Abstract
Sea turtles are migratory animals that travel from foraging grounds to specific nesting beaches every few years and that, therefore, can be influenced by oceanographic conditions in several different habitats. We assessed how sea surface temperature (SST) and chlorophyll-α (Chl-α) within both internesting and foraging areas influence the nesting abundance, phenology and duration of internesting periods of the green turtles (Chelonia mydas) that nest at Cabuyal, Northwest Costa Rica. Specifically, we compared (1) SST and Chl-α in foraging areas to the nesting abundance and median nesting date (MND) registered on the beach over seven nesting seasons and (2) SST in internesting habitats to the observed internesting period (OIP) (number of days between successful nesting events) and the MND. Nesting abundance was strongly correlated to Chl-α concentrations at the main foraging area during the February preceding the nesting season. However, we found no significant effect of SST or Chl-α in either foraging or internesting habitats on the MND. Mean SST values in the internesting habitats and OIP were negatively correlated and were highly variable both between and within years. Oceanographic conditions appear to strongly influence OIP and nesting abundance, but not the nesting phenology of green turtles in this area. The complex nature of the effect of oceanographic conditions on reproduction of the East Pacific green turtle suggests uncertainty in how this species will respond to climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Variability in oceanographic conditions has a significant impact on the reproductive cycles and behavior of countless species, including invertebrates (Angeles-Gonzalez et al. 2017), fish (Potts et al. 2014) and birds (Monticelli et al. 2014). However, not all species react the same way and variation can even exist between con-specific populations (Edwards and Richardson 2004; Both et al. 2009; Mazaris et al. 2013; Bates et al. 2018). Sea turtles are ectotherms that spend their lives in the ocean but nest on tropical, subtropical and temperate beaches. Since they are migratory animals, sea turtles are exposed to different marine environments as they travel between the foraging grounds and the nesting beaches. Therefore, understanding how sea turtle populations respond to climate and oceanographic variations across the multiple habitats that they inhabit is central to assessing how these species will respond to long-term climate variations such as those projected by current climate change models.
Female sea turtles lay several clutches in one nesting season (Hirth 1980). During the time between one successful nesting event and the next in the same nesting season, termed the internesting period (Reina et al. 2002; Blanco et al. 2012), the eggs for the next clutch develop. The rate at which this occurs is influenced by temperature (Sato et al. 1998) and thus, there is an effect of ambient water temperatures on the duration of the internesting period. This effect has been studied in loggerhead turtles (Caretta caretta) in Japan (Sato et al. 1998) and green turtles (Chelonia mydas) in Cyprus and Ascension Island (Hays et al. 2002). Preliminary studies have also shown that in North Pacific Costa Rica, the duration of the internesting period of green turtles increases as the season advances (Santidrián Tomillo et al. 2015); however, it has not been yet confirmed whether this is related to seasonal changes in water temperatures.
Changes in the temperature in the foraging grounds and/or internesting habitats can also affect the phenology of the nesting season, as temperature may serve as a cue that indicates the right time for nesting. Increasing surface temperature seems to lead to earlier nesting, for example, in some populations of green turtles (Weishampel et al. 2010), while others seem to remain unaffected (Pike 2009). This ability to adjust phenology may allow sea turtles to counteract a potential negative effect of high temperatures on their populations (i.e., increased embryo mortality or skewed sex ratios) by nesting earlier (Almpanidou et al. 2018). However, nesting earlier may not always relate to more suitable conditions for nesting.
Oceanographic conditions at the foraging areas can influence reproduction of sea turtles by affecting variability in their reproductive frequency (Broderick et al. 2001; Solow et al. 2002; Vander Zanden et al. 2014; Hart et al. 2015). For example, decreased productivity in the eastern Pacific Ocean during El Niño years associated with high sea surface temperatures (SST), prolong the duration of the remigration interval of leatherback turtles due to poor feeding conditions (Saba et al. 2007). In turn, when conditions are optimal, remigration intervals are reduced, often resulting in more turtles on the nesting beaches. Chlorophyll-α (Chl-α) also serves as an indicator of primary productivity and possible prey availability (Chaloupka et al. 2008). In addition to the effect aforementioned, chlorophyll fronts also affect movements of loggerhead and olive ridley (Lepidochelys olivacea) turtles at their foraging grounds in the west Pacific (Polovina et al. 2004), as well as post-nesting movements of green turtles in the east Pacific Ocean (Seminoff et al. 2008).
Due to the various ways in which oceanographic conditions at different locations affect sea turtle reproductive biology, it makes sense that these different factors are assessed alongside each other. However, most studies tend to focus on a single oceanographic variable and its effect on a single aspect of the reproductive behavior of turtles or at a particular location. To address this issue, we aimed to assess the effect of SST and Chl-α at both nesting and foraging sites on the nesting abundance, phenology and duration of the internesting period of green turtles at Cabuyal, North Pacific Costa Rica. Previous telemetry studies have identified foraging and internesting areas for this particular nesting population (Clyde-Brockway 2014), which facilitates the study. In addition, green turtles in the eastern Pacific feed primarily on marine algae and sea grass and secondarily, on marine invertebrates consumed incidentally, deliberately or opportunistically (Seminoff et al. 2002; Quiñones et al. 2010). As these prey items are strongly tied to oceanographic conditions, we expect to find an important effect of oceanographic features at foraging grounds at least on nesting abundance. Finally, analyzing the effects of temperature and ocean productivity on green turtles at different times during their reproductive cycles (nesting and foraging) may help us to infer the impacts that future climate change may have on their populations.
Materials and methods
We conducted beach patrols at night at Cabuyal (10°40′N, 85°39′W), Northwest Costa Rica, over seven consecutive nesting seasons between 2011/2012 and 2017/2018. We focused our patrolling efforts to the nesting season (September to March) each year as this is when the majority of nesting occurs (Santidrián Tomillo et al. 2015). Because the exact date when we started and ended the monitoring season varied by a few days, we only utilized data collected between September 9th and March 5th (which corresponds to ~ 87% of the total number of nests registered during the season) to make comparisons between seasons.
Night patrols extended from 20:00 to 4:00 during the high season (October to February) and from 21:00 to 3:00 during low season (September and March). In addition, we conducted daily morning surveys to verify all nesting activities from the previous night and to record any nesting activity that may have been missed on the night patrols, so we could estimate beach coverage. When turtles were found, they were identified using both INCONEL metal tags and PIT tags placed in the front right flipper (Santidrián Tomillo et al. 2015). Tagging was only conducted after nesting was completed to minimize the impact on the egg laying process. We measured the curved carapace length (CCL) and width (CCW) of the turtle and recorded if she laid eggs or aborted the nesting attempt. When she laid eggs, we marked the nest with flagging tape and monitored it throughout the season.
To compare the number of turtles among nesting seasons, we had to correct for differences in beach coverage. We estimated the total number of turtles in a season adding the number of turtles identified to the estimated number of unidentified turtles. We used the following equation to estimate the number of unidentified turtles as in Reina et al. (2002): N = (U × S) (1 − B)ECF, where N is the number of unidentified turtles, U the number of body pits for which the turtle was unknown (turtle missed but body pit found in the morning), S the nesting success ratio (successful egg laying events/body pit attempts), B the beach coverage and ECF the mean estimated clutch frequency in a given season. Beach coverage was the ratio between the number of identified turtles to the number of nesting attempts (Table 1).
Internesting and foraging habitats
We were able to define internesting and foraging habitats for the turtles nesting on Cabuyal by referring to satellite tracking studies that have previously been conducted at Cabuyal and nearby nesting beaches (Blanco et al. 2012; Clyde-Brockway 2014; Robinson et al. 2017).
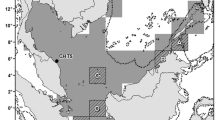
Foraging grounds were divided into two locations (Fig. 1). The northern site comprised an area between the south of Mexico and the Gulf of Papagayo along the Pacific coast, including the Gulf of Fonseca, a major foraging area identified for the green turtles that nest in Northern Costa Rica (Blanco et al. 2012; Clyde-Brockway 2014). The southern area extended between the Gulfs of Papagayo and Panama and was selected because one of the green turtles tracked by Blanco et al. (2012) from Nombre de Jesús moved in that direction. Both areas were delimited using the 200-m isobaths as previous studies have found that green turtles in this area stay close to the coast and mainly within the 100-m isobaths (Blanco et al. 2012). To make sure we included all turtles, we expanded it to the 200-m isobaths (Fig. 2).
Relationship between sea surface temperature (SST) and observed internesting period (OIP) of green turtles (C. mydas) nesting at Playa Cabuyal, Costa Rica, during seven different nesting seasons. a 2011/2012 (R2 = 0.30; p < 0.001); b 2012/2013 (R2 = 0.43; p < 0.001); c 2013/2014 (R2 = 0.25; p < 0.001); d 2014/2015 (R2 = 0.42; p < 0.001); e 2015/2016 (R2 = 0.47; p < 0.001); f 2016/2017 (R2 = 0.45; p > 0.05); g 2017/2018 (R2 = 0.32; p < 0.05)
Daily SST data for the internesting habitats were obtained for the time period 2011–2018 from NASA spacecraft multi-scale ultra-high-resolution (MUR) sensor at a 0.01° × 0.01° spatial grid. SST and Chl-α from the foraging habitats were obtained for the same time period from NOAA spacecraft GOES imager sensor, at a 0.05° × 0.05° spatial grid, for a 3-day composite and from Aqua MODIS sensor at a 4 km × 4 km spatial grid for a 1-day composite, respectively.
Local SST and observed internesting period (OIP)
We calculated the duration of the OIP by counting the number of days between two consecutive nesting events, only when successful egg laying was verified. Values that were double the smallest OIP recorded in each season were excluded, because the clutch could have been missed or the turtles could have nested elsewhere in between the observed nesting events (Reina et al. 2002).
We used daily mean SST within the internesting area to determine the mean temperature for each internesting period which we compared later to the duration of the OIP.
SST, Chl-α, nesting phenology and nesting abundance
Each season, the exact dates that patrolling begins and ends may differ by a few days. Thus, we only used data collected between September 9th and March 5th (period covered in all seasons) to estimate median nesting date (MND) and length of the nesting season (meaning the time between the dates when 25% and 75% of nesting activities were registered). To estimate MND and length of nesting season, we used the number of emergences. Because green turtles in this area have a high rate of nest abandonment (54%, Santidrián Tomillo et al. 2015) and it is not always possible to determine if a turtle laid eggs, we used number of emergences instead of number of verified nests to estimate MND and length of the season. Number of emergences corresponded to the total number of events registered on the beach, including successful and unsuccessful nesting. The season comprises two calendar years as it starts in September of one year and extends to March of the following year. MND and percentiles were calculated using Julian dates for the time period starting on January 1st prior to the beginning of the nesting season (day 1) and until the season ended.
We compared nesting phenology (MND and length of the nesting season) to monthly values of SST and Chl-α levels from the foraging and internesting grounds. Based on Blanco et al. (2012), turtles in this area moved at a rate of 37 km per day after nesting and had short or long migrations that were 62–98 km and 270–1086 km, respectively. Based on that, turtles could spend up to 1 month migrating. However, because return migratory patterns have not yet been identified and we do not know if turtles migrate ahead of time, we have used monthly values of SST and Chl-α levels for 15 months starting in the January of the year the nesting season starts and finishing in the last month of the nesting season (March). Then we compared the correlation values obtained to assess which month gave the stronger signal.
We considered two variables of nesting abundance, the number of nests registered on the beach and the number of female turtles identified during each nesting season. We estimated the total number of nests by multiplying the number of females identified on the beach by the mean estimated clutch frequency (ECF) of the season, which corrects for clutches that could have been missed. Nesting abundance, phenology, and duration of the nesting season were also compared to the monthly SST and Chl-α averages, calculated for the northern and southern foraging areas including several months preceding to each nesting season (January to October), to allow for enough time to store fat reserves and migrate to the nesting beach.
Statistical analysis
We used SPSS v. 23 (IBM 2015) to run all statistical tests. We tested for normality of data using the Shapiro–Wilk test. Data for OIP, SST at internesting grounds and Chl- α at foraging grounds were not normally distributed (Shapiro–Wilk: p < 0.001). However, we could not reject the hypothesis that SST at foraging grounds, number of nests and number of turtles followed a normal distribution (Shapiro–Wilk: p > 0.05). Because some of the data did not have a normal distribution, we used Kruskal–Wallis to compare means with a post hoc analysis to determine where the differences were when present. We used a linear regression to test the relation between local SST and OIP for all seasons together and for each season separately. We ran Kendall correlations to compare MND and total length of the nesting season to SST at internesting areas and SST and Chl-α at the foraging grounds. We also used Kendall correlations to compare SST and Chl-α of the foraging grounds to the number of turtles and the number of nests identified on the beach during each nesting season. We compared monthly SST and Chl-α values between the two foraging sites using a univariate analysis of variance with a random effect to prevent pseudoreplication. We used a nested term as random effect to include the variable month in the variable year.
Results
Local SST and internesting period
Observed internesting period varied between 9 and 21 days (mean ± SD 14.3 ± 2.5) and mean SST between 25.0 and 30.0 °C (mean ± SD 27.5 ± 1.0). Duration of the OIP was negatively correlated to SST during all seasons (linear regression: R2 = 0.52, p < 0.001, Fig. 2), with shorter OIPs during warmer years (Table 2).
SST declined and OIP was longer as the season progressed in all years (Fig. 3). A statistical significant difference was found between both mean SST (Kruskal–Wallis: p < 0.001) and mean OIP (Kruskal–Wallis: p < 0.001) between years. Post hoc tests indicated that these differences were primarily found between the coolest seasons (2011/2012 and 2017/2018) and the warmest ones (2012/2013, 2013/2014, 2014/2015 and 2015/2016, p < 0.05 in all cases). The warmest season (2015/2016) showed significant differences with all other years (p < 0.05 in all cases) (see Table S1 for details of the statistical results).
Seasonal changes in the duration of the observed internesting period (OIP, solid circles) of green turtles (C. mydas) and local sea surface temperature (SST, gray line) along seven different nesting seasons at Cabuyal, Costa Rica. a 2011/2012; b 2012/2013; c 2013/2014; d 2014/2015; e 2015/2016; f 2016/2017; g 2017/2018
SST, Chl-α concentration and nesting phenology
The length of the nesting season ranged between 59 and 121 days (mean ± SD 83.6 ± 23.3 days). Mean local SST during the months of the nesting season (September to March) varied between 27 °C and 29 °C (mean ± SD 27.7 °C ± 0.6), being highest during 2015–2016 (mean ± SD 28.8 ± 1.0) and lowest during 2011/2012 (mean ± SD 27.1 °C ± 1.3) (Table 3). We found no statistically significant correlation between SST and MND (p > 0.05, in all cases) and between SST and length of the season (p > 0.05, in all cases) (see Table S2 for details of the statistical results).
We found statistically significant differences in SST between the northern and southern foraging grounds and between months of the year (p < 0.001 both cases). For Chl-α, we found significant differences between months (p < 0.001), but not between foraging areas (p = 0.099). SST at the foraging areas was lowest in February (mean ± SD 27.4 °C ± 0.5) for the northern area and March (mean ± SD 26.8 °C ± 0.8) for the southern one, and highest in August (mean ± SD 29.6 °C ± 0.5) for the northern area and in May (mean ± SD 28.5 °C ± 0.6) for the southern one (Table 2). We found no statistically significant correlations between MND or length of the season and SST (mean or by month) at either foraging area (p > 0.005 all classes) (see Table S3 for details of correlation results).
In contrast to SST, Chl-α concentration at the foraging areas were highest in March (mean ± SD 1.6 ± 0.9 mg m−3) for the northern area and in February (mean ± SD 1.8 ± 0.4 mg m−3) for the southern one, and lowest in May (mean ± SD 0.4 ± 0.1 mg m−3) for the northern area and in July (mean ± SD 0.7 ± 0.2 mg m−3) for the southern one (Table 4).
Effect of SST and Chl-α concentration at foraging areas on nesting abundance
We found a strongly positive relationship between Chl-α concentrations at the northern foraging site in the month of February preceding the nesting season and the number of turtles (Kendall correlation: r = 0.90, p < 0.01, n = 7) and number of nests registered on the beach (Kendal correlation: r = 0.905, p < 0.01, n = 7), and a weaker but significant relationship between Chl-α levels at the same area in the months of January and March and the number of turtles (Kendall correlation: r = 0.714, p < 0.05, n = 7 and r = 0.714, p < 0.05, n = 7 for January and March, respectively) and number of nests (Kendall correlation: r = 0.714, p < 0.05, n = 7 and r = 0.714, p < 0.05, n = 7, respectively). High Chl-α levels in the month of February resulted in high number of turtles and nests (Fig. 4).
We found no statistically significant relationships between mean Chl-α for any month at the southern foraging site and nesting abundance registered on the beach (p > 0.05 all classes, S 5). Likewise, we did not detect any effect of SST at either foraging site on nesting abundance (p > 0.05 all cases, see Table S6).
Discussion
Effect of local SST on the duration of the internesting period
Observed internesting period of green turtles at Cabuyal varied with SST in all years, increasing towards the end of the season as SST declined. Water temperature in the Gulf of Papagayo is marked by a strong seasonality that is characterized by a dramatic decrease between December and March, with the lowest temperatures in January. This strong change in water temperature is driven by the occurrence of seasonal win patterns that lead to significant deep-water upwelling from November to May (Brenes 2001; Alfaro and Cortés 2012). Thus, turtles arriving early or late in the season may encounter completely different oceanographic conditions, which likely affect their reproduction.
The relationship between SST and OIP has been previously reported for green and loggerhead turtles at other locations (Sato et al. 1998; Hays et al. 2002), which supports the idea that the relationship between these two factors occurs globally at least for these species. Water temperature influences metabolic rate and reproductive cycles of green turtles, as the difference between body and water temperature is minimum (Sato et al. 1998). As a result, cool water temperatures can extend the internesting period. In turn, this could even lead to more time in the reproductive area in cold years or for turtles that nest late in the season. The effect of such an extension on the turtles is unknown. However, since green turtles are mostly aphagic while breeding, spending extra time around the beach could be challenging if lipid reserves drop below critically low levels. On the other hand, this could lead to the atresia and reabsorption of follicles, to be used as an extra source of energy (Limpus et al. 2002; Hamann et al. 2003), reducing the number of clutches but allowing the turtle to stay alive.
The average OIP for the green turtles that nest at Cabuyal was similar to those reported for green turtles nesting in the Atlantic Ocean at Ascension island (14.3 days, Godley et al. 2002) and slightly longer than those reported for turtles nesting in the Mediterranean at Cyprus (13 days, Broderick et al. 2002) and in the Pacific Ocean at Michoacán, Mexico (12 days, Alvarado-Díaz et al. 2003). If OIPs at the different nesting sited were entirely due to changes on SST, local temperature data could be used to infer the intervals for each population.
Oceanography and nesting phenology
Some sea turtle species exhibit a significant response in their phenology to changes in SST (either at internesting or foraging areas), by nesting earlier, e.g., loggerhead turtles in West Florida (Weishampel et al. 2004, 2010; Pike et al. 2006) and Greece (Mazaris et al. 2008, 2009); or nesting later, e.g., loggerheads in south Florida (Lamont and Fujisaki 2014) and leatherback turtles in Costa Rica and Virgin islands (Neeman et al. 2015), also see Robinson et al. (2014). Our results show that, as in other green turtle populations (Pike 2009; Weishampel et al. 2010), the nesting dates did not vary in response to SST or Chl-α changes at the nesting or foraging grounds. As green turtle eggs in the area seem more resistant to high temperatures than other species (Santidrián Tomillo et al. 2017), their phenology may not be affected by SST, as a change in temperature may not relate to a decrease in hatching success. In addition, Cabuyal is highly affected by the cold coastal upwellings of the Gulf of Papagayo during the second part of the nesting season and these conditions could act as an environmental barrier, for adult turtles or hatchlings preventing delays of the nesting season. Future research should investigate the effect that the cold coastal upwellings of the Gulf of Papagayo could have on reproductive success, hatchling survival and dispersal to assess the pros and cons of nesting at the same time the cold fronts occur.
Effect of SST and Chl-α concentration on foraging areas on nesting abundance
The trophic status of green turtles can be a major influencer on their reproductive cycles (clutch size, number of clutches and remigration intervals) because of their mainly herbivorous diet being so tightly related to oceanographic conditions (Broderick et al. 2001). Green turtles foraging areas in the Central American Pacific are also highly influenced by seasonal changes in nutrient concentrations due to coastal upwellings in the gulf of Tehuantepec (Mexico), the gulf of Papagayo (Costa Rica) and the gulf of Panama from November to April (Glynn et al. 1983; Brenes 2001; Alfaro and Cortés 2012). Specifically in February, the cyclonic wind jets from these gulfs cause the thermocline to decrease in depth and have a great influence on Chl-α levels, so that when the wind abates in May, the coastal upwellings recede (Fiedler 2002). The strong positive relationship between Chl-α concentrations in February at the northern foraging grounds, one of the months that registered the highest concentrations in the year, and turtle abundance suggests that turtles take advantage of the high coastal productivity on these months to obtain most of the nutrients they require previous to nesting. This agrees with other studies that show a relationship between green turtles post-nesting movements and Chl-α fronts in oceanic and neritic habitats, suggesting they also benefit from the high productivity found in both environments (Seminoff et al. 2008). The lack of correlation between nesting abundance and Chl-α at the southern foraging grounds is not surprising as previous telemetry studies have shown that most turtles from Cabuyal (Clyde-Brockway 2014) and from nearby Nombre de Jesús (Blanco et al. 2012) migrate toward northern areas after nesting. Some green turtle populations also exhibit high fidelity to foraging grounds (Cheng 2000).
Although we found a strong correlation between Chl-α and the nesting abundance at Cabuyal, some of the interannual variability could be explained by other factors such as cohort effects (i.e., hatchling production in past years and/or number of nesting turtles 3–4 years before). In addition, analyzing smaller and/or more specific foraging areas, as well as using different time periods in future studies, could allow us to better capture the effect of ocean variability on nesting abundance, as relevant changes in oceanographic conditions can occur at very small scales (Schofield et al. 2009; Bates et al. 2018).
SST affects nesting abundance of some sea turtles such as loggerhead turtles in Greece, where the number of nests was negatively correlated to SST at foraging grounds (Mazaris et al. 2009; Patel et al. 2016). However, we found no effect of SST on the green turtles that nest at Cabuyal. SST was used as a proxy of climatic conditions and it is possible that a more throughout analysis on the effect of water column temperatures may show different results. Our study demonstrated that SST on nesting areas can strongly influence OIP, and Chl-α at foraging grounds influenced the number of nesting turtles. Since Cabuyal and the foraging grounds are located in an area highly affected by El Niño Southern Oscillation (ENSO) and both variables (SST and Chl-α) depend on ENSO conditions, further study of the effects of ENSO on the nesting population should be considered as the number of turtles encountered in nesting (Saba et al. 2007) and foraging areas (Quiñones et al. 2010) can vary with these events. Finally, the study of the oceanographic characteristics of the foraging grounds and their influence on sea turtle nesting populations should be kept in consideration for conservation and research initiatives.
References
Alfaro EJ, Cortés J (2012) Atmospheric forcing of cool subsurface water events in Bahía Culebra, Gulf of Papagayo, Costa Rica. Rev Biol Trop 60:173–186
Almpanidou V, Katragkou E, Mazaris AD (2018) The efficiency of phenological shifts as an adaptive response against climate change: a case study of loggerhead sea turtles (Caretta caretta) in the Mediterranean. Mitig Adapt Strateg Glob Change 23(7):1143–1158
Alvarado-Díaz J, Arias-Coyotl E, Delgado-Trejo C (2003) Clutch frequency of the Michoacán green sea turtle. J Herpetol 37:183–185. https://doi.org/10.1670/0022-1511(2003)037%5b0183:CFOTMN%5d2.0.CO;2
Angeles-Gonzalez LE, Calva R, Santos-Valencia J, Avila-Poveda OH, Olivares A, Diaz F, Rosas C (2017) Temperature modulates spatio-temporal variability of the functional reproductive maturation of Octopus maya (Cephalopoda) on the shelf of the Yucatan Peninsula, Mexico. J Molluscan Stud 83:280–288. https://doi.org/10.1093/mollus/eyx013
Bates AE, Helmuth B, Burrows MT, Duncan MI, Garrabou J, Guy-Haim T, Lima F, Queiros AM, Seabra R, Marsh R, Belmaker J, Bensoussan N, Dong Y, Mazaris AD, Smale D, Wahl M, Rilov G (2018) Biologists ignore ocean weather at their peril. Nature 506:299–301
Blanco GS, Morreale SJ, Bailey H, Seminoff JA, Paladino FV, Spotila JR (2012) Post-nesting movements and feeding grounds of a resident East Pacific green turtle Chelonia mydas population from Costa Rica. Endanger Species Res 18:233–245. https://doi.org/10.3354/esr00451
Both C, Van Asch M, Bijlsma RG, Van Den Burg AB, Visser ME (2009) Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? J Anim Ecol 78:73–83. https://doi.org/10.1111/j.1365-2656.2008.01458.x
Brenes CL (2001) Fundamentos de oceanografia descriptiva: aplicaciones al istmo centroamericano. Proyecto para el Desarrollo Integral de la Pesca Artesanal en la Region Autónoma Atlántico Sur (DIPAL), Nicaragua
Broderick AC, Godley BJ, Hays GC (2001) Trophic status drives interannual variability in nesting numbers of marine turtles. Proc R Soc Lond B Biol Sci 268:1481–1487. https://doi.org/10.1098/rspb.2001.1695
Broderick AC, Glen F, Godley BJ, Hays GC (2002) Estimating the number of green and loggerhead turtles nesting annually in the Mediterranean. Oryx 36:227–235. https://doi.org/10.1670/0022-1511(2003)037%5b0183:CFOTMN%5d2.0.CO;2
Chaloupka M, Kamezaki N, Limpus CJ (2008) Is climate change affecting the population dynamics of the endangered Pacific loggerhead sea turtle? J Exp Mar Bio Ecol 356:136–143. https://doi.org/10.1016/j.jembe.2007.12.009
Cheng I (2000) Post-nesting migrations of green turtles (Chelonia mydas) at Wan-An Island, Penghu Archipelago, Taiwan. Mar Biol 137:747–754
Clyde-Brockway CE (2014) Inter-nesting and post-nesting movements and behaviour of East Pacific Green turtles (Chelonia mydas agassizii) from playa Cabuyal, Guanacaste, Costa Rica. Master thesis, Purdue University, Indiana
Edwards M, Richardson AJ (2004) Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430:881–884
Fiedler PC (2002) The annual cycle and biological effects of the Costa Rica Dome. Deep Sea Res 49:321–338
Glynn PW, Druffel EM, Dunbar RB, Glynn Peter, Druffel EM, Dunbar RB (1983) A dead Central American coral reef tract: possible link with the Little Ice Age. J Mar Res 41:605–637. https://doi.org/10.1357/002224083788519740
Godley BJ, Broderick AC, Frauenstein R, Glen F, Hays GC (2002) Reproductive seasonality and sexual dimorphism in green turtles. Mar Ecol Prog Ser 226:125–133
Hamann M, Limpus CJ, Owens DW (2003) Reproductive cycles of males and females. In: Lutz PL, Musick JA, Wyneken J (eds) The biology of sea turtles, vol 2. CRC Press, Florida, pp 135–161
Hart CE, Blanco GS, Coyne MS, Delgado-Trejo C, Godley BJ, Todd Jones T, Resendiz A, Seminoff JA, Witt MJ, Nichols WJ (2015) Multinational tagging efforts illustrate regional scale of distribution and threats for east pacific green turtles (Chelonia mydas agassizii). PLoS ONE. https://doi.org/10.1371/journal.pone.0116225
Hays GC, Broderick AC, Glen F, Godley BJ, Houghton JDR, Metcalfe JD (2002) Water temperature and internesting intervals for loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles. J Therm Biol 27:429–432
Hirth HF (1980) Some aspects of the nesting behavior and reproductive biology of sea turtles. Am Zool 20:507–523
IBM Corp (2015) IBM SPSS Statistics for Windows
Lamont MM, Fujisaki I (2014) Effects of ocean temperature on nesting phenology and fecundity of the loggerhead sea turtle (Caretta caretta). Artic J Herpetol 48:98–102. https://doi.org/10.1670/12-217
Limpus CJ, Hamann M, Whittier JM (2002) Patterns of lipid storage and mobilisation in female green sea turtles (Chelonia mydas). Artic J Comp Physiol B 172:485–493. https://doi.org/10.1007/s00360-002-0271-2
Mazaris AD, Kallimanis AS, Sgardelis SP, Pantis JD (2008) Do long-term changes in sea surface temperature at the breeding areas affect the breeding dates and reproduction performance of Mediterranean loggerhead turtles? Implications for climate change. Exp Mar Biol Ecol 367:219–226. https://doi.org/10.1016/j.jembe.2008.09.025
Mazaris AD, Kallimanis AS, Tzanopoulos J, Sgardelis SP, Pantis JD (2009) Sea surface temperature variations in core foraging grounds drive nesting trends and phenology of loggerhead turtles in the Mediterranean Sea. Exp Mar Biol Ecol 379:23–27. https://doi.org/10.1016/j.jembe.2009.07.026
Mazaris AD, Kallimanis AS, Pantis JD, Hays GC (2013) Phenological response of sea turtles to environmental variation across a species’ northern range. Proc R Soc B Biol Sci 280:20122397. https://doi.org/10.1098/rspb.2012.2397
Monticelli D, Ramos JA, Catry T, Pedro P, Paiva VH (2014) Reproductive parameters of tropical lesser noddies respond to local variations in oceanographic conditions and weather. Estuar Coast Shelf Sci 139:110–118. https://doi.org/10.1016/j.ecss.2013.12.026
Neeman N, Robinson NJ, Paladino FV, Spotila JR, Connor MPO (2015) Phenology shifts in leatherback turtles (Dermochelys coriacea) due to changes in sea surface temperature. J Exp Mar Bio Ecol 462:113–120. https://doi.org/10.1016/j.jembe.2014.10.019
Patel SH, Morreale SJ, Saba VS, Panagopoulou A, Margaritoulis D, Spotila JR (2016) Climate impacts on sea turtle breeding phenology in Greece and associated foraging habitats in the wider Mediterranean region. PLoS ONE 11:157170. https://doi.org/10.1371/journal.pone.0157170
Pike DA (2009) Do green turtles modify their nesting seasons in response to environmental temperatures? Chelonian Conserv Biol 8:43–47. https://doi.org/10.2744/CCB-0726.1
Pike DA, Antworth RL, Stiner JC (2006) Earlier nesting contributes to shorter nesting seasons for the loggerhead sea turtle, Caretta caretta. J Herpetol 40:91–94. https://doi.org/10.1670/100-05N.1
Polovina JJ, George H, Howell EA, Denise M, Seki MP, Peter H (2004) Forage and migration habitat of loggerhead (Caretta caretta) and olive ridley (Lepidochelys olivacea) sea turtles in the central North Pacific Ocean. Fish Oceanogr 13:36–51
Potts WM, Booth AJ, Richardson TJ, Sauer WHH (2014) Ocean warming affects the distribution and abundance of resident fishes by changing their reproductive scope. Rev Fish Biol Fish 24:493–504. https://doi.org/10.1007/s11160-013-9329-3
Quiñones J, González Carman V, Zeballos J, Purca S, Mianzan H (2010) Effects of El Niño-driven environmental variability on black turtle migration to Peruvian foraging grounds. Hydrobiologia 645:69–79. https://doi.org/10.1007/s10750-010-0225-8
Reina RD, Mayor PA, Spotila JR, Piedra R, Paladino FV (2002) Nesting ecology of the leatherback turtle, Dermochelys coriacea, at Parque Nacional Marino Las Baulas, Costa Rica: 1988–1989 to 1999–2000. Copeia 2002:653–664
Robinson NJ, Valentine SE, Santidrián Tomillo P, Saba VS, Spotila JR, Paladino FV (2014) Multidecadal trends in the nesting phenology of Pacific and Atlantic leatherback turtles are associated with population demography. Endanger Species Res 24:197–206. https://doi.org/10.3354/esr00604
Robinson NJ, Paladino FV, Santidrián Tomillo P (2017) Evidence of a green turtle starting its postnesting migration without laying all its vitellogenic follicles. Mar Turt Newsl 152:8–10
Saba VS, Santidrián Tomillo P, Reina RD, Spotila JR, Musick JA, Evans DA, Paladino FV (2007) The effect of the El Niño Southern Oscillation on the reproductive frequency of eastern Pacific leatherback turtles. J Appl Ecol 44:395–404. https://doi.org/10.1111/j.1365-2664.2007.01276.x
Santidrián Tomillo P, Roberts SA, Hernández R, Spotila JR, Paladino FV (2015) Nesting ecology of East Pacific green turtles at Playa Cabuyal, Gulf of Papagayo, Costa Rica. Mar Ecol 36:506–516. https://doi.org/10.1111/maec.12159
Santidrián Tomillo P, Fonseca L, Paladino FV, Spotila JR, Oro D (2017) Are thermal barriers “higher” in deep sea turtle nests? PLoS ONE 12:e0177256. https://doi.org/10.1371/journal.pone.0177256
Sato K, Matsuzawa Y, Tanaka H, Bando T, Minnamikawa S, Sakamoto W, Naito Y (1998) Internesting intervals for loggerhead turtles, Caretta caretta, and green turtles, Chelonia mydas, are affected by temperature. Can J Zool 9:1651
Schofield G, Bishop CM, Katselidis KA, Dimopoulos P, Pantis JD, Hays GC (2009) Microhabitat selection by sea turtles in a dynamic thermal marine environment. J Anim Ecol 78:14–21. https://doi.org/10.1111/j.1365-2656.2007.0
Seminoff JA, Resendiz A, Nichols W (2002) Diet of east Pacific green turtles (Chelonia mydas) in the central Gulf of California, Mexico. J Herpetol 36:447–453
Seminoff JA, Zárate P, Coyne M, Foley DG, Parker D, Lyon BN, Dutton PH (2008) Post-nesting migrations of Galápagos green turtles Chelonia mydas in relation to oceanographic conditions: integrating satellite telemetry with remotely sensed ocean data. Endanger Species Res 4:57–72. https://doi.org/10.3354/esr00066
Solow AR, Bjorndal KA, Bolten AB (2002) Annual variation in nesting numbers of marine turtles: the effect of sea surface temperature on re-migration intervals. Ecol Lett 5:742–746. https://doi.org/10.1046/j.1461-0248.2002.00374.x
Vander Zanden HB, Pfaller JB, Reich KJ, Pajuelo M, Bolten AB, Williams KL, Frick MG, Shamblin BM, Campbell NJ, Bjorndal KA (2014) Foraging areas differentially affect reproductive output and interpretation of trends in abundance of loggerhead turtles. Mar Biol 161:585–598. https://doi.org/10.1007/s00227-013-2361-y
Weishampel JF, Bagley DA, Ehrhart LM (2004) Earlier nesting by loggerhead sea turtles following sea surface warming. Glob Chang Biol 10:1424–1427. https://doi.org/10.1111/j.1365-2486.2004.00817.x
Weishampel JF, Bagley DA, Ehrhart LM, Weishampel AC (2010) Nesting phenologies of two sympatric sea turtle species related to sea surface temperatures. Endangered Species Res 12:41–47. https://doi.org/10.3354/esr00290
Acknowledgements
We would like to thank the research teams that have helped year after year to collect field nesting data at Cabuyal. This research would have been impossible without the hard work of coordinators, research assistants and volunteers. We also thank Roger Blanco and the Guanacaste Conservation Area for granting scientific permits and for their genuine interest in research. We thank Alejandro Martínez Abraín for statistical advice. Finally, we would like to thank the reviewers that helped improved the quality of the study.
Funding
This study was funded by The Leatherback Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Research permits were obtained by the Ministry of Environment and Energy of Costa Rica (MINAE).
Additional information
Responsible Editor: P. Casale.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by J. Bourjea, A. Mazaris and J. Weishampel.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Valverde-Cantillo, V., Robinson, N.J. & Santidrián Tomillo, P. Influence of oceanographic conditions on nesting abundance, phenology and internesting periods of east Pacific green turtles. Mar Biol 166, 93 (2019). https://doi.org/10.1007/s00227-019-3541-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3541-1