Abstract
Structural complexity is a key parameter for fish on reef habitats. Several studies have investigated the influence of this variable on aspects of reef fish population and community dynamics. However, there is a lack of knowledge on the influence of structural complexity on antipredator behavior. Here we studied the effect of habitat type and structural complexity on flight initiation distance (FID) and the escape behaviors of four labrid fishes (Halichoeres brasiliensis, H. penrosei, H. poeyi and Sparisoma axillare) on two different reef habitats (coral and rocky reefs). Habitat type influenced the FID of three of the studied species (H. brasiliensis, H. penrosei and S. axillare), and structural complexity negatively influenced the FID of two species (H. brasiliensis and S. axillare). The frequency of escape behaviors varied between species. All of them showed high frequency of the ‘run away’ behavior and low frequency of the ‘leave the habitat’ behavior. On coral reefs, structural complexity influenced the ‘fled to the holes’ for S. axillare only. Reef ecosystems worldwide are being modified by anthropogenic activities. Our results suggest that if such activities reduce structural complexity, then ‘seascapes of fear’ for labrid fishes will become more widespread, which could lead to negative consequences in the reef ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat structural complexity can influence behavior and distribution of fish (Jones and Syms 1998; Floeter et al. 2007). Some experimental studies indicate that an increase in structural complexity can reduce mortality (Hixon and Beets 1993; Almany 2004). However, changes in prey behaviors due to shifts in complexity are not yet well understood. Krajewski et al. (2010) suggest that substrata with distinctive structure and associated fauna can offer different types of resources (e.g., prey and shelter) and may influence fish activity. Therefore, predator–prey interactions are influenced by the relationship between habitat characteristics and fish behavior.
The flight initiation distance (FID), which is the distance a predator can approach the prey before it flees (Blumstein 2003), is commonly used as a methodology to assess prey decision-making and wariness (Januchowski-Hartley et al. 2011). This metric has been widely used on birds (Blumstein 2003; Blumstein et al. 2003, 2005), lizards (Cooper et al. 2002; Cooper 2008, 2009), and mammals (Runyan and Blumstein 2004; Stankowich 2008). For reef fishes, most studies on FID have focused on the impacts of fishing through comparisons between protected and exploited areas (Feary et al. 2011; Januchowski-Hartley et al. 2011, 2012, 2013). No studies have yet evaluated potential differences in fish FID on habitats with different complexities. Rocky reefs have lower complexity when compared to coral reefs, but also support a rich fauna and flora (Ferreira et al. 2001). There is a need to test if fish can modify the antipredator behaviors according to structural complexity and if that happens in the same way on different habitats.
Caution behavior (preflight behavior) also influences the success of predation. Moreover, it is dependent on species’ trophic groups and life history characteristics (Januchowski-Hartley et al. 2011). On the other hand, escape behaviors have not been evaluated for reef fishes in different types of habitats. These behaviors probably affect the distribution of reef fishes within and among reef habitats and may alter the structure of assemblages.
The fishes of the Labridae family exhibit a great variety of body shapes and several morphological adaptations for feeding. Consequently, they have trophic versatility (Fulton and Belwood 2002), which is important in structuring reef communities (Hobson 1975; Deloach and Humann 1999). Wrasse species of the Halichoeres genus are considered highly diverse and widely distributed in the Atlantic Ocean (Rocha et al. 2010). These species are diurnal, exhibiting opportunistic behavior and feeding on invertebrates (Sazima et al. 1998, 2005; Coni et al. 2007, 2010). According to Nunes et al. (2013), rocky reef complexity influences foraging activity of three Halichoeres species. Yet, there are no studies on the behavior of these species on other reef habitats. Parrotfishes of the Sparisoma genus are recorded only in the Atlantic Ocean, being the most speciose scarid genus in this ocean basin (Bernardi et al. 2000; Streelman et al. 2002). This genus is ecologically diverse, with a wide range of feeding modes and patterns of habitat use (Bernardi et al. 2000; Streelman et al. 2002; Bonaldo et al. 2006).
Here we studied the FID and escape behaviors of two species harvested by spearfishing (Halichoeres brasiliensis and Sparisoma axillare) and two non-harvested (H. penrosei and H. poeyi). The study was carried out on two different habitats: coral and rocky reefs. Specifically, we investigated (1) if labrid fishes on habitats with more structural complexity have shorter FID than on less complex habitats and (2) if escape behaviors are related to reef structural complexity and (3) if the frequencies of escape behaviors vary between reef habitats. Such variation was expected due to the higher availability of refuge resources (e.g., holes at short distances), especially on coral reefs.
Methods
Study area
This study was carried out on reefs in Todos os Santos Bay, Bahia state, Northeast Brazil. We studied FID and escape behaviors of four labrids in six areas, being three of them coral reefs (13°07′S–38°43′W) and three rocky reefs (13°00′S–38°32′W). All sites studied have high fishing pressure. Coral reefs studied were dominated by the corals: Mussismilia spp., Siderastrea sp., Millepora spp. and Porites branneri Rathbun 1887. The coralline algae, macroalgae and the articulated calcareous algae Halimeda sp. were also found within the area (for details see Miranda et al. 2013). Rocky reefs studied were predominantly colonized by filamentous algae, macroalgae and zoanthids [Palythoa caribaeorum (Duchassaing and Michelotti 1860) and Zoanthus sociatus (Ellis and Solander 1786)]). The black sea urchin Echinometra lucunter (Linnaeus 1758), ascidians and colonies of corals Favia gravida (Verrill 1868) and Siderastrea sp. (in lower abundance when compared to coral reefs) were also found within the area (for details see Maia-Nogueira et al. 2010).
Flight initiation distance and escape behaviors
We estimated fish FID through snorkeling. A snorkeler would reproduce spearfisher behavior (according to Januchowski-Hartley et al. 2011, 2012) by swimming directly toward the fish at a constant speed (~0.7 m/s−1, measured using portable GPS at sea surface). When the fish fled, the distance between the end of the speargun and the place where the fish was, prior to fleeing, was measured with a tape measure and represented the estimated FID. The same precautions were taken from the authors cited above, whereby fishes were only targeted if they were feeding or swimming normally (see also Deloach and Humann 1999). We also considered flight to have occurred when the fish increased its swim speed to greater than the approach speed of the diver (Januchowski-Hartley et al. 2011, 2012). We estimated FID of 60 individuals of each of the four species: 30 on coral reefs and 30 on rocky reefs.
There is no consensus on the influences of body size on FID (Gotanda et al. 2009; Feary et al. 2011); therefore, only adults were selected based on size and color. There are considerable differences in color among these labrids, as well as between the life phases within each species (Martha and Jones 2002; Nunes et al. 2013), and only adults were selected based on size and color. Terminal phases (TP) were easily distinguished by their bolder color patterns (except H. poeyi), where changes in morphology and size were utilized.
Fish escape responses usually consist of a C-start maneuver, which involves the unilateral contraction of the body musculature into a ‘C’ shape (Bohorquez-Herrera et al. 2013). Escape behaviors were immediately observed after each FID sample and categorized in: ‘Leave the habitat,’ where fish swam in the direction of unconsolidated substratum (usually sand); ‘Run away,’ where fish fled swimming away; ‘Fled to the hole,’ where the fish sought a hole and went into hiding; ‘Curiosity,’ where the fish fled and curiously returned swimming near the diver; and ‘Fled into group,’ where fish swam into a group. All escape behaviors were observed in the maximum limit of 2 meters from the diver.
Structural complexity
The structural complexity was studied using the Rugosity index (RI) (Graham and Nash 2013). In order to measure rugosity, a 1-m chain was draped over the substrate, conforming as closely as possible to all of its contours and crevices, and a measure of the actual surface distance relative to the linear distance was thus obtained (Luckhurst and Luckhurst 1978). For each FID observation, rugosity was measured where fish fled; thus, 240 samples were obtained. Rugosity index (RI) was calculated as: RI = linear/surface. Linear was the distance covered when the chain was pulled taut, and surface was the linear distance between the start and the end of the chain while over the bottom.
Data analysis
One-way ANOVA was used to test for significant differences in structural complexity (estimated by the Rugosity index) between reef habitats. Analysis of covariance (ANCOVA) was used to investigate if structural complexity (a continuous variable) and type of reef habitats (two categories: coral reefs and rocky reefs) influenced FID (dependent variable) of labrids. The normality and the homogeneity of the data were assessed with quantile–quantile (Q–Q) plots and the Levene’s test, respectively. FID was log (x + 1) transformed. ANOSIM was performed to test if there was any difference in the frequencies of escape behaviors between reef habitats. Bray-Curtis similarity test was utilized for this analysis (Clarke et al. 2006). When escape behaviors varied significantly within species, we used nonlinear logistic regression to evaluate the influence of structural complexity on two escape behaviors (‘fled in group’ and ‘fled to the hole’). Those behaviors were chosen because structural complexity is positively correlated with fish densities and with number of shelters (Hixon and Beets 1993; Graham and Nash 2013). Logistic regression analyses were performed in StatSoft STATISTICA, version 8.0. ANOVA and ANCOVA were performed using Software R version 2.12.1 for Windows R 40 (R Development Core Team 2012). The Primer Software was used for ANOSIM analysis (Clarke and Warwick 2001). For all tests, the α-value adopted was 0.05.
Results
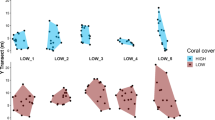
ANCOVA analysis showed significant differences on FID between the studied habitat types (Table 1). We found that the FID of H. brasiliensis, H. penrosei and S. axillare was higher on rocky reefs when compared to coral reefs (Fig. 1 a, b, d). There were significant differences in the structural complexity between the two studied habitats (F = 159; P < 0.01). Higher values of the RI were obtained on coral reefs (2.4 mean ± 0.33 SD) than on rocky reefs (1.8 ± 0.4). Structural complexity negatively influenced FID in H. brasiliensis and S. axillare (Fig. 1; Table 1).
Relationship between habitat types and structural complexity and FID. Black dots represent the measurements on rocky reefs; white dots represent the measurements performed on coral reefs. Continuous line represents best fit for rocky reefs and dotted line for coral reefs. a H. brasiliensis, b H. penrosei, c H. poeyi and d S. axillare. FID was log (x + 1) transformed
The frequencies of escape behaviors varied between species and habitats (Fig. 2). Only S. axillare developed the behavior ‘fled to the hole’ in both habitats, with this behavior being more frequent on coral reefs. ‘Leave the habitat’ and ‘curiosity’ reached higher values for H. poeyi and H. penrosei on both habitat types (Fig. 2 a, b). ‘Run away’ was the most common escape behavior for all species. ‘Fled in group’ was more frequent for H. brasiliensis than for other species. There were significant differences in the frequency of escape behaviors for S. axillare (Global R = 0.071; P = 0.009); however, no differences were found for H. brasiliensis (Global R = −0.017; P = 0.98), H. penrosei (Global R = −0.012; P = 0.79) and H. poeyi (Global R = −0.02; P = 0.95).
Logistic regression showed that the escape behavior ‘fled to the hole’ of S. axillare on coral reefs was influenced by structural complexity, and adults of this species require complex habitats to use holes as a refuge from predators (Table 2). Conversely, this species was not significantly influenced by structural complexity when developed the ‘fled in group’ behavior on coral and rocky reefs and ‘fled to the hole’ on rocky reef habitats.
Discussion
The species of Halichoeres and Sparisoma studied can be found in several types of habitats (Rocha 2004; Rocha et al. 2005; Bonaldo et al. 2006; Nunes et al. 2013), and their FIDs vary according to different habitats. We believe that in complex habitats, the higher density of preys (see Hixon and Beets 1993) could be explained by antipredator behavior and flight capacity. Thus, the antipredator behavior and flight capacity could probably help to understand this pattern described by Hixon and Beets (1993), once the prey species would need to be able to reach the shelters within reef habitats or to keep a safe distance from the predators (when foraging). Habitat use is known to be correlated with swimming ability (Fulton et al. 2001), and it shapes coral reef fish assemblages (Fulton and Bellwood 2004; Fulton et al. 2005). We believe that the capacity to escape and strategies of antipredator behavior to be also correlated with the species’ swimming ability.
The study showed that the FID was strongly associated with structural complexity for two studied species: S. axillare and H. brasiliensis. Results for those species showed the decrease in the FID inhabitats with higher structural complexity, probably because sites with higher structural complexity have more shelter and can provide more possibilities to short escapes than areas with lower structural complexity. On the other hand, H. penrosei and H. poeyi were not influenced by structural complexity. These species inhabit zones with less rugosity, especially H. poeyi, which can be easily found on the edges of coral and rocky reefs (Rocha et al. 2005; Nunes et al. 2013).
We argue that methodology used can provide good results for harvest species, however, to develop studies on FID of non-harvest species; artificial predators might bring better results than human stimulus, since predator recognition is often dependent upon experience (Griffin et al. 2001; Stankowich and Blumstein 2005), and variation in predator lethality influences the predator recognition abilities (Blumstein et al. 2006).
Generally, coral reefs are naturally more complex environments in comparison with rocky reefs. Corals and the other reef builder organisms (such as crustose coralline algae) through their hermatypic actions are constantly acting for the increase in tridimensional complexity, which is directly associated with the increase in biodiversity (Hoegh-Guldberg 2006; Hughes et al. 2007; Graham et al. 2014). In rocky reefs, Ferreira et al. (2001) suggested that complexity is determined mainly by the density of holes, caused by the superposition of boulders. There are different methods to assess structural complexity, from arbitrary visual ratings (Polunin and Roberts 1993) to the more recent digital rugosity (Dustan et al. 2013). We used a fairly common methodology on reef habitats studies, i.e., chain method (Graham and Nash 2013; Chaves and Monteiro-Neto 2009). However, when rugosity is measured using a chain, different surfaces can reach similar rugosity. For instance, one reef with several small crevices (i.e., shelters) may show the same rugosity of another reef with one single depression or one single boulder. Thus, FID and escape behaviors will be likely different in spite of the same estimates of rugosity. Future studies should properly investigate how number of shelters and obstacles can influence FID and escape behaviors.
The most frequent escape behavior of all four species of labrids was ‘run away.’ On coral reefs, several individuals of S. axillare exhibited ‘fled to holes’ as a significant antipredator behavior. Conversely, H. penrosei and H. poeyi never ‘fled to holes.’ Halichoeres species always searched for open areas without obstacles during the escape. The increase in complexity may increase the number of obstacles, making it more difficult to run away from predators. On the other hand, if obstacles are overcome, flight could be facilitated through confounding predators on which way they should follow to find the prey. ‘Leave the habitat’ presented low frequency for all species probably due to higher chances of predation in such areas. Thus, we believe that habitat configuration might be determinant in the choices of antipredator behaviors. The present study strongly suggest that further research involving flight ecology should include escape behaviors as an important way to better understand the indirect consequences of predation.
This paper highlights the importance of structural complexity on FID and escape behaviors. Reef ecosystems are in global decline because of growing pressures on biodiversity (Bellwood et al. 2004; Jackson 2008, 2010). In Brazil, reef ecosystems are following worldwide trends of degradation with impacts including coral diseases (Francini-Filho and Moura 2008), bycatch and overfishing (Floeter et al. 2007; Nunes et al. 2010), nutrient enrichment (Costa et al. 2000), sedimentation (Loiola et al. 2013), as well as the aquarium trade (Gasparini et al. 2005; Sampaio and Nottingham 2008). These human-mediated disturbs directly or indirectly endanger corals (the main reef builder) and favors non-builder and tolerant organisms (such as macroalgae), affecting reef growing and consequently structural complexity (Bryant et al. 2000; Bellwood et al. 2004; Hughes et al. 2007; Halpern et al. 2008). Many coral reefs are changing in composition species due to the different vulnerability and recovery potential of corals and other organisms, consequence of the various human impacts (Graham et al. 2014). These ecosystem modifications could affect the behavior (e.g., FID and escape behaviors) of reef fishes and its ecological relationship (e.g., predation, territorialism, competition for shelter).
‘Landscapes of fear’ are perceptual areas of fear defined topographically by differing levels of predation risk across space and time (Laundré et al. 2010). In the ocean, ‘seascapes of fear’ were recognized for marine mammals (Wirsing et al. 2008). For reef fishes, fear (measured by flight initiation distance) was higher outside marine-protected areas (Januchowski-Hartley et al. 2012). However, there are many gaps involving the ecology of fear for reef fishes. In general, our results suggest that ‘seascapes of fear’ will increase with the increase in habitat degradation (by reduction of structural complexity), and it could have many negative consequences in the reef ecosystems.
References
Almany G (2004) Does increased habitat complexity reduce predation and competition in coral reef fish assemblages? Oikos 106:275–284. doi:10.1111/j.0030-1299.2004.13193.x
Bellwood DR, Hughes TP, Folke C, Nyström M (2004) Confronting the coral reef crisis. Nature 429:827–833. doi:10.1038/nature02691
Bernardi G, Robertson DR, Clifton KE, Azzurro E (2000) Molecular systematics, zoogeography, and evolutionary ecology of the Atlantic genus Sparisoma. Mol Phylogenet Evol 15:292–300. doi:10.1006/mpev.1999.0745
Blumstein DT (2003) Flight initiation distance in birds is dependent on intruder starting distance. J Wildl Manag 67:852–857
Blumstein DT, Harcourt LL, Ross G (2003) Testing a key assumption of wildlife buffer zones: is flight initiation distance a species-specific trait? Biol Conserv 110:97–100. doi:10.1016/S0006-3207(02)00180-5
Blumstein DT, Fernández-Juricic E, Zollber PA, Garity SC (2005) Inter-specific variation in avian responses to human disturbance. J Appl Ecol 42(5):943–953. doi:10.1111/j.1365-2664.2005.01071.x
Blumstein DT, Bitton A, Veiga J (2006) How does the presence of predators influence the persistence of antipredator behavior? J Theor Biol 239:460–468. doi:10.1016/j.jtbi.2005.08.011
Bohorquez-Herrera J, Kawano SM, Domenici P (2013) Foraging behavior delays mechanically-stimulated escape responses in fish. Integr Comp Biol 53:780–786. doi:10.1093/icb/ict031
Bonaldo RM, Krajewski JP, Sazima C, Sazima I (2006) Foraging activity and resource use by three parrotfish species at Fernando de Noronha Archipelago, tropical West Atlantic. Mar Biol 149:423–433. doi:10.1007/s00227-005-0233-9
Bryant D, Burke L, Mcmanus J, Spalding M (2000) Reef at risk: a map-based indicator of threats to the world’s coral reefs. World Resources Institute, Washington, DC, USA (http://www.wri.org/reefsatrisk/)
Chaves LCT, Monteiro-Neto C (2009) Comparative analysis of rocky reef fish community structure in coastal islands of south-eastern Brazil. J Mar Bio Assoc UK 89:609–619. doi:10.1017/S0025315408002695
Clarke KR, Warwick RM (2001) Changes in marine communities: an approach to statistical analysis and interpretation, 2nd edn. Plymouth Marine Laboratory, PRIMER-E 172 p
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J Exp Mar Biol Ecol 330:55–80. doi:10.1016/j.jembe.2005.12.017
Coni EOC, Nunes JACC, Sampaio CLS (2007) Halichoeres penrosei (Labridae), a sporadic cleaner wrasse. Mar Biodivers Rec 1:e82. doi:10.1017/S1755267207008494
Coni EOC, Nunes JACC, Ferreira CM, Maia-Nogueira R, Medeiros DV, Sampaio CLS (2010) The Spanish hogfish Bodianus rufus (Labridae) acting as cleaner of nocturnal fish in the north–east of Brazil. Mar Biodivers Rec 3:e23. doi:10.1017/S1755267210000187
Cooper WE Jr (2008) Visual monitoring of predators: occurrence, cost and benefit for escape. Anim Behav 76:1365–1372
Cooper WE Jr (2009) Flight initiation distance decreases during social activity in lizards (Sceloporusvirgatus). Behav Ecol Sociobiol 63:1765–1771. doi:10.1007/s00265-009-0799-1
Cooper WE Jr, Pérez-Mellado V, Baird T, Baird TA, Caldwell JP, Vitt LJ (2002) Effects of risk, cost, and their interaction on optimal escape by nonrefuging Bonaire whiptail lizards. Cnemidophorus murinus Behav Ecol 14:288–293. doi:10.1093/beheco/14.2.288
Costa OS Jr, Leão ZMAN, Nimmo M, Atrill M (2000) Nutrification impacts on coral reefs from Northern Bahia, Brazil. Hydrobiologia 440:307–316
DeLoach N, Humann P (1999) Reef fish behavior: Florida, Caribbean, Bahamas. New World Publications, Jacksonville 359p
Dustan P, Doherty O, Pardede S (2013) Digital reef rugosity estimates coral reef habitat complexity. PLoS One 8(2):e57386. doi:10.1371/journal.pone.0057386
Feary DA, Graham NAJ, Cinner JE, Januchowski-Hartley FA (2011) The impacts of customary marine closures on fish behaviour with implications for spear fishing success and underwater visual census. Conserv Biol 25:341–349. doi:10.1111/j.1523-1739.2010.01613.x
Ferreira CEL, Gonçalves JEA, Coutinho R (2001) Fish community structure and habitat complexity in a tropical rocky shore. Environ Biol Fish 61:353–369. doi:10.1023/A:1011609617330
Floeter SR, Krohling W, Gasparini JL, Ferreira CEL, Zalmon IL (2007) Reef fish community structure on coastal island of southeastern Brazil: the influence of exposure and benthic cover. Environ Biol Fish 78:147–160. doi:10.1007/s10641-006-9084-6
Francini-Filho RB, Moura RL (2008) Dynamics of fish assemblages on coral reefs subjected to different management regimes in the Abrolhos Bank, eastern Brazil. Aquat Conserv Mar Freshw Ecosys 18:1166–1179. doi:10.1002/aqc.966
Fulton CJ, Bellwood DR (2004) Wave exposure, swimming performance, and the structure of tropical and temperate reef fish assemblages. Mar Biol 144:429–437. doi:10.1007/S00227-003-1216-3
Fulton CJ, Belwood DR (2002) Patterns of foraging in labrid fishes. Mar Ecol Prog Ser 226:135–142. doi:10.3354/meps226135
Fulton CJ, Bellwood DR, Wainwright PC (2001) The relationship between swimming ability and habitat use in wrasses (Labridae). Mar Biol 139:25–33. doi:10.1007/s002270100565
Fulton CJ, Bellwood DR, Wainwright PC (2005) Wave energy and swimming performance shape coral reef fish assemblages. P R Soc Lond B 272:827–832. doi:10.1098/rspb.2004.3029
Gasparini JL, Floeter SR, Ferreira CEL, Sazima I (2005) Marine ornamental trade in Brazil. Biodivers Conserv 14:2883–2899. doi:10.1007/s10531-004-0222-1
Gotanda KM, Turgeon K, Kramer DL (2009) Body size and reserve protection affect flight initiation distance in parrotfishes. Behav Ecol Sociobiol 63:1563–1572. doi:10.1007/s00265-009-0750-5
Graham NAJ, Nash KL (2013) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32:315–326. doi:10.1007/s00338-012-0984-y
Graham NAJ, Cinner JE, Norstrom AV, Nyström M (2014) Coral reefs as novel ecosystems: embracing new futures. Curr Opin Env Sust 7:9–14. doi:10.1016/j.cosust.2013.11.023
Griffin AS, Evans CS, Blumstein DT (2001) Learning specificity in acquired predator recognition. Anim Behav 62:577–589. doi:10.1006/anbe.2001.1781
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Elbert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319:948–952. doi:10.1126/science.1149345
Hixon MA, Beets JP (1993) Predation, prey refuges, and the structure of coral-reef fish assemblages. Ecol Monogr 63:77–101. doi:10.2307/2937124
Hobson ES (1975) Feeding patterns among tropical fishes. Am Sci 63:382–392
Hoegh-Guldberg O (2006) Complexities of coral reef recovery. Science 311:42–43. doi:10.1126/science.1122951
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007) Phase shifts, herbivory and the resilience of coral reefs to climate change. Curr Biol 17:1–6. doi:10.1016/j.cub.2006.12.049
Jackson JBC (2008) Ecological extinction and evolution in the brave new ocean. Proc Natl Acad Sci USA 105:11458–11465. doi:10.1073/pnas.0802812105
Jackson JBC (2010) The future of the oceans past. Philos Trans R Soc B 365:3765–3778. doi:10.1098/rstb.2010.0278
Januchowski-Hartley FA, Graham NAJ, Feary DA, Morove T, Cinner JE (2011) Fear of fishers: human predation explains behavioral changes in coral reef fishes. PLoS One 6(8):e22761. doi:10.1371/journal.pone.0022761
Januchowski-Hartley FA, Nash KL, Lawton RJ (2012) The influence of spear guns, dive gear, and observers on estimating fish flight initiation distance on coral reefs. Mar Ecol Prog Ser 469:113–119. doi:10.3354/meps09971
Januchowski-Hartley FA, Graham NAJ, Cinner JE, Russ GR (2013) Spillover of fish naïveté from marine reserves. Ecol Lett 16:191–197. doi:10.1111/ele.12028
Jones GP, Syms G (1998) Disturbance, habitat structure and the ecology of reef fish on coral reefs. Aust J Ecol 23:287–297. doi:10.1111/j.1442-9993.1998.tb00733.x
Krajewski JP, Floeter SR, Jones G, Leite F (2010) Patterns of variation in behavior within and among reef fishes species on an isolated tropical island: influence of exposure and substratum. J Mar Biol Assoc UK 91:1359–1368. doi:10.1017/S0025315410000111
Laundré JW, Hernández L, Ripple WJ (2010) The landscape of fear: ecological implications of being afraid. Open Ecol J3:1–7. doi:10.2174/1874213001003030001
Loiola M, Oliveira MD, Kikuchi RKP (2013) Tolerance of Brazilian brain coral Mussismilia braziliensis to sediment and organic matter inputs. Mar Pollut Bull 77:55–62. doi:10.1016/j.marpolbul.2013.10.033
Luckhurst BE, Luckhurst K (1978) Analysis of influence of substrate variables on coral reef fish communities. Mar Biol 49:317–324. doi:10.1007/BF00455026
Maia-Nogueira R, Medeiros DV, Jardim A, Nunes JACC, Sampaio CLS (2010) Banded butterflyfish Chaetodon striatus (Chaetodontidae) cleaning the green turtle, Chelonia mydas (Cheloniidae). Mar Biodivers Rec 3:e116. doi:10.1017/S1755267210001041
Martha M, Jones KMM (2002) Behavioural overlap in six Caribbean labrid species: intra- and interspecific similarities. Environ Biol Fish 65:71–81. doi:10.1023/A:1019675323053
Miranda RJ, Cruz ICS, Leão ZMAN (2013) Coral bleaching in the Caramuanas reef (Todosos Santos Bay, Brazil) during the 2010 El Niño event. Lat Am J Aquat Res 41:351–360. doi:10.3856/vol41-issue2-fulltext-14
Nunes JACC, Medeiros DV, Reis-Filho JA, Sampaio CLS, Barros F (2010) Reef fishes captured by recreational spearfishing on reefs of Bahia State, northeast Brazil. Biota Neotrop 12:179–185. http://www.biotaneotropica.org.br/v12n1/pt/abstract?article+bn02012012012
Nunes JACC, Sampaio CLS, Barros F (2013) How wave exposure, group size and habitat complexity influence foraging and population densities in fishes of the genus Halichoeres (Perciformes: Labridae) on tropical rocky shores. Mar Biol 160:2383–2394. doi:10.1007/s00227-013-2233-5
Polunin NVC, Roberts CM (1993) Greater biomass and value of target coral reef fishes in two small Caribbean marine reserves. Mar Ecol Prog Ser 100:167
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN: 3-900051-07-0. http://www.R-project.org/
Rocha LA (2004) Mitochondrial DNA and color pattern variation in three western Atlantic Halichoeres (Labridae), with the revalidation of two species. Copeia 4:770–782. doi:10.1643/CG-04-106
Rocha LA, Robertson DR, Roman J, Bowen BW (2005) Ecological speciation in tropical reef fishes. P R Soc B 272:573–579. doi:10.1098/2004.3005
Rocha LA, Pinheiro TH, Gasparini JL (2010) Description of Halichoeres rubrovirens, a new species of wrasse (Labridae: Perciformes) from the Trindade and Martin Vaz Island group, southeastern Brazil, with a preliminary mtDNA molecular phylogeny of New World Halichoeres. Zootaxa 2422:22–30
Runyan AM, Blumstein DT (2004) Do individual differences influence flight initiation distance? J Wild Manag 68:1124–1129. doi:10.2193/0022-541X(2004)068[1124:DIDIFI]2.0.CO;2
Sampaio CLS, Nottingham MC (2008) Guia para identificação de peixes ornamentais volume I: espécies marinhas. IBAMA, Brasília, p 205
Sazima I, Moura RL, Gasparini JL (1998) The wrasse Halichoeres cyanocephalus (Labridae) as a specialized cleaner fish. Bull Mar Sci 63:605–610
Sazima C, Bonaldo RM, Krajewski JP, Sazima I (2005) The Noronha wrasse: a jack-of-all-trades follower. Aqua J Ichthyol Aquat Biol 9:97–108
Stankowich T (2008) Ungulate flight responses to human disturbance: a review and meta-analysis. Biol Conserv 141:2159–2173. doi:10.1016/j.biocon.2008.06.026
Stankowich T, Blumstein DT (2005) Fear in animals: a meta-analysis and review of risk assessment. Proc R Soc B 272:2627–2634. doi:10.1098/rspb.2005.3251
Streelman JT, Alfaro M, Westneat MW, Bellwood DR, Karl SA (2002) Evolutionary history of the parrotfishes: biogeography, ecomorphology, and comparative diversity. Evolution 56:961–971. doi:10.1111/j.0014-3820.2002.tb01408.x
Wirsing AJ, Heithaus MR, Frid A, Dill LM (2008) Seascapes of fear: evaluating sublethal predator effects experienced and generated by marine mammals. Mar Mam Sci 24:1–15. doi:10.1111/j.1748-7692.2007.00167.x
Acknowledgments
We thank Abraão Nunes (Grupo Nunes, BR) for helping during the field work. Lili Colman (University of Exeter, UK), Miguel Loiola (Universidade Federal da Bahia, BR) and Luciana Leite (University of Cambridge, UK) for help in the revision. Mariana Thevenin (Universidade Federal da Bahia, BR) for her help with the figures and analysis. Fraser A. Januchowski-Hartley (James Cook University, AUS) sent valuable references, and Nick Graham (James Cook University, AUS) and Daniel Blumstein (University of California, Los Angeles, EUA) exchanged ideas with the first author. We also thank CAPES for the financial support to J. A. C. C. N.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Goulet.
Rights and permissions
About this article
Cite this article
Nunes, J.A.C.C., Sampaio, C.L.S. & Barros, F. The influence of structural complexity and reef habitat types on flight initiation distance and escape behaviors in labrid fishes. Mar Biol 162, 493–499 (2015). https://doi.org/10.1007/s00227-014-2578-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2578-4