Abstract
The fear of predators can lead to behavioral changes in their prey, but animals must trade off defensive behavior against other compelling needs. For territorial species, responding to predators may be especially costly, because defense and escape are mutually incompatible. A growing literature documents risk assessment in fish, but a few studies have focused on extremely territorial fish, and nothing is known about how territory size, territorial interactions, habitat structural complexity, and life stage may modify risk assessment. We studied this in a damselfish (Stegastes fuscus) on coral reefs and measured risk assessment using flight initiation distance (FID). We found that only structural complexity explained variation in FID; fish in more complex habitats tolerated closer approach. Our study suggests that individuals in relatively more structurally complex territories are bolder than those that occur in less complex territories. Documenting the relative importance of these variables is important, because antipredator behavior influences not only the predator–prey relationship, but can also generate cascading effects, and influence the structure of the community and potentially entire ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Territorial species must trade off time allocated to territorial behavior with other activities that enhance their fitness (Ydenberg and Krebs 1987). Responding to predatory threats may, indeed, be especially costly, because by doing so individuals are unable to defend their territories. Thus, we expect the decisions about when and how to assess risk and respond to predators to be particularly important.
A commonly used metric to quantify risk is flight initiation distance (FID), which is minimum distance at which a predator approaches a prey before it escapes (Ydenberg and Dill 1986). FID is influenced by a variety of factors, such as predator lethality, group size, approach angle, prey size, and the habitat’s structural complexity (Blumstein et al. 2003; Cooper et al. 2002; Nunes et al. 2015). Optimal escape theory predicts that a prey should begin to flee its approaching predator when it reaches a point where the risk of predation is similar to the cost of escape (Ydenberg and Dill 1986); and reveals that flight initiation distance increases when predators pose a greater threat, and decreases when escape costs increase (Cooper et al. 2002).
Animals often adopt consistent defense strategies when faced with danger—they vary according to temperament or personality. For instance, bold individuals typically behave more actively and may tolerate closer approach before fleeing or actively fight off a predator, whereas shy individuals either flee at greater distances or they may try to avoid detection in the first place by remaining perfectly still. Consistent individual differences in prey behavior, especially in the propensity to take risks (“boldness”), are widespread in the animal kingdom. Theory predicts that individual behavioral types differ, such that bolder individuals may benefit from greater access to resources while paying higher predation-risk costs than less bold animals (Hulthén et al. 2017). Thus, anything that influences the probability of predation may influence the adaptive value of boldness.
In coral reef ecosystems, damselfish influence both benthic patterns and processes (Ceccarelli 2007). Damselfish defend their territories from intruders, and, by doing so, influence the structural makeup of diversity, algal biomass, coral zoning, and the structure of the benthic community within their territory (Helfman and Winkelman 1997; Ferreira et al. 1998; Ceccarelli et al. 2001).
Given the conflicting demands of territory defense and flight, we asked how an extremely territorial damselfish makes these trade-offs. Damselfish could respond two ways to variation in territory size. First, there could be a positive relationship between FID and territory size. This relationship may emerge, because prey are likely farther from their refuge, and by patrolling a larger territory, they are more exposed. Alternatively, there could be a negative relationship between FID and territory size. This could emerge if the number of refuges increases with territory size. Regarding territoriality, we expected a negative relation between flight initiation distance and territoriality (number of agonistic interactions). To more comprehensively understand these trade-offs, we also quantified life stage and habitat structural complexity.
Materials and methods
Species and study area
We studied Stegastes fuscus an endemic damselfish found in the Southwestern Atlantic, and present in the Brazilian coast between the states of Maranhão and Santa Catarina (Sampaio and Nottingham 2008). The species is abundant on reefs and rock shores (Ferreira et al. 2004, 2015). Its territories influence the diversity and biomass of the algal community, and provide hiding places and food resources for several mesoinvertebrate fauna in south-east Brazil (Ferreira et al. 1998). They are found in shallow waters (< 8 m), in both rocky and biogenic reef, and vary in color based on their age (Manegatti et al. 2003). They are diurnal herbivores that are extremely territorial, patrolling their and expelling intruders that enter their territory (Sampaio and Nottingham 2008; Júnior et al. 2010). They are small, at most 15 cm in length, and forage within relatively restricted areas (Ferreira et al. 1998). However, this territory provides space for the construction of their nests, shelter against predators, and food.
We studied damselfish in the reefs off Boa Viagem beach, Todos os Santos Bay, Salvador (TSB), Bahia. Data were collected between October and December 2017 by snorkeling around and identifying damselfish territories that were in water no deeper than 2 m. Fish territories were separated by at least 4 m. This fringing reef is characterized by low wave energy, clear and shallow waters, and easy access. This reef is occupied by filamentous algae, sponges, calcareous algae, sea urchins, and corals (Cruz 2008), as well as several other fish species.
Quantifying agonistic interactions
We quantified aggressive interactions (bites and chases) and estimated territory size by conducting 5 min focal animal samples. Observers were trained to estimate fish body size in the previous studies (Nunes et al. 2015, 2016) and in this by estimating the size of model fish placed on the reef. Once trained, we estimated fish body size visually and classified individuals up to 7 cm as juveniles, and > 7 cm as adults, which correlates with color pattern differences from their corresponding life stages (Sampaio and Notimham 2008). We counted the number of these agonistic interactions each time which a focal individual interacted aggressively with the same or any other species of fish during the 5-min period. Territory size was estimated during this same period of time, where markers were placed in the substrate at six extreme points of the area defended by the sampled individual. The three largest lengths between the points were measured, and from their mean value, it was possible to calculate the circular area of the territory (area = π. r2) (Aued 2012). Ninety focal animals were observed, and following the focal observation, we measured that individual’s flight initiation distance.
Quantifying flight initiation distance
All flight initiation distances began with the observer positioned 2 m from a focal subject. We estimated flight initiation distance by pushing a rod that had a replica of a 30 cm total length predator grouper (Epinephelus sp., Fig. 1) towards a focal subject at ~ 0.7 m/s. The observer swam in direction of the fish while following the bottom and thereby swam horizontally until reaching the target fish, upon which the observer stretched his arm to present the model to target fish. At the time the fish began to flee, the rod was placed on the substrate and the distance between the end of the model predator (mouth) and the place where the fish started to flee (FID) was measured with a measuring tape. It is important to stress that an escape occurred when the individual’s swimming speed increased beyond that of the model predator’s approach speed (Januchowski-Hartley et al. 2011, 2012).
Quantifying habitat complexity
After measuring flight initiation distance (n = 90 fish), we quantified rugosity, frequently used as a proxy of structural complexity, using Luckhurst and Luckhurst’s (1978) chain method, where a chain was placed on the substrate in such a way as to follow as closely as possible all the contours and crevices. This generated a measure of the surface that we expressed in relation to the linear distance (Luckhurst and Luckhurst 1978). The RI was calculated using the formula RI = linear/surface, in which “linear” means the measure of when the chain was stretched (1 m) and “surface” is the distance between the beginning and end of the chain when it is adjusted to all the contours and crevices. Three measurements of rugosity were made within all territories of the chosen fish (i.e., 270 measurements in total). We used the average of these three values as our measure of rugosity.
Statistical analyses
We used a backward stepwise algorithm to select the best model that explained variation in FID. Independent variables included structural complexity, prey size, territory size, and the number of agonistic interactions. Simple linear regressions were used to investigate the potential influence of habitat complexity on the size of the territory and the number of interactions and body size on FID. Normality and homogeneity were investigated using Q–Q plots and the Levene’s test. We set our alpha to 0.05.
Results
Territories averaged 1.71 m−2 (± 0.83 SD; 0.25–3.89; N = 90), fish size averaged 8.7 cm (± 2.43 SD; 4–13; N = 90), and rugosity averaged 1.23 (± 0.1 SD; 1.04–1.52; N = 270). The mean initial flight distance was 35.5 cm (± 13.03 SD; 19–80; N = 90), and the average number of interactions over 5 min was 3.2 (± 3.1 SD; 0–13; N = 90 fish, 286 interactions).
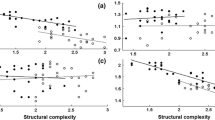
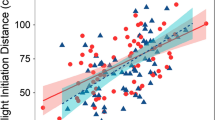
After accounting for non-significant variation in FID explained by territory size, the number of agonistic interactions (F = 0.327; p = 0.569), life phase (F = 0.007; p = 0.934), and body size (F = 1.01; p = 0.317), damselfish tolerated closer approach as rugosity increased (F = 4.28, p = 0.04; Fig. 2; Table 1).
There was no relationship between rugosity and territory size (F = 0.46; p = 0.50; R2 = 0.0052), nor was there a relationship between the number of agonistic interactions and rugosity (F = 1. 30; p = 0.26; R2 = 0.01).
Discussion
We found no relationship between territory size and FID, and thus, our results did not support the hypothesis that larger territories, potentially with more refuges, modulate risk perceptions. However, we found that as rugosity increased fish’s perceptions of risk decreased, corroborating the previous studies with other species (Nunes et al. 2015; Januchowski-Hartley et al. 2015; Benevides et al. 2016). This is likely to occur, because environments that are more complex provide more refuges. As a result, prey are more likely to escape than in environments that are more homogeneous. Therefore, these individuals will adjust their FID in concordance with expectation from optimal escape theory (Cooper and Frederick 2007).
The previous studies have shown that habitat complexity, frequently estimated by rugosity, is positively associated with the abundance and diversity of fish species, precisely because complex environments provide more shelter (e.g., Luckhurst and Luckhurst 1978; Graham and Nash 2012). In addition, greater habitat complexity results in more areas suitable algae and invertebrates to grow and thrive (Nunes et al. 2013). Hence, increasing complexity not only provides more shelters, but can also increase other resources such as food availability (Cecarrelli et al. 2001).
Territories that have higher quality may have a greater value for the fish, since it may provide more shelters and more food (Johnsson et al. 2000). Animals seem to invest relatively more resources defending valuable habitats and are more reluctant to leave these environments, thus reducing their FID as habitat complexity increases. Complexity may, thus, be viewed as a metric of territory “quality”, and we assume that when individual’s make decisions about whether or not to defend a particular location, the rugosity plays an important role. Future studies are required to formally evaluate this hypothesis. For example, analyzing which variables (e.g., differences in shelter, food, and sexual partner availability, and the presence of different predators and competitors) explain the most variance in territory choice will be essential to better understand the importance of rugosity.
Territorial defense is costly, since it decreases individual foraging time, and increases both energetic demands and predation risk (Cleveland 1999). Therefore, the number of agonistic interactions was expected to explain variation the FID, since individuals defending territories must allocate more energy to defense. We hypothesized that as an individual’s number of interactions increase, its energy expenditure would rise, and thus, the prey would only escape when essential, therefore, reducing its FID. We did not find this expected relationship. We used a short measure of agonistic interactions what could contribute to the potential lack of effect, given that there are a variety of variables that influence agonistic interactions.
Individual body size is another variable that can influence flight initiation distance, but how it does so is not uniform (Gotanda et al. 2009; Feary et al. 2011). Our study did not find a significant relationship between these variables in the study area for the species studied. It is possible that, given high fishing pressure in the study area, the abundance of predators was massively reduced (especially groupers), and this limited the opportunity to learn from negative experiences with predators. However, the loss of some predators does not always result in a complete loss of antipredator behavior and the multipredator hypotheses predict, and a variety of species from diverse taxa reveal, that antipredator behavior persists when isolated from some, but not all predators (Blumstein 2006; Rasheed et al. 2017; Carthey and Blumstein 2018).
It is important to know which variables influence fish anti-predatory behavior, because this not only affects predator–prey relationships, but can have cascading effects and influence the entire structure of communities, and even ecosystems. In the case of territorial species such as damselfish, which are considered key species in reef environments, the presence of predators leads to behavioral changes and its consequences can alter the performance of an entire ecosystem.
References
Aued AW (2012) Comportamento territorial e alimentar do peixe-donzela comum, Stegastes fuscus (Pisces: Pomacentridae) ao longo da costa brasileira. Dissertação (Mestrado em Ecologia). Universidade Federal de Santa Catarina (UFSC). http://repositorio.ufsc.br/xmlui/handle/123456789/100442
Basquill SP, Grant JWA (1998) An increase in habitat complexity reduces aggression and monopolization of food by zebra fish (Danio rerio). Can J Zool 76:770–772. https://doi.org/10.1139/z97-232
Benevides LJ, Nunes JACC, Costa TLA, Sampaio CLS (2016) Flight response of the barber surgeonfish, Acanthurus bahianus Castelnau, 1855 (Teleostei: Acanthuridae), to spearfisher presence. Neotrop Ichthyol 14(1):e150010. https://doi.org/10.1590/1982-0224-20150010
Blumstein DT (2003) Flight-Initiation distance in birds is dependent on intruder starting distance. J Wildl Manage 67(4):852–857. https://doi.org/10.2307/3802692
Blumstein DT (2006) The multipredator hypothesis and the evolutionary persistence of antipredator behavior. Ethology 112:209–217. https://doi.org/10.1111/j.1439-0310.2006.01209.x
Blumstein DT, Anthony LL, Harcourt RG, Ross G (2003) Testing a key assumption of wildlife buffer zones: is flight initiation distance a species-specific trait? Biol Conserv 110:97–100. https://doi.org/10.1016/S0006-3207(02)00180-5
Carthey AJR, Blumstein DT (2018) Predicting predator recognition in a changing world. Trends Ecol Evol 33:106–115. https://doi.org/10.1016/j.tree.2017.10.009
Ceccarelli D (2007) Modification of benthic communities by territorial damselfish: a multi species comparison. Coral Reefs 26:853–866. https://doi.org/10.1007/s00338-007-0275-1
Ceccarelli D, Jones GP, McCook LJ (2001) Territorial Damselfish as determinants of the structure of benthic communities on coral reef. Oceanogr Mar Biol Annu Rev 39:355–389
Cleveland A (1999) Energetic costs of agonistic behavior in two herbivorous damselfishes (Stegastes). Copeia 1999:857–867. https://doi.org/10.2307/1447962
Cooper WEJR, Frederick WG (2007) Optimal flight initiation distance. J Theor Biol 244:59–67. https://doi.org/10.1016/j.jtbi.2006.07.011
Cooper WEJR, Mellado VP, Baird T, Baird TA, Caldwell JP, Vitt LJ (2002) Effects of risk, cost, and their interaction on optimal escape by nonrefuging Bonaire whiptail lizards, Cnemidophorus murinus. Behav Ecol 14(2):288–293. https://doi.org/10.1093/beheco/14.2.288
Cruz ICS (2008) Recifes de corais da Baia de Todos os Santos, caracterização, avaliação e identificação de áreas prioritárias para conservação. Dissertação. Universidade Federal da Bahia (UFBA). http://repositorio.ufba.br/ri/handle/ri/12696
Feary DA, Cinner JE, Graham NAJ, Januchowski-Hartley FA (2011) Effects of customary marine closures on fish behavior, spear-fishing success, and underwater visual surveys. Conserv Biol 25(2):341–349. https://doi.org/10.1111/j.1523-1739.2010.01613.x
Ferreira CEL, Gonçalves JEA, Coutinho R, Peret AC (1998) Herbivory by the Dusky Damselfish Stegastes fuscus (Cuvier, 1830) in a tropical rocky shore: effects on the benthic community. J Exp Mar Biol Ecol 229:241–264. https://doi.org/10.1016/S0022-0981(98)00056-2
Ferreira CEL, Floeter SR, Gasparini JL, Joyeux JC, Ferreira BP (2004) Trophic structure patterns of Brazilian reef fishes: a latitudinal comparison. J Biogeogr 31:1093–1106. https://doi.org/10.1111/j.1365-2699.2004.01044.x
Ferreira CM, Coni EOC, Medeiros DV, Sampaio CLS, Reis-Filho JA, Barros F, Loiola M, Nunes JACC (2015) Community structure of shallow rocky shore fish in a tropical bay of the southwestern Atlantic. Braz J Oceanogr 63(4):379–396. https://doi.org/10.1590/S1679-87592015074706304
Gotanda KM, Turgeon K, Kramer DL (2009) Body size and reserve protection affect flight initiation distance in parrotfishes. Behav Ecol Sociobiol 63:1563–1572. https://doi.org/10.1007/s00265-009-0750-5
Graham NAJ, Nash KL (2012) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32:315–326. https://doi.org/10.1007/s00338-012-0984-y
Helfman GS, Winkelman DL (1997) Threat sensitivity in bicolor damselfish: effects of sociality and body size. Ethology 103:369–383
Hulthén K, Chapman BB, Nilsson PA, Hansson LA, Skov C, Brodersen J, Vinterstare J, Brönmark C (2017) A predation cost to bold fsh in the wild. Sci Rep 7:1239. https://doi.org/10.1038/s41598-017-01270-w
Januchowski-Hartley FA, Graham NAJ, Feary DA, Morove T, Cinner JE (2011) Fear of fishers: human predation explains behavioral changes in coral reef fishes. PLoS One 6(8):e22761. https://doi.org/10.1371/journal.pone.0022761
Januchowski-Hartley FA, Nash KL, Lawton RJ (2012) Influence of spear guns, dive gear and observers on estimating fish flight initiation distance on coral reefs. Mar Ecol Prog Ser 469:113–119. https://doi.org/10.3354/meps09971
Januchowski-Hartley FA, Graham NAJ, Cinner JE, Russ GR (2015a) Local fishing influences coral reef fish behavior inside protected áreas of the Indo-Pacific. Biol Conserv 182:8–12. https://doi.org/10.1016/j.biocon.2014.11.024
Januchowski-Hartley FA, Graham NAJ, Cinner JE, Russ GR (2015b) Local fishing influences coral reef fish behavior inside protected áreas of the Indo-Pacific. Biol Conserv 182:8–12. https://doi.org/10.1016/j.biocon.2014.11.024
Jonhsson JI, Carlsson M, Sundstrom LF (2000) Habitat preference increases territorial defence in Brown trout (Salmo trutta). Behav Ecol Sociobiol 48:373–377. https://doi.org/10.1007/s002650000244
Júnior JG, Mendes LF, Sampaio CLS, Lins JE (2010) Biodiversidade marinha da Bacia Potiguar: ictiofauna. Museu Nacional, Rio de Janeiro, p 195
Luckhurst BE, Luckrust K (1978) Analysis of the influence of substrate variables on coral reef fish communities. Mar Biol 49:317–323. https://doi.org/10.1007/BF00455026
Manegatti JV, Vescovi DL, Floeter SR (2003) Interações agonísticas e forrageamento do peixe-donzela, Stegastes fuscus (Peciformes: Pomacentridae). Nat Online 1(2):45–50
Mattos FMG (2016) Uso de habitat e padrões comportamentais do peixe Stegastes fuscus nos recifes costeiros de Porto de Galinhas (PE). Dissertação (Mestrado em Ciências). Universidade Federal de Pernambuco (UFPE). https://repositorio.ufpe.br/handle/123456789/18328
Nunes JACC, Sampaio CLS, Barros F (2013) How wave exposure, group size and habitat complexity influence foraging and population densities in fishes of the genus Halichoeres (Perciformes: Labridae) on tropical rocky shores. Mar Biol 160:2383–2394. https://doi.org/10.1007/s00227-013-2233-5
Nunes JACC, Sampaio CLS, Barros F (2015) The influence of structural complexity and reef habitat types on flight initiation distance and escape behaviors in labrid fishes. Mar Biol 162:493–499. https://doi.org/10.1007/s00227-014-2578-4
Nunes JACC, Loiola M, Miranda RJ, Sampaio CLS, Barros F (2016) Are Abrolhos no-take areas sites of naïve fish? An evaluation using flight initiation distance of labrids. Neotrop Ichthyol 14(4):e160133. https://doi.org/10.1590/1982-0224-20160133
Osório R, Rosa IL, Cabral H (2006) Territorial defence by the Brazilian damsel Stegastes fuscus (Teleostei: Pomacentridae). J Fish Biol 69:233–242. https://doi.org/10.1111/j.1095-8649.2006.01095.x
Rasheed AA, Hambley K, Chan G, de la Rosa CA, Larison B, Blumstein DT (2017) Persistence of antipredator behavior in an island population of California quail. Ethology 2017(00):1–6. https://doi.org/10.1111/eth.12716
Reebs SG (2008) Aggression in fishes. www.howfishbehave.ca. Université de Moncton, Canada
Rupia EJ, Binning SA, Roche DG, Lu W (2016) Fight-flight or freeze-hide? Personality and metabolic phenotype mediate physiological defense responses in flatfish. J Anim Ecol 85(4):927–937. https://doi.org/10.1111/1365-2656.12524
Sampaio CLS, Nottingham MC (2008) Guia para identificação de peixes ornamentais volume I: espécies marinhas. Ibama, Brasília, p 205
Ydenberg RC, Dill LM (1986) The economics of fleeing from predators. Adv Study Behav 16:229–249. https://doi.org/10.1016/S0065-3454(08)60192-8
Ydenberg RC, Krebs JR (1987) The tradeoff between territorial defense and foraging in the great tit (Parus major). Am Zool 27(2):337–346
Acknowledgements
We thank Laboratório de Ecologia Bentônica (LEB-UFBA) team for constant support. F. B. was supported by CNPq fellowships (303897/2011-2; 239978/2012-9) and J.A.C.C.N by CNPq fellowships (150344/2017-1). D.T.B. is currently supported by the US National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study have been followed and all necessary approvals have been obtained. This project was approved by Ethics Committee of the Federal University of Bahia (Brasil) of animal use for research and Sistema de Autorização e Informação em Biodiversidade (44060-1).
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Responsible Editor: D. Goulet.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by D. Kramer and undisclosed experts.
Rights and permissions
About this article
Cite this article
Quadros, A.L.S., Barros, F., Blumstein, D.T. et al. Structural complexity but not territory sizes influences flight initiation distance in a damselfish. Mar Biol 166, 65 (2019). https://doi.org/10.1007/s00227-019-3508-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3508-2