Abstract
Marine communities face continuing and accelerating climate change. Predicting which species will go extinct or persist in future climates requires assessing redistribution potential and tolerance to warming, both of which can depend on dispersal ability. We evaluated biophysical processes that could promote population persistence under changing climatic conditions by (1) promoting poleward dispersal in an Eastern Boundary Current region, where offshore currents flow predominantly equatorward, and (2) increasing the frequency of more thermotolerant phenotypes in marine populations. We paired intensive time-series observations (during 2014 and 2015) of recruitment and thermal tolerance limits for cohorts of marine mussels with simulated larval transport using a high-resolution, 3D coastal circulation model of the northeastern Pacific. We used the modeling results to predict the proportion of individuals in each recruiting cohort that originated from sources south or north of our study site on the USA west coast (45.50°N, 123.95°W) as well as the environmental conditions experienced in the water column. We found that the coastal upwelling index was related to origin of individuals within recruiting cohorts, with poleward recruitment predicted to increase under downwelling conditions. Furthermore, thermal tolerance limits were higher in cohorts predicted to experience higher and more variable temperatures during dispersal. These findings highlight complex links between demographic and physical transport processes as well as the potential for climate-driven changes in wind patterns to indirectly affect species’ abilities to cope with increasing temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The loss of diversity that accompanies extinctions is among the most extreme impacts of climate change (Sala et al. 2000). Extinctions over the past century (Pounds et al. 1999; Parmesan 2006) are minor in comparison to those expected in the future under accelerating climatic shifts (Thomas et al. 2004; Maclean and Wilson 2011; Pacifici et al. 2015). As environmental conditions exceed the tolerances and acclimation capacities of individuals (Deutsch et al. 2008; Somero 2010), persistence of populations and species will increasingly depend on two co-occurring and interacting mechanisms: evolutionary adaptation and redistribution (Jump and Penuelas 2005; Berg et al. 2010). Here, we investigate the potential importance of oceanic conditions in promoting adaptation and redistribution (and, thus, persistence) of marine species in the face of climate change.
Both redistribution and adaptation (because of its reliance on gene flow) depend on dispersal, and dispersal depends on an interplay between physical processes and biological factors (e.g., Pineda et al. 2007; Cowen and Sponaugle 2009; Pineda et al. 2010; Bestion et al. 2015; Benestan et al. 2016). Propagule dispersal (which occurs in 34/40 marine phyla and the majority of marine invertebrate species; Pechenik 1999) is the most likely process allowing compensatory range shifts and gene flow for benthic marine species, with dispersal distances ranging from < 1 m (Kinlan and Gaines 2003) to at least 97 km (e.g., McQuaid and Phillips 2000; Gilg and Hilbish 2003). As compared to more nektonic species, the dispersal of benthic species with planktonic larvae (which typically swim much more slowly than ocean currents due to their small size and the influence of fluid viscosity; Chia et al. 1984) is largely dependent on ocean circulation patterns (Williams and Hastings 2013).
In addition to their role in poleward redistribution, dispersive larvae vary in their ability to promote population persistence by surviving and reproducing once they join adult populations. This variation is due to differences in genotypes, maternal/paternal effects, and pre-settlement acclimation or selection for traits such as environmental stress tolerance (Miller et al. 2009; Marshall and Morgan 2011; Lymbery and Evans 2013; Jensen et al. 2014; Foo et al. 2016). For example, in a laboratory breeding experiment, Foo et al. (2016) found that the response of Antarctic sea urchins to simulated climate change depended on interactive maternal × paternal and environmental (temperature × pH) effects, and responses were positively correlated across life stages. Thus, demographic connectivity patterns could set the stage for adaptive evolution and rescue of populations becoming increasingly maladapted—or unmatched—to environmental conditions as the climate changes (Pringle et al. 2011).
The ability of populations and species of benthic organisms to persist in a changing climate is, therefore, inextricably linked to flow patterns (Gaylord and Gaines 2000; Byers and Pringle 2006; Sorte 2013). Poleward flows (characteristic of Western Boundary Currents) could facilitate redistribution that parallels shifting climates, as well as delivery of propagules from warmer latitudes that are selected for increased thermal tolerance. Dominant equatorward flows (characteristic of Eastern Boundary Currents) might inhibit this poleward redistribution (Keith et al. 2011; Sorte 2013; van Gennip et al. 2017). However, asymmetry in flow and dispersal is not absolute: theoretical models show that heterogeneity in flow patterns can allow larval transport “against the flow” (Byers and Pringle 2006), and this is required for population persistence in advective systems (Pachepsky et al. 2005). The dominant current along the west coast of North America is the California Current, which flows primarily equatorward (north to south; Lynn and Simpson 1987), contrary to the predicted poleward shift in surface temperature clines (Loarie et al. 2009; Burrows et al. 2011). Deviations in this mean flow occur seasonally with the intensification of the Davidson/inshore counter-current (Reid and Schwartzlose 1962; Lynn and Simpson 1987) and on shorter timescales (Kim et al. 2013), particularly on the inner shelf near the shoreline (Washburn and McPhee-Shaw 2013). Coastal wind-driven upwelling and downwelling, mesoscale eddies, and freshwater plumes are common flow features on the continental shelf (Huyer 1983; Strub et al. 1987). The coasts of Oregon and Washington, USA are characterized by upwelling cycles of approximately 3–10 days, with equatorward winds driving upwelling that is interspersed with periods of relaxation or downwelling when winds slow and/or reverse (Landry and Hickey 1989). Variability in currents is a mechanism that might permit poleward dispersal of planktonic larvae. Furthermore, larvae are not passive particles, and swimming behaviors can modify their position in the water column and the influence of flow conditions on horizontal displacement (Poulin et al. 2002; Pineda et al. 2010; Weidberg et al. 2015).
While the direction of flow is likely to affect redistribution, the environmental conditions that larvae encounter during dispersal could influence their demographic rates and growth of the populations as a whole. Matrix population modeling work by Carson et al. (2011) indicated that larval dispersal patterns and juvenile survival—the processes we consider in this study—were two of the most important parameters determining growth of marine mussel populations in southern California, USA. Water masses differ in characteristics including temperature, salinity, and food availability (Hickey et al. 2016), and environmental exposure during pelagic larval stages could lead to differential acclimation and pre-settlement selection. Furthermore, flow patterns can influence both the direction of larval dispersal and of pre-settlement selection for stress tolerance, such that population persistence under climate change might be enhanced by one or both mechanisms. For example, the East Australian Current (a Western Boundary Current) has strengthened, expanding the dispersal and range of a sea urchin to the coast of Tasmania, while also bringing warmer waters that influence urchin demographic rates and population sizes (Ling et al. 2009). Along the coast of Oregon, USA, freshwater fronts formed at the Columbia River mouth are characterized by lower salinity, and these fronts could select for tolerance to low salinity while acting as conduits to move larvae along the coastline during downwelling periods (Giddings et al. 2014). Ultimately, information about tolerance limits can help us to anticipate climate-change sensitivity and which populations and species are likely to be “winners” or “losers” of climate change (Pörtner et al. 2006; Somero 2010).
We investigated the relationship between circulation patterns, dispersal, and environmental tolerances using mussels in the genus Mytilus as our study species. Mussels are benthic bivalves and are dominant foundation species throughout many of the world’s upwelling regions (Ricciardi and Bourget 1999). In the northeastern Pacific, two Mytilus species—M. californianus and M. trossulus—are common and often dominant inhabitants of rocky substrata at intertidal sites between Baja California and Alaska (Suchanek 1981; Sagarin and Gaines 2002; Blanchette et al. 2008). Mussels begin their lives as planktonic larvae, which typically spend 3–5 weeks in the water column (Seed 1969) before recruiting into sessile adult populations. Because Mytilus species are essentially morphologically identical as recruits, we have taken the most conservative approach here of referring to Mytilus spp. (also see e.g., Menge et al. 2009). Previously estimated dispersal distances for mytilid mussels range from an average of 35 km (based on geochemical fingerprinting of M. californianus shells in southern California; Carson et al. 2010) to over 90 km (based on the spread of Mytilus galloprovincialis invasion fronts; McQuaid and Phillips 2000; Gilg and Hilbish 2003).
In this study, we addressed the role of wind-driven flow variability in determining population persistence by influencing the level of poleward dispersal and the delivery of larvae with increased thermal tolerance limits. We combined field and laboratory studies of mussel recruitment rates and recruiting cohort tolerance limits with oceanographic modeling studies to predict recruit origins and environmental conditions along dispersal trajectories. This integrative approach allowed us to address two specific questions:
-
1.
Are transitions in wind and flow patterns related to differences in larval recruitment rates and predicted larval origins (i.e., from the north versus south), and
-
2.
Are thermal tolerance limits of larval recruits related to flow patterns and the environmental conditions experienced along the larval pathway?
Materials and methods
Mussel recruitment and thermal tolerance limits
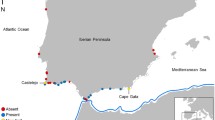
We quantified recruitment and recruit thermal tolerance limits over time-series encompassing variable oceanographic flow patterns. Upon considering the trade-offs of increasing replication (and, thus, ability to generalize) across sites, days, and years, we decided to focus on a single site and maximize temporal replication (multiple days in two separate years). Our study site was Cape Meares (CM; 45.50°N, 123.95°W)—a rocky headland along the northern Oregon coast—chosen because of its location near the center of the region included in our oceanographic model, described below (Fig. 1, S1), and the availability of historical mussel recruitment data (Menge et al. 2009). The mid-intertidal zone at CM is dominated by M. californianus (Bracken et al. 2012; Wallingford and Sorte, unpubl. data). Time periods of our field observations were designed to capture both peak recruitment season (summer–early fall; Menge et al. 2009) and a downwelling transition during the Oregon upwelling season (Landry and Hickey 1989). These periods included 12 days in 2014 (21 August–02 September) and 18 days in 2015 (14–31 July). Mussels recruiting from the water column to the benthic intertidal community were collected in plastic Tuffy® scrubbers (which mimic natural recruitment substrates). On the first day of each sampling period, we attached N = 15 Tuffy® recruitment collectors (except N = 10 used 21–23 August 2014) to the rock at 1.58 ± 0.05 m (mean ± 1 SE) above mean lower-low water, in the middle of the mussel zone. On each subsequent day, we collected and deployed new Tuffies® as soon as they were made accessible by the falling daytime tide, and we used a cooler with ice packs to transport the Tuffies® ~5 km to the processing site where they were immediately examined.
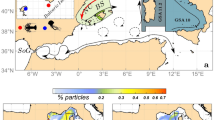
Map of a portion of the modeling domain showing a the initial positions of particles before a particle tracking run and particle positions after b 5 days and c 2 weeks in a representative model run. Particles were released daily from sites to the north (blue) and south (red) of the Cape Meares study site (green triangle in a)
Mussel recruits collected in each Tuffy® were counted under a dissecting scope and scored as live or dead based on observed movement. Recruitment rates were recorded as number of total recruits per collector per day. Live recruits were kept in chilled seawater at approximately ambient ocean temperature (Fig. S1; ~11 °C in 2014 and ~15 °C in 2015) until the start of the thermal tolerance limit assay.
Across the two study periods, we determined the thermal tolerance limits of recruiting cohorts, which were defined as all live mussels arriving at CM on a single day. Thermal tolerance limit assays were conducted in air in 1.5 ml microcentrifuge tubes in dry baths using methods adapted from Jenewein and Gosselin (2013). Tubes contained a seawater-soaked, 1 cm2 chamois to prevent desiccation, and mussels were exposed for 6 h to one of 5 randomly assigned temperatures: ambient (control), 28, 30, 32, and 34 °C. When cohorts were not composed of a multiple of 5, additional individuals were assigned to intermediate (30 and 32 °C) treatments. This assay is designed to simulate heat stress during midday 6 h low tide exposures, with 30 °C corresponding to the 99th percentile of daily average temperatures encountered annually on the central Oregon coast (Helmuth et al. 2002; also see Petes et al. 2008). Survival was assessed after an immersed recovery period via inspection for movement or responsiveness to probing under a dissecting microscope, and mussel length was measured using a stage micrometer.
Number of mussels per cohort varied, depending on daily recruitment rates (Fig. 2), with individuals per temperature averaging 3.80 ± 0.34 in 2014 and 3.75 ± 0.24 in 2015 (Table S1; note: these values represent occasional losses of individuals for which survival could not be assessed). For each daily cohort, we used a generalized linear model to fit a binomial distribution to the survival data (with values of “0” for dead or “1” for alive individuals) as a function of temperature (L. Miller, available at http://lukemiller.org) using R statistical software v. 3.2.2 (R Core Team 2015). We report thermal tolerance limit as the temperature lethal to 50% of individuals (LT50; Table S1), which is the temperature value at which this function predicts a survival probability of 0.5.
a, b Wind, upwelling, and c, d recruitment patterns during our studies conducted in a, c 2014 and b, d 2015. a, b Observed winds were measured by the Stonewall Bank Buoy (black line), and winds from ROMS model runs were from a a time series representative of observed 2014 winds (grey dashed line) or b the actual dates of our study in 2015 (grey solid line). 8-day upwelling indices were calculated as described in Methods. c, d Recruitment values are scaled proportional to the total recruits collected in the observed time series (black line) and predicted by the model (grey bars). Recruits from the particle tracking runs are divided by origin, either south (dark grey bars) or north (light grey bars) of our study site (see Fig. 1)
Comparisons are made within each year because thermal tolerance methods differed slightly between 2014 and 2015. Specifically, in 2014, temperature was increased over a 2-h period at a rate of 1 °C every 5–7 min (depending on treatment temperature), the 6 h exposure time started once the treatment temperature was reached, and recovery was assessed after 24 h. In 2015, temperature was increased at a rate of 1 °C every 2 min, the total thermal exposure (including time of temperature ramp) was 6 h, and recovery was assessed after 18 h.
As described above, we collected and deployed recruitment collectors every 24 h, as soon as they were exposed by the midday (hottest) low tide to minimize post-settlement selection for thermotolerance (see Johnson et al. 2014). This means that collectors underwent two periods of submersion prior to collection, and any recruits arriving to the collectors during the first period of submersion experienced emersed conditions during the nighttime low tide. We assessed potential post-settlement selection pressure based on temperatures recorded every 10 min throughout the sampling periods by N = 2 TidbiT® temperature data loggers (Onset Computer Corp., Bourne, MA; elevated ~2 cm in cages) deployed adjacent to the recruitment collectors. Specifically, we calculated the maximum temperature that an individual in each recruiting cohort could have experienced from the time the collectors were initially submerged to when they were retrieved.
Meteorological data
We compared wind data from two offshore buoys and two onshore weather stations, as shown in Fig. S2. For consistency, and due to good agreement among sites, we used data for coastal winds monitored continuously at Stonewall Bank (Buoy Station 46050; 44.66°N, 124.53°W), 35 km offshore of Newport, Oregon (NOAA National Data Buoy Center 2015). Values are presented as alongshore wind speed, with positive and negative values indicating northward and southward winds, respectively. To characterize the influence of the wind on larval transport, we calculated an 8-day weighted mean upwelling index (W 8d), as defined by Austin and Barth (2002), which has units of m2/s.
Oceanographic model
We conducted a modeling study to predict dispersal trajectories, including recruit origins and environmental conditions in transit. We simulated the transport of mussel larvae to our Cape Meares field site using a high-resolution, three-dimensional, coastal circulation model of the Oregon and Washington shelves and a Lagrangian particle tracking algorithm which has been developed for use with larval dispersal studies (Banas et al. 2009). Velocity fields are from an implementation of the Regional Ocean Modeling System (ROMS; Shchepetkin and McWilliams 2005) on the Pacific Northwest shelf called the “Cascadia” model, developed by the Coastal Modeling Group at the University of Washington. ROMS is a free surface, hydrostatic, primitive equation model, and the Cascadia implementation is forced with realistic atmospheric, tidal, river flow, and boundary conditions (Giddings et al. 2014). The model domain (43–50°N, 127–122°W; Fig. 1) encompasses the coastal ocean and estuaries of Oregon, Washington, and Vancouver Island, British Columbia, with horizontal resolution ranging from 1.5 km at the coast to 4.5 km offshore. Although this model does not include finer-scale (e.g., surf zone and coastal boundary layer; Nickols et al. 2012, 2015) dynamics, it allows us to estimate temporal variability in larval supply. Larval settlement is a function of larval supply, although the two may not be linearly related (Pineda et al. 2010). The model has 40 vertical, terrain-following layers with stretching parameters chosen to enhance resolution near the bottom and in the upper water column. Further details on the physical and ecosystem models—which predict environmental conditions along the larval pathways—as well as comprehensive skill assessments can be found in Davis et al. (2014), Giddings et al. (2014), and Siedlecki et al. (2015).
Hourly output from the Cascadia model was used to predict particle trajectories from an offline particle tracking scheme, which includes advection by currents in three dimensions plus a parameterization of vertical mixing (random displacement model; Visser 1997; North et al. 2008). The particle tracking code was modified to include a depth-seeking vertical swimming behavior following the methods of Drake et al. (2013). Using this approach, vertical swimming speed increases exponentially with distance away from a target depth:
where w max is the maximum swimming speed, sgn is the sign function, d is the target depth, z is the depth of the larva, and λ is a shape parameter set to a value of 12 m. The maximum swimming speed, w max, was set at 0.002 m s−1 and is within the range of maximum swimming speeds found in laboratory settings for Mytilus edulis (Sprung 1984; Troost et al. 2008). The target depth, d, was set to 20 m based on field observations by Shanks and Shearman (2009) showing that abundance of M. californianus larvae peaked at depths from 10–30 m off the Oregon coast (also see Morgan et al. 2009). The inclusion of swimming behavior was appropriate both because of laboratory studies of swimming speeds listed above and because model runs without swimming behavior indicated that particles were carried further offshore than has been observed (Shanks and Shearman 2009) (Fig. S3).
A Cascadia model hindcast of 2015 was used to simulate the period during the concurrent 2015 field experiment at Cape Meares. However, because model output for year 2014 was not available, Cascadia model hindcasts of 2005 and 2006 were used to closely match the 2014 wind conditions measured at Stonewall Bank (Fig. 2a). Using the physical output from ROMS, we virtually “seeded” the model with 3080 mussel larvae (particles) daily along 700 km of coastline, from Heceta Bank, Oregon (43°N) up to Vancouver Island, Canada (50°N) (Fig. 1a), beginning 4 weeks before the start of the field observation periods. We then considered particles that encountered the CM coastline (defined as passing shoreward of the 10 m isobaths in the CM pixel) between 3 and 5 weeks after release (the average larval duration of Mytilus mussels; Seed 1969) as “landed particles” or “recruits”. For each individual particle landing at CM, we determined whether it originated to the north versus south of CM (“origin”) as well as its “age” (i.e., days in the water column). We calculated the proportion of recruits originating from north or south of CM as a ratio to the total number of landed particles.
Modeled physical and biological variables were also extracted along the dispersal pathway to reconstruct the environmental history of each “larval” particle. Water temperature, salinity, and chlorophyll a (a proxy for food availability; available for 2014 only) values were extracted hourly from the ROMS output for each particle along its path, with particle location described as a function of latitude, longitude, and depth. We then calculated the daily minimum, maximum, average, and variance for each of the environmental variables.
Observed and modeled data integration
To assess model performance at predicting total larval recruitment, we used the Willmott Skill Score (WSS; Willmott 1982), defined as
where o i is an observation, m i is the corresponding model value, there are N paired modeled/observed values, and MSE is the mean square error. The WSS is a measure of the level of agreement between the observed and modeled values, with a value of 1 indicating perfect agreement and a value of 0 indicating no agreement.
We used general linear models (Proc GLM in SAS v. 9.4; SAS Institute Inc., Cary, NC, USA) to test the predicted relationships between upwelling, observed recruitment, predicted proportion of recruits originating to the south of CM, LT50, and predicted environmental conditions along the larval pathway summarized for the recruiting cohorts (our experimental units). We tested the assumptions of our statistical models using the Shapiro–Wilk test and visual inspection of residual plots. Data were log10 transformed as necessary.
Results
Both the field observations and particle tracking studies indicated an increase in recruitment following upwelling relaxations and during downwelling periods (Figs. 2, 3, Table S2). Stronger downwelling-favorable winds (described by positive upwelling index values) were associated with an increase in observed recruitment in 2015 (general linear model F 1,16 = 5.93, p = 0.027) and with a similar trend in 2014 (F 1,10 = 3.57, p = 0.088) (Figs. 2, 3). These patterns were particularly evident during the two largest transitions captured in our time-series—at the end of the 2014 study and beginning of the 2015 study (Fig. 2, S4)—while shorter-term fluctuations (e.g., year 2015 days 195–198) were less well resolved.
Relationship between upwelling index and a, b recruitment (observed in black and modeled in gray) and c, d proportion of recruits predicted to originate from the south in a, c 2014 and b, d 2015. Values are as described in Fig. 2. Upwelling and downwelling conditions are indicated by negative and positive index values, respectively
Agreement between the observed recruitment values and modeled estimates of larval supply was higher in the year 2015 (when parallel model runs were available) than in the year 2014 (when we used ROMS model runs from representative time periods), as indicated by the difference in Willmott Skill Scores (0.81 vs. 0.53, respectively). Furthermore, discrepancies in the comparison between observed and predicted recruitment values paralleled discrepancies between winds used to force the oceanographic model and actual wind patterns observed at the Stonewall Bank Buoy. The early 2014 predicted recruitment peak appears to reflect a response from the previous upwelling relaxation (which occurred immediately before our study period). In addition, at the end of 2014, observed winds shifted earlier than the winds from the representative ROMS model runs, and this paralleled an earlier recruitment peak in the observed than predicted recruitment data set (Fig. 2). In 2015, there was one notable discrepancy between observed and modeled recruitment in which predicted (but not observed) recruitment peaked near the end of the study period. Comparing buoy and ROMS winds in Fig. 2 shows a period of downwelling predicted by the model on year day 206 and 207, whereas the downwelling threshold was only minimally exceeded based on wind observations at the buoy. This downwelling-favorable transition observed primarily in the ROMS winds was also reflected in the salinity data: ROMS predicted a decline in salinity, indicative of a wind-driven freshwater front (Fig. S5).
The particle tracking study results also indicated links between wind-driven circulation and both recruitment rates and dispersal direction. In both years, more positive upwelling indices were associated with an increase in the proportion of recruits predicted to originate south of our study site (2014 F 1,9 = 9.59, p = 0.013; 2015 F 1,15 = 9.66, p = 0.0072) (Figs. 2, 3, Table S2). Thus, downwelling-favorable winds were related to an increase in predicted poleward dispersal in this location.
Our experimental results showed that thermal tolerance limits within the recruiting cohorts also varied concurrent with flow patterns (Fig. 4). LT50 values were higher in cohorts arriving during periods of greater upwelling (as indicated by more negative upwelling index values) (2014 F 1,7 = 6.02, p = 0.044; 2015 F 1,15 = 3.70, p = 0.074). At the same time, LT50 was negatively related to the proportion of recruits originating to the south in 2015 (F 1,14 = 5.47, p = 0.035), but did not differ with predicted origins in 2014 (p = 0.629) (Tables S1, S2).
We assessed environmental conditions along the larval pathways to evaluate pre-settlement selection as a potential mechanism linking flow conditions and variation in cohort thermal tolerances. We found that thermal tolerance limits were significantly related to predicted conditions along the larval pathway in 2015; these trends were consistent, but not significant in 2014 (when values were predicted from representative, rather than actual, model conditions) (Fig. 5, Table S3). In particular, LT50 increased when recruits were predicted to encounter more variable (F 1,14 = 7.67, p = 0.015) and higher maximum (F 1,14 = 5.26, p = 0.038) temperatures (Fig. 5). Of the environmental variables considered (Table S3), temperature variance was most strongly related to thermal tolerance limit, explaining 35.4% of variability in LT50 values in 2015. The effect of recruit size on thermal tolerance limit differed between years: LT50 was higher for larger recruits in 2014 (p < 0.03) but did not depend on size in 2015 (p > 0.10). In neither year were LT50 values related to the predicted age of recruits (p = 0.509 in 2014, and p = 0.792 in 2015) or to the maximum temperatures that recruits could have experienced during the nighttime low tide measured by adjacent data loggers on the day of cohort collection (p = 0.616 in 2014, and p = 0.577 in 2015). Importantly, the direction of all relationships was consistent between years.
Discussion
Our study uncovered associations between physical transport and demographic processes, suggesting complex links between wind-driven flow variability and mechanisms of population persistence. Prior studies of recruitment variability over time have also shown increases in recruitment associated with upwelling relaxations (e.g., Roughgarden et al. 1988; Farrell et al. 1991; Wing et al. 1995; Shanks et al. 2000; but see Mace and Morgan 2006; Narváez et al. 2006; Morgan et al. 2012 for species demonstrating opposing recruitment patterns). Our findings corroborate the importance of wind-driven circulation while also indicating the potential importance of freshwater plumes (Vargas et al. 2006; Banas et al. 2009; Ayata et al. 2011; Giddings et al. 2014) for particle transport and delivery (also see Kirincich et al. 2005; Morgan et al. 2009; Pfaff et al. 2015). While predicting the precise location of the Columbia River plume with the model is challenging, entrainment of particles in freshwater plumes is a potentially important mechanism for alongshore transport (e.g., Giddings et al. 2014). Comparisons between observed and modeled recruitment patterns, as well as discrepancies between measured and modeled winds that translate into discrepancies in recruitment, underline the dynamicity of processes that drive larval dispersal.
To the degree that our model accurately predicts dispersal trajectories, these results suggest that upwelling relaxations and downwelling conditions might promote poleward larval dispersal and redistribution in this Eastern Boundary Current region (also see Farrell et al. 1991; Wing et al. 1995; Morgan et al. 2012; Hameed et al. 2016). In fact, because downwelling winds were associated with increased overall recruitment rate and poleward dispersal, the majority of recruits arriving over our 2014 and 2015 study periods were predicted to originate south of the study site. Outside of the upwelling season, which typically runs from April to October along the Oregon and Washington coasts (Strub et al. 1987), downwelling conditions are even more frequent. Any role of wind-driven flow variability in promoting poleward dispersal and shifts in abundance distributions is likely to differ by species, with variation in behaviors determining their vertical and horizontal location in the water column and, consequently, dispersal direction (Morgan et al. 2009; Morgan and Fisher 2010) and distance (Largier 2003; Nickols et al. 2012, 2015). The potential for poleward dispersal can also depend on timing of reproduction (see Byers and Pringle 2006). While spawning has historically been known to occur during winter pulses for M. trossulus and year-round for M. californianus (Suchanek 1981), the seasonality of Mytilus spp. recruitment appears to be shifting, possibly driven by recent changes in large-scale flow patterns (Menge et al. 2009). Due to difficulty of species identification for Mytilus recruits, such shifts in reproductive timing may also reflect species-specific responses, which could confound results in time-series studies—including ours—if they span recruitment from spawning pulses of both species.
Perhaps the most intriguing finding of our study was the variation in cohort thermal tolerance limits that was associated temporally with flow conditions. Potential mechanisms underlying variable tolerance limits include differential acclimation or mortality (due to pre- or post-settlement selection and associated trade-offs) and traits inherited from different parent populations (Pörtner et al. 2006; Marshall and Morgan 2011; Johnson et al. 2014; Benestan et al. 2016; Samani and Bell 2016). Our results support a mechanism involving differential acclimation or pre-settlement selection for increased thermal tolerance limits during the pelagic larval stage, given higher LT50 values for cohorts predicted to have experienced higher and more variable temperatures during this stage. Hayhurst and Rawson (2009) also showed differential larval survival during a ~1 month exposure to this range of temperatures—with higher mortality at 15 °C than 10 °C—for two Mytilus species on the USA east coast. Furthermore, conditions during larval development have been shown to influence benthic performance in marine invertebrates (Pechenik 1999; Phillips 2004; Allen and Marshall 2010; Crean et al. 2011). For example, Giménez (2010) and Rey et al. (2016) demonstrated links between oceanographic conditions and size at metamorphosis and performance (growth and survival) of green crabs (Carcinus maenas) in later life stages.
Several alternate mechanisms for differential thermal tolerance limits were not supported. First, larval condition did not appear to differ with thermal tolerance limit, as the latter was not related to chlorophyll a levels (an index of food availability) along the larval pathway in 2014 nor size of individuals (an index of larval condition). Second, thermal tolerance limit was not related to maximum onshore temperatures, suggesting that differences between cohorts were not driven by post-settlement mortality. Third, for this variation to be explained by genetic differences between potential parent populations, thermal tolerance limits should be higher in populations to the north (the predicted origin of more tolerant larvae recruiting during upwelling) than south of our study site. The genetic basis of larval phenotype (e.g., thermal tolerance) is largely unknown across marine invertebrate taxa (Sanford and Kelly 2011), but the opposite trend is best supported: M. californianus thermal tolerances tend to increase from north to south along the USA west coast, based on studies of 7 populations across 33° of latitude (Logan et al. 2012). It is possible that the parents of recruits arriving from the north inhabit “hot spots” (Helmuth et al. 2002), where body temperatures and, potentially, selection for increased thermal tolerances are relatively high. However, a preliminary investigation of 5 populations spanning 100 km and the Cape Meares field site indicated that tolerance limit differences were small and did not vary linearly with latitude (C. Sorte, unpubl. data).
In summary, our integrated field and modeling work uncovered correlations between wind and flow transitions and the number, source, and thermal tolerance phenotypes of mussel recruits across two time series on the central Oregon coast. All three of these responses are indicators of persistence potential. Over the next century, warming sea surface temperatures are likely to influence development (and, thus, dispersal patterns) of marine invertebrates (Byrne 2011). At the same time, climate change is expected to increase the strength of alongshore winds and intensify coastal upwelling in most Eastern Boundary Upwelling Systems, and the upwelling season is projected to start earlier and end later at higher latitudes (Snyder et al. 2003; Sydeman et al. 2014; Wang et al. 2015). By decreasing downwelling and opportunities for poleward dispersal, this may have implications not only for compensatory poleward range shifts, but for local population persistence along this advective coastline (Hastings and Botsford 2006).
References
Allen RM, Marshall DJ (2010) The larval legacy: cascading effects of recruit phenotype on post-recruitment interactions. Oikos 199:1977–1983
Austin JA, Barth JA (2002) Variation in the position of the upwelling front on the Oregon shelf. J Geophys Res Oceans 107:C11
Ayata SD, Stolba R, Comtet T, Thiébaut É (2011) Meroplankton distribution and its relationship to coastal mesoscale hydrological structure in the northern Bay of Biscay (NE Atlantic). J Plankton Res 33:1193–1211
Banas N, McDonald P, Armstrong D (2009) Green crab larval retention in Willapa Bay, Washington: an intensive Lagrangian modeling approach. Estuar Coasts 32:893–905
Benestan L, Quinn BK, Maaroufi H, Laporte M, Clark K, Greenwood SJ, Rochette R, Bernatchez L (2016) Seascape genomics provides evidence for thermal adaptation and current-mediated population structure in American lobster (Homarus americanus). Mol Ecol 25:5073–5092
Berg MP, Kiers ET, Driessen G, van der Heijden M, Kooi BW, Kuenen F, Liefting M, Verhoef HA, Ellers J (2010) Adapt or disperse: understanding species persistence in a changing world. Glob Change Biol 16:587–598
Bestion E, Clobert J, Cote J (2015) Dispersal response to climate change: scaling down to intraspecific variation. Ecol Lett 18:1226–1233
Blanchette CA, Miner CM, Raimondi PT, Lohse D, Heady KE, Broitman BR (2008) Biogeographical patterns of rocky intertidal communities along the Pacific coast of North America. J Biogeogr 35:1593–1607
Bracken MES, Menge BA, Foley MM, Sorte CJB, Lubchenco J, Schiel DR (2012) Mussel selectivity for high-quality food drives carbon inputs into open-coast intertidal ecosystems. Mar Ecol Progr Ser 459:53–62
Burrows MT, Schoeman DS, Buckley LB, Moore P, Poloczanska ES, Brander KM, Brown C, Bruno JF, Duarte CM, Halpern BS, Holding J, Kappel CV, Kiessling W, O’Connor MI, Pandolfi JM, Parmesan C, Schwing FB, Sydeman WJ, Richardson AJ (2011) The pace of shifting climate in marine and terrestrial ecosystems. Science 334:652–655
Byers JE, Pringle JM (2006) Going against the flow: retention, range limits and invasions in advective environments. Mar Ecol Progr Ser 313:27–41
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Annu Rev 49:1–42
Carson HS, López-Duarte PC, Rasmussen L, Wang D, Levin LA (2010) Reproductive timing alters population connectivity in marine metapopulations. Curr Biol 20:1926–1931
Carson HS, Cook GS, Lopez-Duarte PC, Levin LA (2011) Evaluating the importance of demographic connectivity in a marine metapopulation. Ecology 92:1972–1984
Chia F-S, Buckland-Nicks J, Young CM (1984) Locomotion of marine invertebrate larvae: a review. Can J Zool 62:1205–1222
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Annu Rev Mar Sci 1:443–466
Crean AJ, Monro K, Marshall DJ (2011) Fitness consequences of larval traits persist across the metamorphic boundary. Evolution 65:3079–3089
Davis KA, Banas NS, Giddings SN, Siedlecki SA, MacCready P, Lessard EJ, Kudela RM, Hickey BM (2014) Estuary-enhanced upwelling of marine nutrients fuels coastal productivity in the US Pacific Northwest. J Geophys Res Oceans 119:8778–8799
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105:6668–6672
Drake PT, Edwards CA, Morgan SG, Dever EP (2013) Influence of larval behavior on transport and population connectivity in a realistic simulation of the California Current System. J Mar Res 71:317–350
Farrell TM, Bracher D, Roughgarden J (1991) Cross-shelf transport causes recruitment to intertidal populations in central California. Limnol Oceanogr 36:279–288
Foo SA, Sparks KM, Uthicke S, Karelitz S, Barker M, Byrne M, Lamare M (2016) Contributions of genetic and environmental variance in early development of the Antarctic sea urchin Sterechinus neumayeri in response to increased ocean temperature and acidification. Mar Biol 163:1–11
Gaylord B, Gaines SD (2000) Temperature or transport? Range limits in marine species mediated solely by flow. Am Nat 155:769–789
Giddings SN, MacCready P, Hickey BM, Banas NS, Davis KA, Siedlecki SA, Trainer VL, Kudela RM, Pelland NA, Connolly TP (2014) Hindcasts of potential harmful algal bloom transport pathways on the Pacific Northwest coast. J Geophys Res Oceans 119:2439–2461
Gilg MR, Hilbish TJ (2003) The geography of marine larval dispersal: coupling genetics with fine-scale physical oceanography. Ecology 84:2989–2998
Giménez L (2010) Relationships between habitat conditions, larval traits, and juvenile performance in a marine invertebrate. Ecology 91:1401–1413
Hameed SO, White JW, Miller SH, Nickols KJ, Morgan SG (2016) Inverse approach to estimating larval dispersal reveals limited population connectivity along 700 km of wave-swept open coast. Proc R Soc B Biol Sci 283:20160370
Hastings A, Botsford LW (2006) Persistence of spatial populations depends on returning home. Proc Natl Acad Sci USA 103:6067–6072
Hayhurst S, Rawson PD (2009) Species-specific variation in larval survival and patterns of distribution for the blue mussels Mytilus edulis and Mytilus trossulus in the Gulf of Maine. J Molluscan Stud 75:215–222
Helmuth B, Harley CDG, Halpin PM, O’Donnell M, Hofmann GE, Blanchette CA (2002) Climate change and latitudinal patterns of intertidal thermal stress. Science 298:1015–1017
Hickey B, Geier S, Kachel N, Ramp S, Kosro PM, Connolly T (2016) Alongcoast structure and interannual variability of seasonal midshelf water properties and velocity in the Northern California Current System. J Geophys Res Oceans 121:7408–7430
Huyer A (1983) Coastal upwelling in the California current system. Progr Oceanogr 12:259–284
Jenewein BT, Gosselin LA (2013) Ontogenetic shift in stress tolerance thresholds of Mytilus trossulus: effects of desiccation and heat on juvenile mortality. Mar Ecol Progr Ser 481:147–159
Jensen N, Allen RM, Marshall DJ (2014) Adaptive maternal and paternal effects: gamete plasticity in response to parental stress. Funct Ecol 28:724–733
Johnson DW, Grorud-Colvert K, Sponaugle S, Semmens BX (2014) Phenotypic variation and selective mortality as major drivers of recruitment variability in fishes. Ecol Lett 17:743–755
Jump AS, Penuelas J (2005) Running to stand still: adaptation and the response of plants to rapid climate change. Ecol Lett 8:1010–1020
Keith SA, Herbert RJ, Norton PA, Hawkins SJ, Newton AC (2011) Individualistic species limitations of climate-induced range expansions generated by mesoscale dispersal barriers. Divers Distrib 17:275–286
Kim SY, Cornuelle BD, Terrill EJ, Jones B et al (2013) Poleward propagating subinertial alongshore surface currents off the U.S. West Coast. J Geophys Res Oceans 118:6791–6806
Kinlan BP, Gaines SD (2003) Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84:2007–2020
Kirincich AR, Barth JA, Grantham BA, Menge BA, Lubchenco J (2005) Wind-driven inner-shelf circulation off central Oregon during summer. J Geophys Res Oceans 110:17
Landry MR, Hickey BM (1989) Coastal oceanography of Washington and Oregon. Elsevier, Amsterdam
Largier JL (2003) Considerations in estimating larval dispersal distances from oceanographic data. Ecol Appl 13:S71–S89
Ling SD, Johnson CR, Ridgway K, Hobday AJ, Haddon M (2009) Climate-driven range extension of a sea urchin: inferring future trends by analysis of recent population dynamics. Glob Change Biol 15:719–731
Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD (2009) The velocity of climate change. Nature 462:1052–1057
Logan CA, Kost LE, Somero GN (2012) Latitudinal differences in Mytilus californianus thermal physiology. Mar Ecol Progr Ser 450:93–105
Lymbery RA, Evans JP (2013) Genetic variation underlies temperature tolerance of embryos in the sea urchin Heliocidaris erythrogramma armigera. J Evol Biol 26:2271–2282
Lynn RJ, Simpson JJ (1987) The California Current system: the seasonal variability of its physical characteristics. J Geophys Res Oceans 92:12947–12966
Mace AJ, Morgan SG (2006) Biological and physical coupling in the lee of a small headland: contrasting transport mechanisms for crab larvae in an upwelling region. Mar Ecol Progr Ser 324:185–196
Maclean IMD, Wilson RJ (2011) Recent ecological responses to climate change support predictions of high extinction risk. Proc Natl Acad Sci USA 108:12337–12342
Marshall DJ, Morgan SG (2011) Ecological and evolutionary consequences of linked life-history stages in the sea. Curr Biol 21:R718–R725
McQuaid CD, Phillips TE (2000) Limited wind-driven dispersal of intertidal mussel larvae: in situ evidence from the plankton and the spread of the invasive species Mytilus galloprovincialis in South Africa. Mar Ecol Progr Ser 201:211–220
Menge BA, Chan F, Nielsen KJ, Lorenso ED, Lubchenco J (2009) Climatic variation alters supply-side ecology: impact of climate patterns on phytoplankton and mussel recruitment. Ecol Monogr 79:379–395
Miller KJ, Maynard BT, Mundy CN (2009) Genetic diversity and gene flow in collapsed and healthy abalone fisheries. Mol Ecol 18:200–211
Morgan SG, Fisher JL (2010) Larval behavior regulates nearshore retention and offshore migration in an upwelling shadow and along the open coast. Mar Ecol Progr Ser 404:109–126
Morgan SG, Fisher JL, Mace AJ (2009) Larval recruitment in a region of strong, persistent upwelling and recruitment limitation. Mar Ecol Progr Ser 394:79–99
Morgan SG, Fisher JL, McAfee ST, Largier JL, Halle CM (2012) Limited recruitment during relaxation events: larval advection and behavior in an upwelling system. Limnol Oceanogr 57:457–470
Narváez DA, Navarrete SA, Largier J, Vargas CA (2006) Onshore advection of warm water, larval invertebrate settlement, and relaxation of upwelling off central Chile. Mar Ecol Progr Ser 309:159–173
Nickols KJ, White JW, Largier JL, Gaylord B, Associate Editor: Wolf MM, Editor: Judith LB (2015) Marine population connectivity: reconciling large-scale dispersal and high self-retention. Am Nat 185:196–211
Nickols KJ, Gaylord B, Largier JL (2012) The coastal boundary layer: predictable current structure decreases alongshore transport and alters scales of dispersal. Mar Ecol Progr Ser 464:17–35
North EW, Schlag Z, Hood RR, Li M, Zhong L, Gross T, Kennedy VS (2008) Vertical swimming behavior influences the dispersal of simulated oyster larvae in a coupled particle-tracking and hydrodynamic model of Chesapeake Bay. Mar Ecol Progr Ser 359:99
Pachepsky E, Lutscher F, Nisbet RM, Lewis MA (2005) Persistence, spread and the drift paradox. Theor Pop Biol 67:61–73
Pacifici M, Foden WB, Visconti P, Watson JEM, Butchart SHM, Kovacs KM, Scheffers BR, Hole DG, Martin TG, Akcakaya HR, Corlett RT, Huntley B, Bickford D, Carr JA, Hoffmann AA, Midgley GF, Pearce-Kelly P, Pearson RG, Williams SE, Willis SG, Young B, Rondinini C (2015) Assessing species vulnerability to climate change. Nat Clim Change 5:215–224
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Ann Rev Ecol Evol Syst 37:637–669
Pechenik JA (1999) On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Mar Ecol Progr Ser 177:269–297
Petes LE, Mouchka ME, Milston-Clements RH, Momoda TS, Menge BA (2008) Effects of environmental stress on intertidal mussels and their sea star predators. Oecologia 156:671–680
Pfaff MC, Branch GM, Fisher JL, Hoffmann V, Ellis AG, Largier JL (2015) Delivery of marine larvae to shore requires multiple sequential transport mechanisms. Ecology 96:1399–1410
Phillips NE (2004) Variable timing of larval food has consequences for early juvenile performance in a marine mussel. Ecology 85:2341–2346
Pineda J, Hare JA, Sponaungle S (2007) Larval transport and dispersal in the coastal ocean and consequences for population connectivity. Oceanography 20:22–39
Pineda J, Porri F, Starczak V, Blythe J (2010) Causes of decoupling between larval supply and settlement and consequences for understanding recruitment and population connectivity. J Exp Mar Biol Ecol 392:9–21
Pörtner HO, Bennett AF, Bozinovic F, Clarke A, Lardies MA, Lucassen M, Pelster B, Schiemer F, Stillman JH (2006) Trade-offs in thermal adaptation: the need for a molecular to ecological integration. Physiol Biochem Zool 79:295–313
Poulin E, Palma AT, Leiva G, Narvaez D, Pacheco R, Navarrete SA, Castilla JC (2002) Avoiding offshore transport of competent larvae during upwelling events: the case of the gastropod Concholepas concholepas in Central Chile. Limnol Oceanogr 47:1248–1255
Pounds JA, Fogden MP, Campbell JH (1999) Biological response to climate change on a tropical mountain. Nature 398:611–615
Pringle JM, Blakeslee AMH, Byers JE, Roman J (2011) Asymmetric dispersal allows an upstream region to control population structure throughout a species’ range. Proc Natl Acad Sci USA 108:15288–15293
Reid JL, Schwartzlose RA (1962) Direct measurements of the Davidson Current off central California. J Geophys Res 67:2491–2497
Rey F, Silva Neto GM, Brandao C, Ramos D, Silva B, Rosa R, Queiroga H, Calado R (2016) Contrasting oceanographic conditions during larval development influence the benthic performance of a marine invertebrate with a bi-phasic life cycle. Mar Ecol Progr Ser 546:135–146
Ricciardi A, Bourget E (1999) Global patterns of macro invertebrate biomass in marine intertidal communities. Mar Ecol Progr Ser 185:21–35
Roughgarden J, Gaines S, Possingham H (1988) Recruitment dynamics in complex life cycles. Science 241:1460–1466
Sagarin RD, Gaines SD (2002) Geographical abundance distributions of coastal invertebrates: using one-dimensional ranges to test biogeographic hypotheses. J Biogeogr 29:985–997
Sala OE, Stuart Chapin F III, Armesto JJ, Berlow E, Bloom J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff L, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Samani P, Bell G (2016) The ghosts of selection past reduces the probability of plastic rescue but increases the likelihood of evolutionary rescue to novel stressors in experimental populations of wild yeast. Ecol Lett 19:289–298
Sanford E, Kelly MW (2011) Local adaptation in marine invertebrates. Ann Rev Mar Sci 3:509–535
Seed R (1969) The ecology of Mytilus edulis L. (Lamellibranchiata) on exposed rocky shores. Oecologia 3:277–316
Shanks AL, Shearman RK (2009) Paradigm lost? Cross-shelf distributions of intertidal invertebrate larvae are unaffected by upwelling or downwelling. Mar Ecol Progr Ser 385:189–204
Shanks AL, Largier J, Brink L, Brubaker J, Hooff R (2000) Demonstration of the onshore transport of larval invertebrates by the shoreward movement of an upwelling front. Limnol Oceanogr 45:230–236
Shchepetkin AF, McWilliams JC (2005) The regional oceanic modeling system (ROMS): a split-explicit, free-surface, topography-following-coordinate oceanic model. Ocean Model 9:347–404
Siedlecki SA, Banas NS, Davis KA, Giddings SN, Hickey BM, MacCready P, Connolly T, Geier S (2015) Seasonal and interannual oxygen variability on the Washington and Oregon continental shelves. J Geophys Res Oceans 120:608–633
Snyder MA, Sloan LC, Diffenbaugh NS, Bell JL (2003) Future climate change and upwelling in the California Current. Geophys Res Lett 30:1823
Somero GN (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213:912–920
Sorte CJB (2013) Predicting persistence in a changing climate: flow direction and limitations to redistribution. Oikos 122:161–170
Sprung M (1984) Physiological energetics of mussel larvae (Mytilus edulis). I. Shell growth and biomass. Mar Ecol Progr Ser 17:283–293
Strub PT, Allen JS, Huyer A, Smith RL, Beardsley RC (1987) Seasonal cycles of currents, temperatures, winds, and sea level over the northeast Pacific continental shelf: 35°N to 48°N. J Geophys Res Oceans 92:1507–1526
Suchanek TH (1981) The role of disturbance in the evolution of life history strategies in the intertidal mussels Mytilus edulis and Mytilus californianus. Oecologia 50:143–152
Sydeman WJ, García-Reyes M, Schoeman DS, Rykaczewski RR, Thompson SA, Black BA, Bograd SJ (2014) Climate change and wind intensification in coastal upwelling ecosystems. Science 345:77–80
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, Ferreira de Siqueira M, Grainger A, Hannah L, Hughes L, Huntley B, van Jaarsveld AS, Midgley GF, Miles L, Ortega-Huerta MA, Townsend Peterson A, Phillips OL, Williams SE (2004) Extinction risk from climate change. Nature 427:145–147
Troost K, Veldhuizen R, Stamhuis EJ, Wolff WJ (2008) Can bivalve veligers escape feeding currents of adult bivalves? J Exp Mar Biol Ecol 358:185–196
van Gennip SJ, Popova EE, Yool A, Pecl GT, Hobday AJ, Sorte CJB (2017) Going with the flow: the role of ocean circulation in global marine ecosystems under a changing climate. Global Change Biol. https://doi.org/10.1111/gcb.13586
Vargas CA, Narváez DA, Piñones A, Navarrete SA, Lagos NA (2006) River plume dynamic influences transport of barnacle larvae in the inner shelf off central Chile. J Mar Biol Assoc UK 86:1057–1065
Visser AW (1997) Using random walk models to simulate the vertical distribution of particles in a turbulent water column. Mar Ecol Progr Ser 158:275–281
Wang D, Gouhier TC, Menge BA, Ganguly AR (2015) Intensification and spatial homogenization of coastal upwelling under climate change. Nature 518:390–394
Washburn L, McPhee-Shaw E (2013) Coastal transport processes affecting inner-shelf ecosystems in the California Current system. Oceanography 26:34–43
Weidberg N, Porri F, Von der Meden CEO, Jackson JM, Goschen W, McQuaid CD (2015) Mechanisms of nearshore retention and offshore export of mussel larvae over the Agulhas Bank. J Mar Syst 144:70–80
Williams PD, Hastings A (2013) Stochastic dispersal and population persistence in marine organisms. Am Nat 182:271–282
Willmott CJ (1982) Some comments on the evaluation of model performance. Bull Am Meteorol Soc 63:1309–1313
Wing SR, Botsford LW, Largier JL, Morgan LE (1995) Spatial structure of relaxation events and crab settlement in the northern California upwelling system. Mar Ecol Progr Ser 128:199–211
Acknowledgements
We thank G. Bernatchez, G. McGann, L. Elsberry, M. Bracken, and F. Bracken-Sorte for their assistance with the fieldwork and tolerance limit assays. N. Banas, S. Maurel and B. Wu assisted with coding the particle tracking model. Members of the Sorte and Davis Labs gave helpful feedback on the project and manuscript. Funding was provided by the UCI Data Science Initiative (to LP) and via the Interdisciplinary Innovation Initiative (to KD and CS), with support from the UCI Henry Samueli School of Engineering, Department of Ecology and Evolutionary Biology, and School of Biological Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the University of California, Irvine through the Data Science Initiative (to LP) and the Interdisciplinary Innovation Initiative (to KD and CS), with support from the UCI Henry Samueli School of Engineering, Department of Ecology and Evolutionary Biology, and School of Biological Sciences.
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: F. Bulleri.
Reviewed by N. Mieszkowska and undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sorte, C.J.B., Pandori, L.L.M., Cai, S. et al. Predicting persistence in benthic marine species with complex life cycles: linking dispersal dynamics to redistribution potential and thermal tolerance limits. Mar Biol 165, 20 (2018). https://doi.org/10.1007/s00227-017-3269-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3269-8