be discovered, especially when they are rare [8].
The aim of this study was to analyse the safety profile of individual AEDs in an Italian spontaneous reporting database over the period January 1988–June 2005 and also to evaluate their use in Italy during the period January 2003–June 2005.
Methods
In this study the database of the Italian Interregional Group of Pharmacovigilance (GIF) was analysed. This database collects all spontaneous reports of suspected adverse drug reactions (ADRs) from six Italian regions: Veneto and Autonomous District of Trento (since 1988), Lombardy (since 1993), Sicily (since 1996), Emilia Romagna (since 2000) and Friuli Venezia Giulia (since 2003). This area has a population of about 24 million inhabitants (about 43% of the total Italian population). More than 60% of all Italian spontaneous reports came from these regions where the reporting rate is two times greater than the other Italian regions [11, 12].
In the GIF database reports associated with antiepileptic drugs [Anatomical Therapeutic Chemical (ATC) code N03A] were selected. Benzodiazepines have been excluded since they refer also to other ATC groups. The toxicity profile was evaluated only for antiepileptic drugs associated with at least 30 reports. Each report was classified according to the World Health Organization (WHO) criteria for causality assessment [13], and only reports with a “certain”, “probable” or “possible” causality assessment were included. The drugs were classified following the Italian System Codifa linked to the ATC classification. The reactions were classified according to the WHO adverse reaction terminology and were classified as “serious” or “non-serious” events on the basis of the WHO critical term list [14]. A report was considered serious if it contained one reaction which had been fatal or life-threatening, or had caused or prolonged hospitalisation, or resulted in persistent or significant disability or incapacity or if it contained one ADR on the WHO critical term list.
Both national and regional drug sales data (including unbranded drugs), related to the period January 2003–June 2005, were supplied by Institute for Medical Statistics (IMS) Health. This private company gathers data from different sources including manufacturers, wholesalers and pharmacies. Drug consumption data were expressed as defined daily dose (DDD, average dosage per day for the main indication in adult patients) per 1,000 inhabitants per day [15]. National and regional population data were supplied by the National Institute of Statistics (ISTAT) [16].
We related the number of reports received from all the GIF regions in the period January 2003–June 2005 with the drug use in the same regions and in the same time period to calculate the spontaneous reporting rate (as number of reports/1,000,000 DDD per year) for each AED.
The chi-square test for statistical analysis was used to compare the distribution of causality assessment and adverse reactions among the selected drugs.
Results
In the database 37,906 reports (up to June 2005) were present; 6,131 of them (16.1%) were related to vaccines. The analysis included 666 reports (1.76%) related to at least one antiepileptic drug: 645 of them (96.8%) reported only one suspected AED, 20 reported two and 1 reported three different AEDs. Almost all the reports were sent by physicians (64% by hospital physicians, 35% by general practitioners, 1% by pharmacists). Causality assessment was “certain” in 5% of included reports, “probable” in 60% and “possible” in 34%, with no significant differences among single drugs.
The AED with the highest number of reports was carbamazepine (208 reports) followed by phenobarbital (98 reports) and gabapentin (80 reports) (Table 1). The total number of reports is clearly affected by the length of time it has been on the market and also by how much it is used. Vigabatrin had the highest percentage of serious reactions (97.1%), almost all related to a visual field deficit, whereas gabapentin had the lowest (31.3%).
Table 2 presents AED utilization data in the study area during the period January 2003–June 2005. Phenobarbital was the most commonly used drug (4.26 DDD/1,000 inhabitants per day) followed by carbamazepine (1.97), valproic acid (1.33) and gabapentin (1.10). During the same time period AED national use was similar.
Vigabatrin had the highest reporting rate (10.5 reports/1,000,000 DDD per year) followed by lamotrigine (2.6), phenytoin (2.2), carbamazepine (1.9), gabapentin (1.3), oxcarbazepine (1.3), valproic acid (0.7) and phenobarbital (0.4).
Table 3 shows the distribution of the reported ADRs according to the involved system organ classes (SOC).
The distribution of ADRs was significantly (p < 0.01) different within each SOC and drug. Skin reactions were the most frequently reported ADRs, followed by haematological, general condition, hepatic, neurological and gastrointestinal adverse reactions.
Phenobarbital, lamotrigine, carbamazepine and phenytoin had the highest percentage of skin reactions. Serious skin reactions included 34 cases of Stevens-Johnson syndrome (SJS) and 13 cases of toxic epidermal necrolysis (TEN), mainly associated with phenobarbital (10 SJS, 7 TEN), carbamazepine (13 SJS, 1 TEN), phenytoin (7 SJS, 1 TEN) and lamotrigine (4 SJS, 1 TEN). One case of SJS caused hospitalization and one had a fatal outcome; two cases of TEN required hospitalization and five cases had a fatal outcome. Other serious cutaneous ADRs included erythema multiforme (16), exfoliative dermatitis (8), skin exfoliation (6), eruption with blisters (3) and photosensitivity reaction (1).
Many haematological reactions were reported for each AED (the highest percentage was related to valproic acid). Serious haematological reactions, associated with all the AEDs considered except with vigabatrin, included leukopaenia (22), thrombocytopaenia (17), purpura (15), granulocytopaenia (12), bone marrow depression/aplasia (3) and agranulocytosis (3). Four cases had a fatal outcome: one bone marrow aplasia related to phenytoin, two granulocytopaenias related to phenobarbital and one agranulocytosis related to carbamazepine.
Vigabatrin was associated with the highest percentage of reactions related to hearing, vision and other senses; almost all the reports described vision disorders, including 30 visual field deficits and one optic neuritis.
Hepatic adverse effects were reported for almost all the selected AEDs. Thirty-three percent of hepatic adverse effects were mild, which were alterations of liver enzymes in most cases. The most frequent serious hepatic reaction was hepatitis (24 reports), followed by cholestatic hepatitis (7 reports). Phenytoin and valproic acid had the highest proportion of hepatic adverse reactions. Phenytoin-related reports included nine cases of hepatitis (five cholestatic hepatitis), one of hepatic insufficiency and one of hepatocellular injury; one fatal outcome was reported. Valproic acid-related reports, all of which occurred in paediatric patients, included one case of coma and eight cases of hepatitis (two cholestatic hepatitis); no fatal outcome was reported.
Twelve reports of endocrine system adverse reactions were related to oxcarbazepine, nine of which were cases of hyponatraemia and two of inappropriate secretion of antidiuretic hormone (SIADH).
Gabapentin had the highest proportion of neurological, psychiatric, gastrointestinal and urinary reactions. The highest number of gabapentin-related reports involved the nervous system (21 reports), 5 of which were serious. The most frequently reported neurological reactions were ataxia (five reports) and dizziness (three reports). Seventeen cases of psychiatric reactions were also related to the same drug, three of them were serious (two hallucinations and one suicide attempt). The psychiatric reactions reported most often were somnolence (six reports), followed by confusion (three) and agitation (three). Gabapentin was associated with 17 reports of gastrointestinal reactions, which included 1 case of acute pancreatitis.
Gabapentin had a significantly higher percentage of urinary adverse reactions, including three reports of pollakiuria and one acute renal failure.
Phenobarbital had a significantly higher percentage of musculoskeletal ADRs; six reports were serious including five cases of Dupuytren’s disease.
Discussion
In Italy during the period 2003–2005 the total national AED consumption was 8.41 DDD/1,000 inhabitants per day; differences between the northern (7.51), central (9.50) and southern regions (including isles) (9.00) of Italy have been reported [4, 17]. Our results showed that in the period January 2003–June 2005, both in the study area and in the whole of Italy, phenobarbital was the most frequently used AED followed by carbamazepine and valproic acid. Few studies are available in the literature on AED utilization and they had the main purpose of estimating the prevalence of epilepsy from prescription data [18–20]. In two recent studies conducted in the Netherlands in the period 1995–2001, the three most frequently prescribed AEDs were carbamazepine, phenytoin and valproic acid [21, 22]; in Denmark during the period 1993–2002 the most prescribed AEDs were carbamazepine, phenobarbital and oxcarbazepine [23, 24]. Thus in Italy phenobarbital is used more than in these two countries. Antiepileptic drug therapy appears to be still dominated by traditional drugs; one factor that probably contributes to the modest utilization volume of newer AEDs is their higher cost. In our study gabapentin was used the most among these drugs. This is probably due to its wide use in the treatment of neuropathic pain [25]. Only 5% of gabapentin-associated reports indicated the treatment of epilepsy as the drug’s indication; this could also explain the highest mean age in this group.
The limitations of our study are strictly related to spontaneous reporting. Underreporting is probably the main disadvantage, since the absolute number of ADR reports is not truly known. Variations in underreporting for different drugs and types of ADRs in different populations and at different points in time could also be present. Data from the spontaneous reports when taken alone do not accurately quantify the risk associated with a drug. Estimation of risk requires adequate denominator information on drug utilization, but this is commonly taken from sales data, which may not accurately reflect prescribing and usage levels. The reporting rate may vary over time and be influenced by factors such as media attention. However, in pharmacovigilance it is often useful and necessary to compare safety profile of two or more drugs of the same class. When the drug consumption data are available and the reporting rate is acceptable, we have the opportunity to learn more about the safety of a group of drugs, as from published studies [26, 27].
When related to drug consumption, our data suggest higher reaction rates for vigabatrin and lamotrigine. However, as discussed above, many confounders could influence these values. It is well accepted that the reporting rate of a drug is higher in the first years of marketing (“Weber effect”) and that the number of reports increases when a drug-related adverse event is highlighted by the media (“notoriety bias”) [28, 29].
In the GIF database skin reactions, as for other drug categories [30], were the most frequently reported adverse reactions for anticonvulsant drugs. Serious cutaneous reactions include SJS and TEN. It is well known that AEDs are among the numerous drugs (more than 100) that have been associated with SJS and TEN [31–33]. Risk has been reported to be higher for phenobarbital and lamotrigine [32, 34]. Valproic acid does not seem to increase the risk of SJS and TEN, being associated with these diseases only when used together with lamotrigine [33–35]. In our database phenobarbital, phenytoin and lamotrigine had the highest proportion of SJS and TEN.
In our database one report of TEN, with fatal outcome, was related to gabapentin; the patient was exposed to other suspected drugs and the causal relationship has been classified as “possible”. In published studies no reports of serious rash have been associated with gabapentin treatment [36–38]. In the Italian leaflet SJS or TEN are not reported among skin reactions related to gabapentin. However, in the US package insert, SJS is listed among the adverse events reported from post-marketing experience.
The risk of AED-induced SJS and TEN is largely confined to the first 8 weeks of therapy [32, 34]. In our database all the AED-related TEN cases occurred within the first 38 days of therapy (median: 23 days), whereas all the SJS cases (with only one exception) occurred within the first 65 days (median: 16 days).
AEDs have been associated with severe haematological reactions including leukopaenia, aplastic anaemia, agranulocytosis, and thrombocytopaenia [39, 40]. The incidence of aplastic anaemia and agranulocytosis appears to be 5–8 times greater in patients treated with carbamazepine than in the general population, and it does not appear to be dose related [39]. In our study valproic acid had the highest percentage of haematological reactions. The most widely reported reaction was thrombocytopaenia, known to be usually dose related, moderate and relatively frequent. Occasional fatal bone marrow failure has also been seen in patients taking valproic acid [39] and we found one case in the GIF database.
In the GIF database two reports of leukopaenia were associated with gabapentin. Even if this reaction is present in the drug leaflet, no case report can be found in the literature. In a large post-marketing study, none of the observed haematological events was considered to be associated with gabapentin treatment [37].
In our database almost all reports associated with vigabatrin noted a visual field deficit. About 30% of patients treated with this drug are at risk of irreversible visual field defects. Visual field constriction is now a well-known vigabatrin-related adverse effect; however, many questions related to the incidence and to the mechanism of this association remain unanswered. The prevalence of vigabatrin-associated field defects seems to be lower in children [41–43].
In our study phenytoin and valproic acid had the highest percentages of hepatic reactions. In the literature valproic acid has been reported as the most common AED related to fatal hepatotoxicity. The highest risk (1:500) has been found in children under 2 years of age with complex neurological disorders receiving polytherapy [44, 45]. The risk declines significantly with age and in monotherapy; fatalities beyond 20 years of age are exceedingly rare [11]. In our study about 31% of the total reports associated with valproic acid referred to paediatric patients. However, all the valproic acid-related hepatic reactions occurred in children, even if no fatal outcome was reported.
One case of gabapentin-related hepatitis was present in the GIF database. In two reviews [39, 46] this drug was not associated with severe hepatotoxicity, whereas two case reports of acute liver toxicity induced by gabapentin have been published [47, 48]. In the Italian and US package inserts of gabapentin, only altered liver function tests are reported.
Hyponatraemia and SIADH appear to be common problems with both oxcarbazepine and carbamazepine even if they may rarely lead to serious complications. This effect seems to have been noted more often during treatment with oxcarbazepine [49].
Neurological events have been reported as frequent possible side effects of gabapentin [50, 51]. In a prescription-event monitoring (PEM) study on gabapentin in the UK, as in our study, the most commonly reported adverse events were neurological events; ataxia was ranked sixth with the highest incidence in the first month of treatment. In 50% of cases this reaction caused therapy to be stopped. Gabapentin-related hallucinations, one of the reported psychiatric reactions, was also detected during the PEM study, although in only a small number of patients [37].
Gabapentin was the AED with the highest proportion of urinary adverse reactions. Urinary adverse reactions are not reported in the Italian leaflet for gabapentin. Two case reports described reversible renal dysfunction associated with this drug [52, 53].
In our database five reports of Dupuytren’s disease related to phenobarbital are present. This disease is a progressive fibrotic condition affecting the palmar and digital fascia [54]. In the summary of product characteristics for phenobarbital, connective tissue disorders are not reported as possible adverse side effects. The development of Dupuytren’s contractures has been associated with the use of AEDs, particularly phenobarbital, in many retrospective studies and case reports, with incidence varying between 8 and 57% [55, 56]. However, in a recent case-control study [57], conducted by using the UK General Practice Research Database (GPRD), none of the considered AEDs (carbamazepine, phenytoin, valproic acid and phenobarbital) were significantly associated with Dupuytren’s disease.
Conclusions
In Italy antiepileptic drug therapy appears to be still dominated by traditional drugs. Our analysis showed a different toxicity profile related to each AED. Clinicians should consider carefully this situation and should tailor each AED to individual patients’ characteristics. Finally, some of the drug-adverse reaction associations discussed are not included in the drug leaflets or have not been reported before in the literature.
References
Hauser WA, Annegers JF, Kurland LT (1993) Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 34:453–468
Lega Italiana contro l’Epilessia (LICE) Working Group (1992) Manuale italiano di epilettologia (Ed. Piccin):334–339
Perucca E (2005) An introduction to antiepileptic drugs. Epilepsia 46(Suppl 4):31–37
L’uso dei farmaci in Italia. Rapporto nazionale OsMed. http://www.agenziafarmaco.it (accessed September 2006)
Beghi E (2004) Efficacy and tolerability of the new antiepileptic drugs: comparison of two recent guidelines. Lancet Neurol 3:618–621
LaRoche SM, Helmers SL (2004) The new antiepileptic drugs: scientific review. JAMA 291(5):605–614
Perucca E, Meador KJ (2005) Adverse effects of antiepileptic drugs. Acta Neurol Scand 112(Suppl 181):30–35
Perucca E, Beghi E, Dulac O et al (2000) Assessing risk to benefit ratio in antiepileptic drug therapy. Epilepsy Res 41:107–139
Acharya NV, Pickering RM, Wilton LW, Shakir SA (2005) The safety and effectiveness of newer antiepileptics: a comparative postmarketing cohort study. J Clin Pharmacol 45:385–393
Wong IC, Lhatoo SD (2000) Adverse reactions to new anticonvulsant drugs. Drug Saf 23(1):35–56
Leone R, Conforti A, Venegoni M et al (2005) Drug-induced anaphylaxis case/non-case study based on an Italian pharmacovigilance database. Drug Saf 28(6):547–556
Interregional Group of Pharmacovigilance http://www.gruppogif.org/download/giftot2004.pdf (accessed March 2006)
Olsson S (1998) Role of WHO programme on international drug monitoring in co-ordinating world-wide drug safety efforts. Drug Saf 19:1–10
Uppsala Monitoring Centre (2000) Safety monitoring of medicinal products: guidelines for setting up and running a pharmacovigilance centre. The Uppsala Monitoring Centre, WHO Collaborating Centre for International Drug Monitoring, Uppsala, Sweden
World Health Organization, Collaborating Centre for Drug Statistics Methodology (1998) Guidelines for ATC classification and DDD assignment
National Institute of Statistics. http://demo.istat.it
http://www.ministerosalute.it/medicinali/resources/documenti/osmed/rapporti/consumi_farmaceutici_italia_2004.xls (accessed September 2006)
Banfi R, Borselli G, Marinai C et al (1995) Epidemiological study of epilepsy by monitoring prescriptions of antiepileptic drugs. Pharm World Sci 17:138–140
Lammers MW, Hekster YA, Keyser A et al (1996) Use of antiepileptic drugs in a community-dwelling Dutch population. Neurology 46:62–67
Roberts SJ, Feely M, Bateman DN (1998) Prescribing of antiepileptic drugs in the northern and Yorkshire region: 1992–1995. Seizure 7:127–132
Knoester PD, Belitser SV, Deckers CLP et al (2004) Diffusion of the new antiepileptic drug lamotrigine in Dutch clinical practice. Eur J Clin Pharmacol 60:751–758
Knoester P, Deckers C, van der Vaart R et al (2005) Volume and market share of antiepileptic drugs in the Netherlands: impact of new drugs. Pharm World Sci 27:129–134
Rochat P, Hallas J, Gaist D, Friis ML (2001) Antiepileptic drug utilization: a Danish prescription database analysis. Acta Neurol Scand 104:6–11
Tsiropoulos B, Gichangi A, Andersen M et al (2006) Trends in utilization of antiepileptic drugs in Denmark. Acta Neurol Scand 113(6):405–411
Wiffen PJ, McQuay HJ, Edwards JE, Moore RA (2005) Gabapentin for acute and chronic pain (review). The Cochrane Database of Systematic Reviews (3):CD005452
Almenoff J, Tonning JM, Gould AL et al (2005) Perspectives on the use of data mining in pharmaco-vigilance. Drug Saf 28(11):981–1007
de Vries TW, de Langen-Wouterse JJ, van Puijenbroek E et al (2006) Reported adverse drug reactions during the use of inhaled steroids in children with asthma in the Netherlands. Eur J Clin Pharmacol 62(5):343–346
Hartnell NR, Wilson JP (2004) Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy 24(6):743–749
de Graaf L, Fabius MA, Diemont WL, van Puijenbroek EP (2003) The Weber-curve pitfall: effects of a forced introduction on reporting rates and reported adverse reaction profiles. Pharm World Sci 25(6):260–263
Naldi L, Conforti A, Venegoni M et al (1999) Cutaneous reactions to drugs. An analysis of spontaneous reports in four Italian regions. J Clin Pharmacol 48:839–846
Wolf R, Orion E, Marcos B, Matz H (2005) Life-threatening acute adverse cutaneous reactions. Clin Dermatol 23:171–181
Roujeau JC, Kelly JP, Naldi L et al (1995) Medication use and the risk of Steven-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 333(24):1600–1607
Rzany B, Correia O, Kelly JP et al (1999) Risk of Steven-Johnson syndrome or toxic epidermal necrolysis during first weeks of antiepileptic therapy: a case-control study. Lancet 353:2190–2194
Mockenaupt M, Messenheimer J, Tennis P, Schlingmann J (2005) Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis in new users of antiepileptics. Neurology 64:1134–1138
Dunn N, Wilton L, Shakir S (1999) Stevens-Johnson syndrome and antiepileptics. Lancet 354:1033
Chadwick DW, Anhut H, Greiner MJ et al (1998) A double blind trial of gabapentin monotherapy for newly diagnosed partial epilepsy. Neurology 51(5):1282–1288
Wilton L, Shakir S (2002) A postmarketing surveillance study of gabapentin as add-on therapy for 3100 patients in England. Epilepsia 43(9):983–992
Sabers A, Gram L (2000) Newer anticonvulsants: comparative review of drug interactions and adverse effects. Drugs 60(1):23–33
Arroyo S, de la Morena A (2001) Life-threatening adverse events of antiepileptic drugs. Epilepsy Res 47:155–174
Blackbum SC, Oliart AD, Garcia Rodriguez LA, Perez Gutthann S (1998) Antiepileptics and blood dyscrasias: a cohort study. Pharmacotherapy 18(6):1277–1283
Duncan JS (2002) The promise of new antiepileptic drugs. Br J Clin Pharmacol 53(2):123–131
Eke T, Talbot JF, Lawden MC (1997) Severe persistent visual field constriction associated with vigabatrin. Br Med J 314:180–181
Wilton LV, Stephens MD, Mann RD (1999) Visual field defect associated with vigabatrin: observational cohort study. Br Med J 319:1165–1166
König SA, Siemes H, Blazer F et al (1994) Severe hepatotoxicity during valproate therapy: an update and report of eight new fatalities. Epilepsia 35:1005–1015
Bryant AE, Dreyfuss FE (1996) Valproic acid hepatic fatalities III: US experience since 1986. Neurology 2:465–469
Ahmed SN, Siddiqi ZA (2006) Antiepileptic drugs and liver disease. Seizure 15(3):156–164
Singh BK, White-Scott S (1997) Side effects of add-on gabapentin in individuals with epilepsy, mental retardation, and developmental disabilities. Epilepsia 38(Suppl 8):180
Lasso de la Vega MC, Zapater P, Such J et al (2001) Gabapentin-associated hepatotoxicity. Am J Gastroenterol 96(12):3460–3461
Schmidt D, Elger CE (2004) What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs? Epilepsy Behav 5:627–635
Chadwick D (1994) Gabapentin. Lancet 343:89–91
Lhatoo SD, Wong ICK, Polizzi G et al (2000) Long-term retention rates of lamotrigine, gabapentin, and topiramate in chronic epilepsy. Epilepsia 41:1592–1596
Grunze H, Dittert S, Bungert M, Erfurth A (1998) Renal impairment as a possible side effect of gabapentin. Neuropsychology 38:198–199
Gallay B, de Mattos A, Norman D (2000) Reversible acute renal allograft dysfunction due to gabapentin. Transplantation 70(1):208–209
Hart MG, Hooper G (2005) Clinical associations of Dupuytren’s disease. Postgrad Med J 81:425–428
Arafa M, Noble S, Royle G et al (1992) Dupuytren’s and epilepsy revisited. J Hand Surg [Br] 17B: 221–224
Coral P, Zanatta A, Teive HA et al (1999) Dupuytren’s and Ledderhose’s diseases associated with chronic use of anticonvulsants. Case report (in Portuguese). Arq Neuropsiquiatr 57(3B):860–862
Geoghegan JM, Forbes J, Clark DI et al (2004) Dupuytren’s disease risk factors. J Hand Surg [Br] 29B(5):423–426
Acknowledgements
We thank the Pharmaceutical Departments of Emilia Romagna, Friuli Venezia Giulia, Lombardy, Sicily, Autonomous District of Trento, and the Veneto, and their local Health Districts, for collecting the adverse reaction forms. We also thank GlaxoSmithKline for having provided IMS drug sales data. We are very grateful to Dr. G. De Carli and Dr. E. Formenti for their help and great availability. The authors have no conflicts of interest directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iorio, M.L., Moretti, U., Colcera, S. et al. Use and safety profile of antiepileptic drugs in Italy. Eur J Clin Pharmacol 63, 409–415 (2007). https://doi.org/10.1007/s00228-006-0236-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0236-6