Abstract
Objectives
CYP2D6 drug-metabolising enzyme has been shown to be involved in fluoxetine metabolism in vitro and in vivo. CYP2C9 has also been shown to influence the metabolism of fluoxetine in vitro; however, this relationship has not been studied in humans. The aim of the present study was to evaluate the influence of CYP2D6 and CYP2C9 genotypes on the plasma concentration of fluoxetine and norfluoxetine in psychiatric patients during steady-state conditions.
Methods
White European psychiatric patients (n=64) receiving antidepressant monotherapy with fluoxetine were studied. CYP2D6 and CYP2C9 genotypes were determined by polymerase chain reaction-specific methods. The plasma concentrations of fluoxetine and its metabolite, norfluoxetine, were measured by high-performance liquid chromatography.
Results
The dose-corrected plasma concentrations of fluoxetine were related (P<0.01, r=−0.36) to CYP2D6 genotypes (number of active genes). The fluoxetine/norfluoxetine ratio also correlated (P<0.01, r=−0.39) with the number of active CYP2D6 genes. Among patients with two CYP2D6 active genes, the dose-corrected plasma concentrations of fluoxetine and active moiety (fluoxetine plus norfluoxetine) were significantly (P<0.05) higher in the CYP2C9*1/*2 and CYP2C9*1/*3 genotype groups than in CYP2C9*1/*1. However, dose-corrected (C/D) plasma concentrations of fluoxetine, active moiety and fluoxetine/norfluoxetine ratios were not highly different in the individuals with two mutated alleles as compared with those heterozygous for *2 or *3.
Conclusion
The present results show that CYP2D6 and potentially CYP2C9 genotypes seem to influence fluoxetine plasma concentration during steady-state conditions in patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluoxetine is a widely used selective serotonin reuptake inhibitor (SSRI) antidepressant drug prescribed for a variety of psychopathological conditions, including mood and eating disorders, obsessive–compulsive disorders, and dysthymia [1]. Generally, fluoxetine is regarded as a safe antidepressant medication, although side effects, such as gastrointestinal symptoms, nervousness, anxiety, insomnia or tremor, may frequently occur [2]. Fluoxetine has also been reported to be involved in potentially dangerous pharmacokinetic interactions with warfarin [3, 4, 5], phenytoin [6, 7, 8], tricyclic antidepressants [9, 10, 11] or antipsychotic drugs [12, 13]. Two recent studies have shown that the concurrent use of SSRIs, including fluoxetine, and non-steroid anti-inflammatory drugs (NSAIDs), increased the risk of upper gastrointestinal bleeding [14, 15]. Therefore, these interactions are of major concern, due to the long elimination half-lives of fluoxetine and its major active metabolite, norfluoxetine. As a result, the clinical management of these potential drug interactions may not be easy.
In humans, fluoxetine is mainly metabolised to N-demethylfluoxetine (norfluoxetine), which seems to have a similar pharmacological activity to the parent compound [1]. Other metabolic pathways include glucuronidation of both fluoxetine and norfluoxetine and the formation of multiple poorly characterised metabolites [16]. A great inter-individual variability exists in the plasma concentrations of fluoxetine and norfluoxetine after administration of the same dose of the drug, which may be partly related to the different activity of the drug-metabolising cytochrome P 450 enzymes CYP2D6 and CYP2C9 [16, 17, 18, 19, 20].
CYP2D6 has been shown to determine the plasma concentration of several important drugs (such us antiarrhytmics, tricyclic antidepressants, SSRIs and antipsychotic drugs, inter alia) [21]. CYP2D6 has also been shown to be related to the metabolism of fluoxetine to norfluoxetine in vitro and among healthy volunteers after a single dose [17, 19, 22]. In patients, the plasma concentrations of fluoxetine and norfluoxetine have been shown to be related to CYP2D6 genotype [23]. Furthermore, fluoxetine has been reported to be a potent inhibitor of the enzyme both in vitro and in vivo [24, 25, 26, 27]. Five to ten percent of Caucasians have a genetically determined decreased capacity of the CYP2D6 enzyme due to several inactivating alleles [21, 28]; thus, in those patients, the disposition of fluoxetine may be altered.
The polymorphic CYP2C9 enzyme is also involved in the disposition of such widely used drugs as warfarin, phenytoin, losartan, perazine and NSAIDs [29, 30]. In vitro, fluoxetine is metabolised by CYP2C9 and also inhibits the activity of the enzyme [18, 22, 31]. Thus far, no data about fluoxetine disposition in different CYP2C9 genotypes in humans are available. We have recently described that the frequencies of CYP2C9 allelic variants (*1, *2, *3) in Spaniards are similar to those found among other white European populations [32]. To the best of our knowledge, no study has yet evaluated the effect of both CYP2D6 and CYP2C9 genotypes on the metabolism of fluoxetine among psychiatric patients.
The aim of the present study was to evaluate the influence of CYP2D6 and CYP2C9 genotypes on the plasma concentration of fluoxetine and norfluoxetine in psychiatric patients during steady-state conditions.
Materials and methods
White European psychiatric patients (n=64) without any relevant organic disease were studied. They were outpatients visiting the Mental Health Center of Don Benito (Extremadura, Spain). Ten patients (15.6%) were classified as tobacco smokers, defined as smoking more than ten cigarettes per day. The patients were on continuous oral antidepressant monotherapy treatment with the same fluoxetine dose for at least 45 days (mean±SD: 130±104 days; range 45–365 days) before the study. The concomitant drugs that 40 of the patients were taking were not known as inhibitors or substrates of CYP2D6 or CYP2C9. The concomitant drugs included: acetylsalicylic acid, amiloride, enalapril, atenolol, benzodiazepines, hidroaldosterone, l-dopa, nimodipine, nitroglycerine, spironolactone, sulpiride, tiamazol, zolpiclone and zolpidem. The fluoxetine dose range was from 10 mg/day to 60 mg/day and the average dose was 25.2±10.0 mg/day (mean±SD). The mean age of the patients was 51±15 years (range: 18–77 years). Patients gave their prior written consent to participate in the study. The study was performed according to the Helsinki Declaration and was approved by the ethics committee of the Extremadura University Hospital.
CYP2D6 and CYP2C9 genotyping
Blood samples for CYP2D6 and CYP2C9 genotyping were available for 64 patients. CYP2D6 genotype was determined using genomic DNA purified from peripheral blood leukocytes using the QIAamp DNA Mini Kit (Quiagen, Hilden, Germany). The AmpliTaq Gold System (Perkin-Elmer Inc., Wellesley, USA) was used to amplify the CYP2D6*3, CYP2D6*4 and CYP2D6*6 alleles by tetra-primer polymerase chain reaction (PCR), and the Expand Long Template PCR System (Roche Diagnostics, Basel, Switzerland) was used to detect the CYP2D6*5 allele by multiplex PCR. Amplifications were performed with a PTC-100 thermocycler (MJ Research, Inc. Watertown, USA). Oligonucleotide sequences and PCR conditions for the detection of the CYP2D6*3, *4, *5 and *6 alleles have been described previously [33, 34, 35]. Duplicated CYP2D6 genes (ultrarapid metabolisers) were determined according to a previously published method [36]. PCR products were separated by electrophoresis in agarose gels and visualised by staining with ethidium bromide.
The CYP2C9 genotypes were determined by a PCR-restriction fragment length polymorphism method, as described previously, allowing identification of the allelic variants CYP2C9*1, CYP2C9*2 and CYP2C9*3 [32].
Plasma concentrations of fluoxetine and norfluoxetine
Blood sampling was performed before the morning fluoxetine dose, and the plasma samples were stored at −20°C until measurement. Plasma concentrations of fluoxetine and its metabolite, norfluoxetine, were measured by a validated previously published high-performance liquid chromatography method [37].
Data analysis
Kruskal-Wallis non-parametric test was used to compare the different groups, and Dunn’s post-test was applied for multiple comparisons. Correlations of different variables were tested by non-parametric Spearman’s test. Statistical calculations were carried out using GraphPad Prism 3.02 software (GraphPad Software Inc., http://www.graphpad.com); P<0.05 was considered as significant.
Results
The CYP2D6 and CYP2C9 genotypes of the patients are given in Table 1 and Table 2. There was a great inter-individual variability in plasma concentrations of fluoxetine and norfluoxetine. The steady-state C/D plasma concentrations of fluoxetine showed a higher than 16-fold inter-individual variation (from 2.1 nmol/l/mg to 33.2 nmol/l/mg), while the C/D plasma concentrations of norfluoxetine varied from 2.5 nmol/l/mg to 43.5 nmol/l/mg, (17.4-fold variation). The C/D plasma concentrations of fluoxetine plus norfluoxetine (active moiety) also showed substantial inter-individual variability (4.6 nmol/l/mg to 74.2 nmol/l/mg, 16-fold variation).
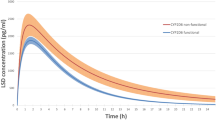
The C/D plasma concentrations of fluoxetine were overall significantly (P<0.01, r=−0.36) related to the number of active CYP2D6 genes. The mean±SD C/D plasma concentrations of fluoxetine (nmol/l/mg) were 16.7 for the only patient with zero active genes, 17.2±8.2 for patients with one active gene (n=19), 13.0±7.6 with two active genes (n=41) and 7.3±4.5 with more than two active genes (n=3). No significant correlation was found between the C/D plasma concentrations of norfluoxetine or active moiety and the number of active genes. The fluoxetine/norfluoxetine ratio was overall significantly correlated (P<0.01, r=−0.39) with the number of active CYP2D6 genes (Fig. 1).
To eliminate the possible confounding effect of CYP2D6, the influence of CYP2C9 was evaluated among patients with two CYP2D6 active genes (CYP2D6*1/*1 genotype) and statistical analyses were carried out among patients carrying CYP2C9*1/*1, CYP2C9*1/*2, or CYP2C9*1/*3 genotypes (n=38). The C/D plasma concentrations of fluoxetine and the active moiety were higher (P<0.05) in the CYP2C9*1/*2 or CYP2C9*1/*3 genotype groups compared with the CYP2C9*1/*1 genotype, while the C/D plasma concentrations of norfluoxetine were higher (P<0.05) in the CYP2C9*1/*3 genotype in comparison with the homozygous CYP2C9 wild-type patients (Table 3). Three patients with genotypes CYP2C9*2/*2, *2/*3 and *3/*3 were found among patients with two CYP2D6 active genes. The C/D plasma concentrations of fluoxetine of these patients were 21.6, 13.0 and 14.1 nmol/l/mg for CYP2C9*2/*2, *2/*3 and *3/*3 genotypes, respectively. The corresponding C/D plasma concentrations of norfluoxetine were 14.8, 19.7 and 14.7 nmol/l/mg. The C/D plasma concentrations of active moiety were 36.4, 32.7 and 28.8 nmol/l/mg. The fluoxetine/norfluoxetine ratio was 1.45 for CYP2C9*2/*2, 0.66 for CYP2C9*2/*3 and 0.96 for CYP2C9*3/*3 genotype patients. Smoking habits had no significant effect on any of the parameters studied (results not shown).
Discussion
There has been an increasing recognition of the importance of CYP2C9 enzyme in the metabolism of several important drugs (such as anticoagulants, losartan, phenytoin, perazine and NSAIDs) [29]. To the best of our knowledge, this is the first study that shows the potential effect of CYP2C9 genotypes on the fluoxetine plasma concentration among psychiatric patients during steady-state conditions; the present results also confirm the influence of CYP2D6 genotypes.
The plasma concentrations of fluoxetine and norfluoxetine in the present patient population were within the range reported in the literature [20, 38], although a great inter-individual variability was found in both fluoxetine and norfluoxetine plasma concentrations as well as in the fluoxetine/norfluoxetine ratio.
The present study suggests the potential involvement of CYP2C9 in fluoxetine metabolism in psychiatric patients, which has only been reported previously for in vitro studies [18, 31]. CYP2C9 genotypes seem to have influence on fluoxetine metabolism, since both fluoxetine and the active moiety plasma concentrations were higher in patients with CYP2C9*1/*2 or CYP2C9*1/*3 genotype than in those with CYP2C9*1/*1 genotype. However, C/D plasma concentrations of fluoxetine, active moiety and fluoxetine/norfluoxetine ratios were not highly different in the individuals with two mutated alleles as compared with those heterozygous for *2 or *3. Thus, data from these single individuals do not clearly support any strong influence of CYP2C9 genotype. We acknowledge that the low number of patients with CYP2C9*3/*3 genotype is a limitation of the present study to conclude the influence of CYP2C9 genotype on fluoxetine plasma concentrations.
Nevertheless, CYP2C9 enzyme activity impairment may also result from concomitant medication, with an inhibitor or substrate of CYP2C9, and a consequence of this interaction could be an alteration of the disposition of fluoxetine and/or the concomitant drug. In light of the present results, the previously reported drug interactions during concomitant administration of fluoxetine with CYP2C9 substrates might be explained [2, 3, 4, 5, 6, 7, 8].
In the present study, fluoxetine/norfluoxetine ratio was correlated with CYP2D6 genotypes and the plasma concentration of fluoxetine was influenced by the number of CYP2D6 active genes. These results and a previous report [23] support that the CYP2D6 enzyme is involved in the metabolism of fluoxetine to norfluoxetine in patients under steady-state conditions. Since the activity of CYP2D6 is polymorphic [21], and, in addition, fluoxetine and norfluoxetine are potent inhibitors of CYP2D6 [17, 18, 19, 20, 22, 23, 24, 25, 26], inter-individual variability of drug and metabolite plasma concentrations might occur during fluoxetine treatment.
Fluoxetine is administered as a racemic drug containing 50% of each of the S- and R-enantiomers of fluoxetine. It would appear that both CYP2D6 and CYP2C9 contribute to the formation of R-norfluoxetine, whereas only CYP2D6 is involved for the conversion to S-norfluoxetine [19, 22, 39]. In the present study, the stereospecific metabolism of fluoxetine enantiomers was not studied, but, rather, the overall effect of CYP2C9 and CYP2D6 enzymes under clinical conditions was evaluated. In a recent study, the plasma levels of individual enantiomers and the active moiety (sum of the concentrations of R-fluoxetine, S-fluoxetine and S-norfluoxetine) in responder patients did not differ significantly from those found in patients with an unsatisfactory therapeutic response [40]. It has also been reported that the concentrations of individual enantiomers and of the active moiety are similar in patients with or without adverse effects [40]. This finding suggests that fluoxetine enantiomers may not be clinically relevant in patients, although this question requires further studies.
In conclusion, CYP2D6 and potentially CYP2C9 genotypes may influence the plasma concentrations of fluoxetine. Since both CYP2C9 and CYP2D6 are involved in the metabolism of several commonly used drugs, interactions with fluoxetine may occur when these drugs are administered together.
References
Altamura AC, Moro AR, Percudani M (1994) Clinical pharmacokinetics of fluoxetine. Clin Pharmacokinet 26:201–204
Preskorn SH (2000) The adverse effect profiles of the selective serotonin reuptake inhibitors: relationship to in vitro pharmacology J Pract Psych Behav Health 6:153–157
Claire RJ, Servis ME, Cram DL Jr (1991) Potential interaction between warfarin sodium and fluoxetine. Am J Psychiatry 148:1604
Woolfrey S, Gammack NS, Dewar MS, Brown PJ (1993) Fluoxetine-warfarin interaction. BMJ 307:241
Dent LA, Orrock MW (1997) Warfarin-fluoxetine and diazepam-fluoxetine interaction. Pharmacotherapy 17:170–172
Jalil P (1992) Toxic reaction following the combined administration of fluoxetine and phenytoin: two case reports. J Neurol Neurosurg Psychiatry 55:412–413
Darley J (1994) Interaction between phenytoin and fluoxetine. Seizure 3:151–152
Woods DJ, Coulter DM, Pillans P (1994) Interaction of phenytoin and fluoxetine. N Z Med J 107:19
Preskorn SH, Beber JH, Faul JC, Hirschfeld RM (1990) Serious adverse effects of combining fluoxetine and tricyclic antidepressants. Am J Psychiatry 147:532
Balant-Gorgia AE, Ries C, Balant LP (1996) Metabolic interaction between fluoxetine and clomipramine: a case report. Pharmacopsychiatry 29:38–41
Preskorn SH, Baker B (1997) Fatality associated with combined fluoxetine-amitriptyline therapy. JAMA 277:1682
Michalets EL, Smith LK, Van Tassel ED (1998) Torsade de pointes resulting from the addition of droperidol to an existing cytochrome P 450 drug interaction. Ann Pharmacother 32:761–765
Spina E, Avenoso A, Scordo MG, Ancione M, Madia A, Gatti G, Perucca E (2002) Inhibition of risperidone metabolism by fluoxetine in patients with schizophrenia: a clinically relevant pharmacokinetic drug interaction. J Clin Psychopharmacol 22:419–423
Dalton SO, Johansen C, Mellemkjaer L, Norgard B, Sorensen HT, Olsen JH (2003) Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding: a population-based cohort study. Arch Intern Med 163:59–64
de Abajo FJ, Rodriguez LA, Montero D (1999) Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case-control study. BMJ 319:1106–1109
Hiemke C, Hartter S (2000) Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther 85:11–28
Hamelin BA, Turgeon J, Vallee F, Belanger PM, Paquet F, LeBel M (1996) The disposition of fluoxetine but not sertraline is altered in poor metabolizers of debrisoquin. Clin Pharmacol Ther 60:512–521
von Moltke LL, Greenblatt DJ, Duan SX, Schmider J, Wright CE, Harmatz JS, Shader RI (1997) Human cytochromes mediating N-demethylation of fluoxetine in vitro. Psychopharmacology 132:402–407
Margolis JM, O’Donnell JP, Mankowski DC, Ekins S, Obach RS (2000) (R)-, (S)-, and racemic fluoxetine N-demethylation by human cytochrome P 450 enzymes. Drug Metab Dispos 28:1187–1191
Lundmark J, Reis M, Bengtsson F (2001) Serum concentrations of fluoxetine in the clinical treatment setting. Ther Drug Monit 23:139–147
LLerena A, Cobaleda J, Martínez C, Benítez J (1996) Interethnic differences in drug metabolism: influence of sex-related and environmental factors on debrisoquine hydroxylation phenotype. Eur J Drug Metab Pharmacokinet 21:129–138
Ring BJ, Eckstein JA, Gillespie JS, Binkley SN, Vandenbranden M, Wrighton SA (2001) Identification of the human cytochromes P 450 responsible for in vitro formation of R- and S-norfluoxetine. J Pharmacol Exp Ther 297:1044–1050
Eap CB, Bondolfi G, Zullino D, Savary-Cosendai L, Powell-Golay K, Kosel M, Baumann P (2001) Concentrations of the enantiomers of fluoxetine and norfluoxetine after multiple doses of fluoxetine in cytochrome P 4502D6 poor and extensive metabolizers. J Clin Psychopharmacol 21:330–334
Skjelbo E, Brosen K (1992) Inhibitors of imipramine metabolism by human liver microsomes. Br J Clin Pharmacol 34:256–261
Crewe HK, Lennard MS, Tucker GT, Woods FR, Haddock RE (1992) The effect of selective serotonin re-uptake inhibitors on cytochrome P 450 2D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol 34:262–265
Alfaro CL, Lam YW, Simpson J, Ereshefsky L (2000) CYP2D6 inhibition by fluoxetine, paroxetine, sertraline, and venlafaxine in a crossover study: intraindividual variability and plasma concentration correlations. J Clin Pharmacol 40:58–66
Vandel S, Bertschy G, Baumann P, Bouquet S, Bonin B, Francois T, Sechter D, Bizouard P (1995) Fluvoxamine and fluoxetine: interaction studies with amitriptyline, clomipramine and neuroleptics in phenotyped patients. Pharmacol Res 31:347–353
Bertilsson L, Dahl ML, Dalen P, Al-Shurbaji A (2002) Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol 53:111–122
Miners JO, Birkett DJ (1998) Cytochrome P 450 2C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol 45:525–538
Dahl ML (2002) Cytochrome P 450 phenotyping/genotyping in patients receiving antipsychotics: useful aid to prescribing? Clin Pharmacokinet 41:453–470
Schmider J, Greenblatt DJ, von Moltke LL, Karsov D, Shader RI (1997) Inhibition of CYP2C9 by selective serotonin reuptake inhibitors in vitro: studies of phenytoin p-hydroxylation. Br J Clin Pharmacol 44:495–498
Dorado P, Berecz R, Norberto MJ, Yasar Ü, Dahl ML, LLerena A (2003) CYP2C9 genotype and diclofenac metabolism in healthy Spanish volunteers. Eur J Clin Pharmacol 59:221–225
Marez D, Legrand M, Sabbagh N, Guidice JM, Spire C, Lafitte JJ, Meyer UA, Broly F (1997) Polymorphism of the cytochrome P 450 CYP2D6 gene in a European population: characterization of 48 mutations and 53 alleles, their frequencies and evolution. Pharmacogenetics 7:193–202
Gaedigk A, Gotschall RR, Forbes NS, Simon SD, Kearns GL, Leeder JS (1999) Optimization of cytochrome P 450 2D6 (CYP2D6) phenotype assignment using a genotyping algorithm based on allele frequency data. Pharmacogenetics 9:669–682
Hersberger M, Marti-Jaun J, Rentsch K, Hanseler E (2000) Rapid detection of the CYP2D6*3, CYP2D6*4, and CYP2D6*6 alleles by tetra-primer PCR and of the CYP2D6*5 allele by multiplex long PCR. Clin Chem 8:1072–1077
Lundqvist E, Johansson I, Ingelman-Sundberg M (1999) Genetic mechanisms for duplication and multiduplication of the human CYP2D6 gene and methods for detection of duplicated CYP2D6 genes. Gene 226:327–338
LLerena A, Dorado P, Berecz R, Gonzalez A, Norberto MJ, de la Rubia A, Caceres M (2003) Determination of fluoxetine and norfluoxetine in human plasma by high-performance liquid chromatography with ultraviolet detection in psychiatric patients. J Chromatogr B 783:25–31
Kelly MW, Perry PJ, Holstad SG, Garvey MJ (1989) Serum fluoxetine and norfluoxetine concentrations and antidepressant response. Ther Drug Monit 11:165–170
Fjordside L, Jeppesen U, Eap CB, Powell K, Baumann P, Brosen K (1999) The stereoselective metabolism of fluoxetine in poor and extensive metabolizers of sparteine. Pharmacogenetics 9:55–60
Jannuzzi G, Gatti G, Magni P, Spina E, Pacifici R, Zuccaro P, Torta R, Guarneri L, Perucca E (2002) Plasma concentrations of the enantiomers of fluoxetine and norfluoxetine: sources of variability and preliminary observations on relations with clinical response. Ther Drug Monit 24:616–627
Acknowledgements
The collaboration of Macarena C. Cáceres and Alfredo de la Rubia is acknowledged. This study was supported by a grant from the Spanish Ministry of Health (Instituto Carlos III, FIS 01/0699). R. Berecz was supported by the Hungarian-Spanish Intergovernmental Scientific and Technology Cooperation project (E-45/2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
LLerena, A., Dorado, P., Berecz, R. et al. Effect of CYP2D6 and CYP2C9 genotypes on fluoxetine and norfluoxetine plasma concentrations during steady-state conditions. Eur J Clin Pharmacol 59, 869–873 (2004). https://doi.org/10.1007/s00228-003-0707-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-003-0707-y