Abstract
Invasive species are generally believed to be more tolerant to varying abiotic conditions than native species. Here, we report the combined effect of temperature (5, 15, 20 and 25 °C) and salinity (33, 24, 18, 12 and 6 SA) on the performance of the invasive kelp Undaria pinnatifida and two native kelp species (Lessonia variegata, Ecklonia radiata) from Tauranga Harbour, New Zealand (37°38′S, 176°10′E, 2014). Vegetative blade discs were exposed to temperature and salinity treatments in a 10-day laboratory experiment, and the physiological response was assessed employing photosynthetic (Fv/Fm, ETRmax) as well as biochemical parameters (chlorophyll a, xanthophylls and antioxidant pool size). U. pinnatifida sustained a high photosynthetic quantum yield in most treatments, with a negative synergistic effect on photosynthetic yield observed at 25 °C and low salinities (12, 6 SA). E. radiata died in salinities below 18 SA, except at 5 °C and L. variegata was highly susceptible to elevated temperatures (20, 25 °C). Antioxidant pool size showed species-specific responses to the experimental conditions, being most resilient in U. pinnatifida. Overall, U. pinnatifida displayed broader tolerance to the experimental salinity and temperature conditions than native kelps. The abilities to cope with a wide range in abiotic factors and to thrive in estuarine conditions might contribute to higher competitive strength compared to native kelps leading to its invasion success, especially with regard to ocean warming.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The introduction of species into new habitats is a phenomenon of growing ecological and economical concern (Ruiz et al. 1997; Kolar and Lodge 2001; Perrings et al. 2002). As an outcome of global trade, transportation and tourism, the connectivity of previously isolated ecosystems is rapidly increasing and therefore facilitating the introduction of non-indigenous species (NIS) in marine environments (Carlton and Geller 1993; Carlton 1995). In addition, world aquaculture production has dramatically increased in the past decade, including the annual harvesting of 25 million tonnes of seaweed and algae (FAO 2014). As a consequence, aquaculture is now regarded as one of the main vectors for the introduction of NIS (Welcomme 1992).
The immense ecological importance of species invasions is reflected by a growing body of the literature (Kolar and Lodge 2001; Lowry et al. 2013), and seaweeds represent a considerable proportion (up to 38 %) of marine NIS (Schaffelke et al. 2007). An introduced species is not necessarily an “invasive” species unless it has an impact on the community, ecosystem or economy (Davis and Thompson 2000, 2002). Under such conditions, the invader may substantially affect structure and function of the indigenous community (Ruiz et al. 1999; Williams and Smith 2007). Predicting future invasions, as well as understanding conditions, vectors and traits that facilitate successful establishment are current challenges to invasion research (Carlton 1996; Byers et al. 2002).
Invasive seaweeds tend to exhibit both, rapid growth rates and the ability for short- and long-distance dispersal (Valentine et al. 2007; Andreakis and Schaffelke 2012). A prominent example of the detrimental impact of invasive seaweeds is the introduction of the siphonous chlorophyte Caulerpa taxifolia in the Mediterranean (Meinesz et al. 1993, 2001), which was associated with a marked loss in biodiversity at the infested sites (Boudouresque et al. 1995). Worldwide, 407 seaweed introduction events have been recorded so far, encompassing 277 different species (Williams and Smith 2007).
Listed among the “100 worst alien species” (Lowe et al. 2000) the invasive Asian kelp Undaria pinnatifida (Harvey) Suringar (Laminariales) has been introduced to many coastlines worldwide, including different countries in Europe, New Zealand, Australia, Argentina, California and Mexico (ICES 2007 and references therein). In Europe, U. pinnatifida was first observed in 1971 (Etang de Thau, France) after its accidental introduction with imported oysters from Japan (Perez et al. 1981) and as a target species of aquaculture activities was deliberately established in other areas (Floc’h et al. 1991; ICES 2007). In New Zealand, the invasive kelp most likely arrived with international shipping (Hay and Luckens 1987). The vegetative gametophytes of U. pinnatifida are capable of surviving extended periods of darkness (tom Dieck 1993) and exhibit a broad temperature tolerance (tom Dieck 1993; Henkel and Hofmann 2008), enabling the kelp to be transported over long distances in ballast water tanks. Furthermore, sporophytes of U. pinnatifida might reach new habitats as fouling organisms on hulls of vessels (Hay 1990; Farrell and Fletcher 2004). From its initial introduction sites U. pinnatifida most likely spreads naturally through spore dispersal or drifting sporophytes, as observed along the New Zealand coast (Forrest et al. 2000).
Globally, ecological concerns are associated with the establishment of U. pinnatifida in new environments as it potentially competes with native seaweed species for habitat space and light (Curiel et al. 1998; Farrell and Fletcher 2006). Consequently, U. pinnatifida was observed to monopolize space (Battershill et al. 1998) and cause decreases in native seaweed density and diversity (Casas et al. 2004). Differences in the associated fauna and feeding preferences of native grazers might induce further alterations to natural community composition and impact trophic relationships (Raffo et al. 2009; Jiménez et al. 2015). In Argentina, a significant decrease in fish abundance was observed in some reefs covered by U. pinnatifida (Irigoyen et al. 2011a), and serious economical concerns may arise if commercial species are affected (Orensanz et al. 2002). However, the extent and type of effect may be community or regionally dependent. Irigoyen et al. (2011b) suggested an increase in prey abundance due to the provision of a structurally complex habitat by U. pinnatifida.
Invasive species are generally believed to be more tolerant to changing abiotic factors than natives (Zerebecki and Sorte 2011). Physiological tolerance to environmental conditions, such as temperature and salinity, determines the geographic distribution of many species (Crain et al. 2004; Bozinovic et al. 2011) and regulates invasion success (Levine 2008). To adapt to the projected changing climatic conditions, i.e. rising surface temperature and ocean acidification (IPCC 2014), broad physiological tolerance might be advantageous (Dukes and Mooney 1999). Compared to invasive species within the same habitat, less tolerant natives are suggested to be disproportionally negatively affected by the impacts of climate change (Sorte et al. 2010; Zerebecki and Sorte 2011). Competitive interactions between native and invasive species may also be affected due to alterations in physiological optima and limited resource availability (Dukes and Mooney 1999; Occhipinti-Ambrogi 2007).
Relatively few studies have assessed the comparative tolerance of native and invasive seaweed species. A study by Liu and Pang (2010) demonstrated that the invasive Grateloupia turuturu was more tolerant to changing environmental conditions than the morphologically similar non-invasive Palmaria palmata. Similarly, high resistance to sedimentation, desiccation and varying nutrient conditions, enabled the non-native red alga Gracilaria vermiculophylla to dominate a lagoon environment (Thomsen et al. 2006).
Photosynthetic quantum yield and antioxidative potential are commonly used as proxies for comparative stress susceptibility (Maxwell and Johnson 2000; Arora et al. 2002). Based on species-specific stress tolerance predictions can be made on inter-specific competition and the potential for future establishment and range expansion.
In this study, we compare species-specific physiological traits under combinations of varying temperature and salinity regimes of the invasive U. pinnatifida and two native New Zealand kelps in order to assess differences in stress tolerance and competitive strength. Our specific aim was to investigate how abiotic stress is reflected in photophysiological and biochemical properties of different kelps. We hypothesized that U. pinnatifida would be more tolerant to different experimental conditions, which might provide a competitive advantage for its future distribution.
Materials and methods
Study site and kelp species
Specimens of the invasive U. pinnatifida and two native kelps (Ecklonia radiata (C. Agardh) J. Agardh, Laminariales; Lessonia variegata J. Agardh, Laminariales) were collected from Tauranga Harbour, New Zealand (37°38′S, 176°10′E) in October 2014. Average surface water temperatures in the harbour range from about 15 °C in August and September up to 20–22 °C in February (Chappell 2013; MetOcean View). Seawater salinities in some parts of the harbour might be reduced down to 2.5–5 SA following a freshwater inflow event from Wairoa River (Pritchard et al. 2009) but at the collection site salinities are above 32.5 SA.
In New Zealand, U. pinnatifida grows at the mean low-water line (Hay and Luckens 1987; Curiel et al. 1998) as well as in intertidal rock pools (Russell et al. 2008), but may also be present down to 15 m water depth (Saito 1975; Hay and Villouta 1993). E. radiata and L. variegata might occur on a vertical range from 0 to 20 m, but show highest abundances in intermediate depths (E. radiata: 4–10 m; L. variegata: 3–15 m) (Schiel 1990; Schiel and Nelson 1990).
Sampling and Experimental Set-Up
Thallus discs of five individuals per species were cut from the median part of the blade using a 2.05-cm diameter cork-borer. For U. pinnatifida, discs were taken proximal to the midrib, at least 20 cm above the sporophyll and 10 cm below the distal thallus end. A total of at least 400 discs were cut from each species (approximately 80 discs per individual). All vegetative blade discs were carefully cleaned, removing epibiota. To allow for recovery from the cutting process and for acclimation to culture conditions seaweed discs (separated by species) were cultivated under constant bubbling in seawater (15 °C) for 24 h prior to the experimental exposure. Experimental treatments were set-up in temperature-controlled incubators at four temperatures (5, 15, 20 and 25 °C). Temperatures were chosen to replicate mean summer (20 °C) and winter (15 °C, ambient water temperature at the collection time) water temperatures in the harbour. The 5 and 25 °C treatments were selected in order to test temperatures close to minimal and maximal extremes from the literature. Upper critical temperatures recorded for U. pinnatifida sporophytes from laboratory studies range from 22–27 °C (Morita et al. 2003; Gao et al. 2013) suggesting that 25 °C might induce temperature stress for the specimens. E. radiata is considered to be a warm-temperate species; however, studies investigating thermal tolerance of its sporophytes are scarce. The optimal temperature range of E. radiata gametophytes lies somewhere between 12 and 20 °C (Novaczek 1984), while the upper thermal limit was detected to be 27–28 °C (tom Dieck 1993). Salinities (absolute salinity, SA) (6, 12, 18, 24, 33 SA) were obtained by dilution of 1-µm filtered seawater with distilled (reverse osmosis) water. 33 SA refers to ambient seawater salinity at the sampling site, reduced salinities correlate with salinities that might occur inside Tauranga Harbour due to riverine input following rainfall events (Pritchard et al. 2009).
Three flasks were prepared for each temperature–salinity combination (one for each species), prior to the addition of 20 monospecific kelp discs. We consider each single disc as a replicate. Discs were maintained in 300 mL of medium continuously bubbled with air in 70–80 µmol m−2 s−1 photon irradiance on a 12:12-h light cycle throughout the experimental exposure. Discs for biochemical analyses (pigment and antioxidant analysis) were stored at −80 °C after 10 d of exposure for later processing. When no photosynthetic signal was detected from the kelp discs, storage for biochemical analyses was accomplished on the specific experimental day. Levels of replication for each analysis are explained below.
Photosynthetic parameters
Chlorophyll (Chl) a fluorescence parameters (Fv/Fm, maximum quantum yield) were recorded after 1, 3, 6 and 10 d of experimental exposure using a pulse amplitude-modulated fluorometer (DivingPAM; Walz, Effeltrich, Germany). Ten randomly selected kelp discs (replicates) from each treatment were assessed after dark adaption (5 min). For the generation of photosynthesis versus irradiance curves (PE curves), three kelp discs for each treatment were exposed to a series of gradually increasing actinic irradiances at 30-s intervals 10 d after the initial exposure. The position of the fibre optic was held constant during the measurement procedure using “Magnet Sample Holders DIVING-MLC” (Walz). Relative electron transport rate (ETR) was calculated from PE curves as described by Schreiber et al. (1994). Subsequently, maximum electron transport rate (ETRmax) was defined by PE curve fitting after Jassby and Platt (1976).
Pigment analysis
Pigment analysis was performed in quintuplicate for each experimental treatment. Deep-frozen kelp discs were lyophilized for 24 h and pulverized at 4 m s−1 for 20 s in a high-speed benchtop homogenizer (FastPrep®-24; MP Biomedicals, Solon, OH, USA). Pigments were extracted from the resulting seaweed powder in 1.5 mL of 90 % acetone for 24 h at 4 °C in darkness. Samples were vortexed and centrifuged (5 min, 4 °C, 16,000 g) before the supernatant was filtered through a cellulose acetate (CA) membrane filter (45 µm). Pigments were analysed by high-performance liquid chromatography (HPLC) on a LaChromElite® system equipped with a chilled autosampler L-2200 and a DAD detector L-2450 (VWR-Hitachi International GmbH, Darmstadt, Germany). A reversed phase Spherisorb ODS-2 column (25 cm × 4.6 mm, 5 µm particle size; Waters, Milford, MA) was used for the separation of pigments according to the method described by Wright and Jeffrey (1997) using the modified procedure for the Spectra Physics system. Pigments were identified by co-chromatography with pigment standards for Chl a, Chl b, lutein, antheraxanthin, zeaxanthin, violaxanthin, neoxanthin and β-carotene obtained from DHI Laboratory Products (Hørsholm, Denmark) using the software EZChrom Elite ver. 3.1.3. Chl a concentration is expressed in relation to thallus dry weight (DW) (µg Chl a mg−1 DW). Pool size of xanthophyll cycle pigments (∑VAZ) was calculated from the sum of concentrations of xanthophylls involved in the cycle (∑VAZ = violaxanthin + antheraxanthin + zeaxanthin) and is expressed in relation to Chl a concentration (µg µg−1 Chl a).
Antioxidant activity
DPPH (2,2-diphenyl-1-picrylhydrasyl) radical scavenging activity was determined using modified protocols of Brand-Williams et al. (1995) and Cruces et al. (2012) for five replicate kelp discs per treatment. Deep-frozen seaweed discs were freeze-dried and pulverized at 4 m s−1 for 20 s in a high-speed benchtop homogenizer (FastPrep ®-24; MP Biomedicals, Solon, OH, USA). Antioxidants were extracted from the samples (approximately 10 mg DW) in 5 mL of 70 % acetone for 24 h under constant shaking at 4 °C in darkness. After centrifugation (5 min, 500 rpm, 4 °C), 22 µL of the supernatant of each sample was mixed with 200 µL DPPH solution (150 µM prepared in 100 % ethanol) in a 96-well microtiter plate (trifold determination for each replicate). Absorbance was measured at 520 nm after 45 min, when the reaction was finished, employing a photometer and the software FLUOstar OPTIMA (FLUOstar OPTIMA, BMG Labtech, Ortenberg, Germany). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as a standard, activity of the antioxidant pool is expressed as µg Trolox equivalents (TE) per dry weight (µg TE mg−1 DW).
Statistics
All statistical analyses were conducted using the statistic programme “R” (R Development Core Team 2008). Multi-factorial analyses of variance (ANOVA) were applied to assess the impact of experimental factors on the respective variable. Since the assumption of normally distributed data was not met, nonparametric statistical analyses according to Brunner and Munzel (2013) were applied involving replacement of variable values by ranks prior to the ANOVA.
For each experimental variable, the general dependence on factors was approached employing a four-way ANOVA (variable: Fv/Fm; factors: species, measurement day, temperature and salinity) and three-way ANOVAs (variable: ETRmax, Chl a concentration, ∑VAZ, TE; factors: species, temperature, salinity), respectively. Significant interactions between specific factors were encountered for all analyses indicating a combined effect of these factors on the experimental variable. However, as the interpretation of main effects may be incomplete or misleading in the presence of significant interactions, testing “simple effects” of one factor at a fixed level of another factor is a common follow-up method in the case of significant interactions (Keppel and Wickens 2004). Therefore, a three-way ANOVA (variable: Fv/Fm; factors: measurement day, temperature and salinity) and two-way ANOVAs (variables: ETRmax, Chl a concentration, ∑VAZ, TE; factors: temperature, salinity) were applied separately for each kelp species. In presence of significant interactions differences between cell means were tested in a one factorial design (simple effects). Therefore, levels of two factors were combined before applying a one-way ANOVA (factor: temperature_salinity). When high numbers of factor levels made this procedure inappropriate due to extremely low α-errors, a graphical comparison of cell means was accomplished.
In order to detect inter-specific differences in ambient conditions (15 °C, 33 SA), a one-way ANOVA (variables: Fv/Fm, ETRmax, Chl a concentration, ∑VAZ, TE; factor: species) was applied for each experimental variable at the specific factor levels.
For post hoc analyses, Tukey’s honest significant difference (HSD) test was applied. Significant differences between group means are denoted by different lower cases for temperature, capital letters for salinity and italic small letters for the combined factor (temperature_salinity) in the text.
Results
Photosynthetic parameters
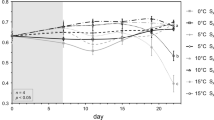
Significant interactions between factors were detected by the four-way ANOVA and the three-way ANOVAs for each species indicating that the impact of one factor on photosynthetic quantum yield depended on the level of another factor (Table 1). Due to the high number of factor levels, a graphical comparison of cell means was accomplished. Photosynthetic quantum yield of U. pinnatifida was sustained on a high level after one day of experimental exposure and was only impacted by the combination of highest temperature (25 °C) and low salinities (12, 6 SA) (Fig. 1). Similar patterns were observed on day 3, 6 and at the end of the experimental exposure (day 10) when no photosynthetic signal could be detected for the aforementioned treatments. Contrasting this, photosynthetic quantum yield of E. radiata drastically decreased over the experimental duration. Specimens of E. radiata disintegrated or did not exhibit measurable photosynthetic signals in salinities down from 18 SA in all temperatures except for the 5 °C treatment after 10 d of exposure. Similarly, only one day after the start of the experimental exposure, a reduction of 84 % was evident for the photosynthetic quantum yield of L. variegata in 25 °C and 6 SA compared to the photosynthetic yield measured in ambient conditions (15 °C, 33 SA). By day 6, no photosynthetic quantum yield could be measured in any of the L. variegata specimens in 25 °C and the yield of individuals in 20 °C was considerably reduced by up to 70 %.
Maximum electron transport rate (ETRmax) ranged from 23.56 (E. radiata, 5 °C, 12 SA) to 109.97 μmol e− m−2 s−1 (E. radiata, 5 °C, 33 SA) (Table 2). Due to significant interactions between factors (species and salinity) in the three-factorial analysis (Table 3) separate two-way ANOVAs were accomplished for each species. Two-way ANOVA for U. pinnatifida detected a significant interaction between temperature and salinity. Subsequent one-way ANOVA for simple effects (F(15/38) = 3.01, p < 0.01) indicated differences between cell means. ETRmax values were observed to decrease with increasing temperatures in ambient seawater salinity (33 SA) resulting in a reduction of ETRmax of 22 % in 25 °C compared to ambient water temperature (15 °C). However, this pattern was not statistically significant (HSD test for simple effects: 5 °C ab, 15 °C ab, 20 °C no data available (NA), 25 °C b). ETRmax values of U. pinnatifida describe an optimum curve along the experimental salinities in 5 °C with the highest value of ETRmax occurring in 24 SA (104.5 μmol e− m−2 s−1) (HSD test for simple effects: 33 SA ab, 24 SA a, 18 SA ab, 12 SA ab, 6 SA ab). The same pattern, but less pronounced, was indicated for L. variegata in 5 °C with the highest ETRmax value at 24 SA (88.48 μmol e− m−2 s−1). However, the effect of salinity on ETRmax was not statistically significant for L. variegata. Temperature impacted ETRmax of L. variegata with highest ETRmax occurring in 5 °C (HSD test: 5 °C a, 15 °C b, 20 °C ab, 25 °C NA). Two-way ANOVA detected no effect of temperature on ETRmax of E. radiata; however, ETRmax significantly varied with salinity. The lowest measured value of ETRmax occurred in 5 °C and 12 SA (23.6 µmol e− m−2 s−1) but HSD test did not detect significant differences between temperature means.
Pigment analysis
Three-way ANOVA detected significant interactions between factors (species, temperature and salinity) for the Chl a concentration per dry weight (Table 3). Chl a concentration at ambient conditions (15 °C, 33 SA) was highest in U. pinnatifida (one-way ANOVA: F(2/14) = 4.53, p = 0.03) exhibiting 2-fold concentrations compared to native kelps (Fig. 2).
Tissue chlorophyll (Chl) a concentrations (boxplots, n = 5) of Undaria pinnatifida, Ecklonia radiata and Lessonia variegata exposed to various temperatures (5, 15, 20 and 25 °C) and salinities (33, 24, 18, 12 and 6 SA) after 10 days of experimental exposure. Chlorophyll a concentration is expressed in relation to thallus dry weight (DW). Bottom and top of the boxes in the plot depict the first and third quartiles, respectively. The median is represented by the band inside the box. The ends of the whiskers are designed to show minimum and maximum values still within 1.5 × Interquartile range (IQR) of the lower and upper quartiles. Values outlying these ranges are displayed by single dots. Details on statistical analyses and results are given in the text
The two-way ANOVA detected a significant interaction between factors for U. pinnatifida. Subsequent one-way analysis of simple effects (F(18/65) = 5.24, p < 0.001) and HSD test detected differences in Chl a concentration between temperatures with highest Chl a concentrations occurring in 5 °C, except for the 24 SA-treatment where no differences between temperatures were indicated. At ambient levels of seawater salinity, a distinct pattern of decreasing Chl a concentration with increasing temperature was apparent for U. pinnatifida (HSD test for simple effects: 5 °C a, 15 °C ab, 20 °C ab, 25 °C b) with a reduction of 38 % in Chl a content in 25 °C compared to the 5 °C-temperature treatment. A high rate of disc disintegration occurred in the combination of 25 °C and low salinities (12, 6 SA). Both factors, temperature and salinity, significantly influenced Chl a content of native kelp species as displayed by the results of the two-way ANOVA. Chl a content of L. variegata displayed a continual decrease with decreasing salinities (HSD test: 33 SA A, 24 SA B 18 SA B, 12 SA B, 6 SA C), exhibiting an averaged reduction of 58 % from ambient seawater salinity to the lowest experimental salinity treatment. Highest Chl a concentration for L. variegata was detected in 15 °C, lowest concentration in 25 °C (HSD test: 5 °C ab, 15 °C a, 20 °C ab, 25 °C b). No clear pattern could be identified for the Chl a content of E. radiata, the highest value being 235 µg Chl a mg−1 DW (25 °C, 18 SA).
Significant interactions between factors (species, temperature and salinity) were detected for ∑VAZ (Table 3). In ambient conditions (15 °C, 33 SA), no difference in ∑VAZ was detected between species (one-way ANOVA: F(2/14) = 2.46, p = 0.12). A significant effect of temperature and salinity on ∑VAZ was identified for U. pinnatifida and E. radiata (two-way ANOVA). ∑VAZ was continuously reduced with decreasing salinities for the invasive kelp (HSD test: 33 SA A, 24 SA AB, 18 SA AB, 12 SA AB, 6 SA B) and E. radiata (HSD test: 33 SA A, 24 SA B, 18 SA AB, 12 SA B, 6 SA B), displaying an average reduction of 21 % and 42 % in 6 SA compared to 33 SA, respectively (Table 4). ∑VAZ of E. radiata was highest in 5 °C treatments (HSD test: 5 °C a, 15 °C b, 20 °C b, 25 °C b). For L. variegata, the two-way ANOVA detected significant interactions between factors. One-way ANOVA (F(19/75) = 7.32, p < 0.001) indicated differences between cell means and subsequent HSD test for simple effects detected highest ∑VAZ for L. variegata in 5 and 15 °C (33 SA). In 25 °C, ∑VAZ of L. variegata was reduced compared to the ambient water temperature.
Antioxidant activity
Detected antioxidant values ranged from 7 to 54 µg TE mg−1 DW (Fig. 3). Three-way ANOVA identified significant interactions between all factors (species, temperature and salinity) (Table 3). L. variegata exhibited the lowest antioxidant concentrations in relation to thallus dry weight (one-way ANOVA: F(2/11) = 17.18, p < 0.001), 49 % and 58 % lower compared with U. pinnatifida and E. radiata, respectively (15 °C, 33 SA). Two-way ANOVAs for each species identified interactions between factors (temperature and salinity) and differences between cell means were indicated when testing for simple effects (U. pinnatifida: F(19/68) = 7.93, p < 0.001, E. radiata: F(18/59) = 6.36, p < 0.001, L. variegata: F(19/75) = 9.68, p < 0.001). The antioxidant pool of U. pinnatifida revealed the least reductions over the range of experimental conditions. Average antioxidant pool size of U. pinnatifida displayed a distinct optimum curve for temperature at each salinity level, except for the 6 SA treatment, with highest pool sizes occurring in 15 °C. This pattern was, however, not statistically significant (HSD test for simple effects). HSD test of simple effects indicated consistently decreasing antioxidant pool size with lower salinities for U. pinnatifida, exhibiting 29 % reduction in 6 SA compared to ambient seawater salinity. However, the antioxidant pool of U. pinnatifida was never depleted. Antioxidant levels of E. radiata and L. variegata displayed dramatic decreases. The combination of low salinities (18–6 SA) and elevated temperatures (20, 25 °C) completely exhausted antioxidant levels in L. variegata. Average antioxidant pool size was reduced by 72 % for L. variegata and 53 % for E. radiata in the lowest salinity treatment compared with ambient seawater salinities. For the two native kelps HSD test for simple effects indicated the most resilient antioxidant pool in 5 °C, antioxidant levels were entirely depleted in L. variegata in 25 °C.
Antioxidant pool size (boxplots, n = 5) of Undaria pinnatifida, Ecklonia radiata and Lessonia variegata exposed to various temperatures (5, 15, 20 and 25 °C) and salinities (33, 24, 18, 12 and 6 SA) after 10 days of experimental exposure. Pool size is expressed as Trolox equivalents (TE) per dry weight (DW). Bottom and top of the boxes in the plot depict the first and third quartiles, respectively. The median is represented by the band inside the box. The ends of the whiskers are designed to show minimum and maximum values still within 1.5 × Interquartile range (IQR) of the lower and upper quartiles. Values outlying these ranges are displayed by single dots. Details on statistical analyses and results are given in the text
Discussion
Species-specific physiological response
Displaying least reductions in photosynthetic quantum yield, as a proxy of plant stress, the invasive U. pinnatifida was more tolerant to the various experimental conditions applied in this study than the investigated native kelp species. Each single experimental factor, temperature and salinity, did not impact photosynthetic quantum yield; however, the combined effects of highest temperature and low salinity treatments affected the performance of U. pinnatifida specimens. The deleterious effect of several combined stress factors most often exceeds the simple additive effect of their single action, an effect known as “cross-synergism” (Alexieva et al. 2003). However, a reduction in maximum electron transport was observed as a consequence of elevated temperatures for U. pinnatifida. Thus, high temperatures affected photosynthetic rates but did not affect efficiency of photosystem II (PSII) or survival of U. pinnatifida. In contrast, specimens of the endemic L. variegata were strongly impacted by elevated temperatures displaying no photosynthetic signal and massive disintegration in 25 °C.
Most striking differences in the physiological reaction to abiotic factors between kelp species occurred with measured antioxidant capacity in this study. While pool size of the invasive kelp was most resilient and never depleted, drastic reductions were detected for the two native kelps in response to the experimental conditions. New Zealand’s endemic L. variegata displayed complete exhaustion of antioxidants in 25 °C as well as in the combination of low salinities (18–6 SA) and various temperatures (15, 20, 25 °C). Exhausted antioxidant levels indicate the occurrence of oxidative stress and the generation of reactive oxygen species (ROS), potentially leading to inhibition and destruction of the photosynthetic apparatus, DNA, proteins and cell membranes (Kumar et al. 2014). The availability of antioxidants is a crucial defence mechanism to protect from cellular damage (Burritt et al. 2002). Therefore, high antioxidant levels are suggested to represent a physiological adaption (Kumar et al. 2014) to extreme environmental conditions, such as those occurring in elevated shore habitats. Consistently, higher antioxidant levels have been detected in intertidal macroalgae compared to subtidal species (Ross and Van Alstyne 2007). Thus, the resilient antioxidant pool of U. pinnatifida, observed in this study might promote its tolerance against various physico-chemical stresses, such as temperature and salinity alterations.
L. variegata displayed high susceptibility to elevated experimental temperatures (25 °C). Rapid exhaustion of its antioxidant pool in 25 °C is consistent with high sensitivity to elevated temperatures displayed by the photosynthetic quantum yield. Additionally, reduced chlorophyll a concentration and xanthophyll pool size in 25 °C confirm the negative impact of elevated water temperatures on the physiological condition of L. variegata. Data on salinity and temperature tolerance for L. variegata is scarce. However, Nelson (2005) tested sporophyte growth of L. variegata at three temperatures (10, 12 and 15 °C) and detected highest growth rates in 15 °C. Our results of photosynthetic quantum yield, chlorophyll a concentration and antioxidant capacity demonstrate higher susceptibility to reduced salinities in 20 °C compared to 15 °C, suggesting an optimum physiological performance of L. variegata in 15 °C. Our observations, however, indicate that 25 °C exceeds the tolerance limit of L. variegata sporophytes.
In ambient seawater salinity, the physiological condition of E. radiata was not impacted by experimental temperatures. However, reduced salinities impacted all investigated parameters in E. radiata. Drastically reduced photosynthetic quantum yield as well as impacted antioxidant and pigment concentrations indicate that low salinities down from 18 SA induce stress in E. radiata sporophytes. This is consistent with Burridge et al. (1999), who observed zoospore germination and gametophyte growth of E. radiata to be negatively impacted by reduced salinities. In the lowest temperature treatment E. radiata displayed the most resilient photosynthetic quantum yield and antioxidant concentration in reduced salinities, suggesting that it exhibits the highest acclimation potential in 5 °C.
Tolerance to salinity changes is one critical factor for vertical distribution limits of seaweed species in the intertidal zone (Kumar et al. 2014). From studies on different algal species, hyposaline conditions are known to influence growth (Bjærke and Rueness 2004), water content (Luo and Liu 2011), photosynthetic activity and chlorophyll a concentrations (Karsten 2007), respiration rates (Ogata and Takada 1968) as well as carbon and nitrogen metabolism (Kakinuma et al. 2006). In our study, reduced salinities did not limit survival or markedly impact chlorophyll a concentration and photosynthetic quantum yield of U. pinnatifida. However, reduced antioxidant levels and xanthophyll pool sizes reflected the potential generation of ROS as consequence of low salinities. While zoospores of U. pinnatifida germinated in salinities down to 8 SA (Bite 2001) and microscopic gametophytes remain viable at salinities as low as 6 SA (Peteiro and Sánchez 2012) young sporophytes (up to 4 mm long) only survived salinities down to 16 SA (Peteiro and Sánchez 2012). Our results suggest that adult sporophytes of U. pinnatifida are capable of enduring hyposaline conditions down to 6 SA, at least for short time periods.
Carbon dioxide solubility increases with decreasing salinities (Weiss 1974), potentially enhancing the availability of carbon dioxide to photosynthesis in reduced salinities. Elevated CO2 levels are known to increase the photosynthetic performance of macroalgae (Johnson et al. 2012; Olischläger et al. 2012). This correlation might explain the observation that U. pinnatifida exhibited highest ETRmax values at lower than ambient seawater salinities (24 SA). When salinity is further reduced, physiological stress might exceed the positive effect of hyposaline conditions.
Ecological implications
Temperature is a main driver in seaweed biogeography, and ocean water temperature is projected to increase continuously in the coming decades (IPCC 2014). Organisms susceptible to temperature changes or living close to their critical temperature limit might experience restrictions in physiological performance, as predicted for corals (Fitt et al. 2001). Schiel et al. (2004) reported a decrease in abundance of temperature-sensitive algae over the duration of ten years in response to artificial warming induced by the thermal outfall of a power-generating station. Our findings suggest that physiological conditions of the endemic L. variegata will be strongly impacted if water temperatures exceed 25 °C for multiple days, potentially leading to reduced abundances in the intertidal. In contrast, we did not detect major restrictions to the physiological performance of U. pinnatifida in 25 °C indicating that the kelp is capable of withstanding 25 °C water temperature. Based on satellite-derived Sea Surface Temperature (SST) data James et al. (2015) suggested that extensive areas of the world coastlines might be suitable for the invasion of U. pinnatifida.
Salinities in Tauranga Harbour can be significantly lower than the ocean outside the harbour (Pritchard et al. 2009). Close to the inlet of Wairoa river, the main freshwater source of Tauranga Harbour, extremely low salinities (0 – 5 SA) might be experienced for at least short periods of time (Pritchard et al. 2009). The pronounced tolerance to salinity of U. pinnatifida sporophytes, found in our study, potentially enables the kelp to further expand its range into less saline areas of Tauranga Harbour and elsewhere. In Venice, lagoon invasive stands of U. pinnatifida are already established, experiencing salinities of 16 to 38 SA (Curiel et al. 1998). However, timescale and frequency of salinity fluctuations will be important factors determining acclimation rates for the invasion success (Lee and Bell 1999) in environments of varying salinities, e.g. lagoons. Experiments comparing short- and long-term salinity exposure may add valuable information to predict future distribution patterns.
Physiological impairment of E. radiata and the endemic L. variegata in high temperatures, as observed in this study, might favour the spread of the less impacted U. pinnatifida in a competitive situation (e.g. for space), eventually resulting in local dominance of U. pinnatifida in the intertidal. U. pinnatifida has been shown to benefit from disturbance of native canopy forming kelps (Valentine and Johnson 2003, 2004). Therefore, impaired performance of native species may be a critical invasion factor.
In our study area, Tauranga Harbour (Te Awanui) located in the Bay of Plenty, New Zealand, U. pinnatifida was first observed in 2005 (Russell et al. 2008). The invader vigorously grows on hard substrata in close vicinity to the examined native kelp species. U. pinnatifida within Tauranga Harbour currently co-occurs with other macrophytes such as Carpophyllum maschalocarpum, C. flexuosum and Sargassum spp. as well as E. radiata. Together with subcanopy species such as Ulva, Codium, Corallina spp. as well as a number of small foliose red algae. It currently is not present to any great extent on the outer coast, but given the rapid spread observed in coastal regions adjacent to ports such as Wellington, Napier and Gisborne (the latter two with similar salinity and temperature profiles to Tauranga), it is expected to be interacting with coastal E. radiata and L. variegata imminently (Hay and Luckens 1987; Hay 1990; Battershill et al. 1998). Indeed, the relatively low abundance of E. radiata inside the harbour in areas of U. pinnatifida presence and on reef habitat that would normally otherwise support E. radiata could signal a competitive displacement already in action.
In conclusion, this study demonstrates that adult sporophytes of U. pinnatifida exhibit a wide physiological tolerance to synergistic effects of temperature and salinity, suggesting the potential invasion of brackish environments. Species-specific physiological reactions to abiotic stress have been observed, strikingly demonstrating the importance of antioxidant pool size for stress regulation in kelps. U. pinnatifida exhibited considerably higher tolerance to abiotic factors than the native kelps, supporting the generally accepted assumption that invasive species are more tolerant to abiotic stresses than natives. Especially with regard to its performance in elevated temperature conditions, U. pinnatifida might experience a competitive advantage in a warming ocean, and further expand its invaded range.
References
Alexieva V, Ivanov S, Sergiev I, Karanov E (2003) Interaction between stresses. Bulg J Physiol Special Issue 1–17
Andreakis N, Schaffelke B (2012) Invasive Marine Seaweeds: Pest or Prize? In: Wiencke C, Bischof K (eds) Seaweed Biology, Ecological Studies 219. Springer, New York, pp 235–262
Arora A, Sairam RK, Srivastava GC (2002) Oxidative stress and antioxidative system in plants. Curr Sci India 82(10):1227–1238
Battershill C, Miller K, Cole R (1998) The understorey of marine invasions. Seafood New Zeal 6:31–33
Bite JS (2001) The ecology and demography of the introduced macroalga Undaria pinnatifida (Harvey) Suringar in Port Phillip Bay, Victoria, Australia. Dissertation, Victoria University
Bjærke MR, Rueness J (2004) Effects of temperature and salinity on growth, reproduction and survival in the introduced red alga Heterosiphonia japonica (Ceramiales, Rhodophyta). Bot Mar 47:373–380. doi:10.1515/BOT.2004.055
Boudouresque CF, Meinesz A, Ribera AM, Ballesteros E (1995) Spread of the green alga Caulerpa taxifolia (Caulerpales, Chlorophyta) in the Mediterranean: possible consequences of a major ecological event. Sci Mar 59(S1):21–29
Bozinovic F, Calosi P, Spicer JI (2011) Physiological Correlates of Geographic Range in Animals. Annu Rev Ecol Evol Syst 42:155–179. doi:10.1146/annurev-ecolsys-102710-145055
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30. doi:10.1016/S0023-6438(95)80008-5
Brunner E, Munzel U (2013) Nichtparametrische Datenanalyse, Unverbundene Stichproben. 2nd edition. Springer-Verlag Berlin Heidelberg. doi:10.1007/978-3-642-37184-4
Burridge T, Campbell S, Bidwell J (1999) Use of the kelp Ecklonia radiata (Laminariales: Phaeophyta) in routine toxicity testing of sewage effluents. Australasian J Ecotoxicology 5:133–140
Burritt DJ, Larkindale J, Hurd CL (2002) Antioxidant metabolism in the intertidal red seaweed Stictosiphonia arbuscula following desiccation. Planta 215:829–838. doi:10.1007/s00425-002-0805-6
Byers JE, Reichard S, Randall JM, Parker IM, Smith CS, Lonsdale WM, Atkinson IAE, Seastedt TR, Williamson M, Chornesky E, Hayes D (2002) Directing Research to Reduce the Impacts of Nonindigenous Species. Conserv Biol 16(3):630–640
Carlton JT (1995) Biological Invasions and Cryptogenic Species. Ecology 77(6):1653–1655
Carlton JT (1996) Pattern, process, and prediction in marine invasion ecology. Biol Conserv 78:97–106
Carlton JT, Geller JB (1993) Ecological roulette: the global transport of non-indigenous marine organisms. Science 261:78–82
Casas G, Scrosati R, Piriz ML (2004) The invasive kelp Undaria pinnatifida (Phaeophyceae, Laminariales) reduces native seaweed diversity in Nuevo Gulf (Patagonia, Argentina). Biol Invasions 6:411–416. doi:10.1023/B:BINV.0000041555.29305.41
Chappell, PR (2013) The climate and weather of Bay of Plenty, Niwa Science and technology series, 3rd edition
Crain CM, Silliman BR, Bertness SL, Bertness MD (2004) Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology 85:2539–2549. doi:10.1890/03-0745
Cruces E, Huovinen P, Gomez I (2012) Phlorotannin and Antioxidant Responses Upon Short-term Exposure to UV Radiation and Elevated Temperature in Three South Pacific Kelps. Photochem Photobiol 88:58–66. doi:10.1111/j.1751-1097.2011.01013.x
Curiel D, Bellemo G, Marzocchi M, Scattolin M, Parisi G (1998) Distribution of introduced Japanese macroalgae Undaria pinnatifida, Sargassum muticum (Phaeophyta) and Antithamnion Pectinatum (Rhodophyta) in the Lagoon of Venice. Hydrobiologia 385:17–22. doi:10.1023/A:1003437105147
Davis MA, Thompson K (2000) Eight ways to be a colonizer; two ways to be an invader: a proposed nomenclature scheme for invasion ecology. Bull Ecol Soc Am 81:226–230
Davis MA, Thompson K (2002) ‘‘Newcomers’’ invade the field of invasion ecology: question the field’s future. Bull Ecol Soc Am 83:196–197
Dukes JS, Mooney HA (1999) Does global change increase the success of biological invaders? Trends Ecol Evol 14(4):135–139. doi:10.1016/S0169-5347(98)01554-7
FAO (2014) The State of World Fisheries and Aquaculture 2014, Rome (http://www.fao.org/3/a-i3720e.pdf). Accessed 14 March 2016
Farrell P, Fletcher R (2004) Boats as a vector for the introduction and spread of a fouling alga, Undaria pinnatifida in the UK. Porcupine Marine Natural History Society Newsletter 15:48–52
Farrell P, Fletcher RL (2006) An investigation of dispersal of the introduced brown alga Undaria pinnatifida (Harvey) Suringar and its competition with some species on the man-made structures of Torquay Marina (Devon, UK). J Exp Mar Biol Ecol 334(2):236–243. doi:10.1016/j.jembe.2006.02.006
Fitt WK, Brown BE, Warner ME, Dunne RP (2001) Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 20:51–65. doi:10.1007/s003380100146
Floc’h JY, Pajot R, Wallentinus I (1991) The Japanese brown alga Undaria pinnatifida on the coast of France and its possible establishment in European waters. J Cons int Explor Mer 47:379–390
Forrest BM, Brown SN, Taylor MD, Hurd CL, Hay CH (2000) The role of natural dispersal mechanisms in the spread of Undaria pinnatifida (Laminariales, Phaeophyceae). Phycologia 39(6):547–553. doi:10.2216/i0031-8884-39-6-547.1
Gao X, Endo H, Taniguchi K (2013) Agatsuma Y (2013) Combined effects of seawater temperature and nutrient condition on growth and survival of juvenile sporophytes of the kelp Undaria pinnatifida (Laminariales; Phaeophyta) cultivated in northern Honshu, Japan. J Appl Phycol 25:269–275. doi:10.1007/s10811-012-9861-x
Hay CH (1990) The dispersal of sporophytes of Undaria pinnatifida by coastal shipping in New Zealand, and implications for further dispersal of Undaria in France. Brit Phycol J 25:301–313
Hay CH, Luckens PA (1987) The Asian kelp Undaria pinnatifida (Phaeophyta: Laminariales) found in a New Zealand harbour. New Zeal J Bot 25(2):329–332
Hay CH, Villouta E (1993) Seasonality of the Adventive Asian Kelp Undaria pinnatifida in New Zealand. Bot Mar 36(5):461–476. doi:10.1515/botm.1993.36.5.461
Henkel SK, Hofmann GE (2008) Thermal ecophysiology of gametophytes cultured from invasive Undaria pinnatifida (Harvey) Suringar in coastal California harbors. J Exp Mar Biol Ecol 367(2):164–173. doi:10.1016/j.jembe.2008.09.010
ICES (2007) Alien Species Alert: Undaria pinnatifida (wakame or Japanese kelp). ICES Cooperative Research Report 283:1–38
IPCC (2014) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri RK, Meyer LA (eds.)]. IPCC, Geneva, Switzerland, 151 pp
Irigoyen AJ, Eyras C, Parma AM (2011a) Alien algae Undaria pinnatifida causes habitat loss for rocky reef fishes in north Patagonia. Biol Invasions 13(1):17–24. doi:10.1007/s10530-010-9780-1
Irigoyen AJ, Trobbiani G, Sgarlatta MP, Raffo MP (2011b) Effects of the alien algae Undaria pinnatifida (Phaeophyceae, Laminariales) on the diversity and abundance of benthic macrofauna in Golfo Nuevo (Patagonia, Argentina): potential implications for local food webs. Biol Invasions 13(7):1521–1532. doi:10.1007/s10530-010-9910-9
James K, Kibele J, Shears NT (2015) Using satellite-derived sea surface temperature to predict the potential global range and phenology of the invasive kelp Undaria pinnatifida. Biol Invasions 17(12):3393–3408. doi:10.1007/s10530-015-0965-5
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Jiménez RS, Hepburn CD, Hyndes GA, McLeod RJ, Hurd CL (2015) Contributions of an annual invasive kelp to native algal assemblages: algal resource allocation and seasonal connectivity across ecotones. Phycologia 54(5):530–544. doi:10.2216/15-39.1
Johnson V, Russell B, Fabricius KE, Brownlee C, Hall-Spencer JM (2012) Temperate and tropical brown macroalgae thrive, despite decalcification, along natural CO2 gradients. Global Change Biol 18:2792–2803. doi:10.1111/j.1365-2486.2012.02716.x
Kakinuma M, Coury DA, Kuno Y, Itoh S, Kozawa Y, Inagaki E, Yoshiura Y, Amano H (2006) Physiological and biochemical responses to thermal and salinity stresses in a sterile mutant of Ulva pertusa (Ulvales, Chlorophyta). Mar Biol 149:97–106. doi:10.1007/s00227-005-0215-y
Karsten U (2007) Salinity tolerance of Arctic kelps from Spitsbergen. Phycol Res 2007(55):257–262. doi:10.1111/j.1440-1835.2007.00468.x
Keppel G, Wickens TD (2004) Design and Analysis: A Researcher’s Handbook, 4th edn. Pearson Prentice Hall, New Jersey
Kolar SK, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16(4):199–204
Kumar M, Kumari P, Reddy CRK, Jha B (2014) Salinity and Desiccation Induced Oxidative Stress Acclimation in Seaweeds. In: Salinity and desiccation induced oxidative stress acclimation in seaweeds. In: Sea Plants 71 (Adv Bot Res), 1st edition (Bourgougnon N ed). San Diego, CA: Academic Press Publisher, Elsevier Ltd., 91–123
Lee CE, Bell MA (1999) Causes and consequences of recent freshwater invasions by saltwater animals. Trends Ecol Evol 14:284–288
Levine JM (2008) Biological invasions. Curr Biol 18(2):R57–R60. doi:10.1016/j.cub.2007.11.030
Liu F, Pang SJ (2010) Stress tolerance and antioxidant enzymatic activities in the metabolisms of the reactive oxygen species in two intertidal red algae Grateloupia turuturu and Palmaria palmata. J Exp Mar Biol Ecol 382:82–87. doi:10.1016/j.jembe.2009.11.005
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the World’s Worst Invasive Alien Species A selection from the Global Invasive Species Database. The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN)
Lowry E, Rollinson EJ, Laybourn AJ, Scott TE, Aiello-Lammens ME, Gray SM, Mickley J, Gurevitch J (2013) Biological invasions: a field synopsis, systematic review, and database of the literature. Ecol Evol 3(1):182–196. doi:10.1002/ece3.431
Luo MB, Liu F (2011) Salinity-induced oxidative stress and regulation of antioxidant defense system in the marine macroalga Ulva prolifera. J Exp Mar Biol Ecol 409(1–2):223–228. doi:10.1016/j.jembe.2011.08.023
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51(345):659–668. doi:10.1093/jexbot/51.345.659
MetOcean View: https://hindcast.metoceanview.com/, accessed 19.05.2016
Meinesz A, de Vaugelas J, Hesse B, Mari X (1993) Spread of the introduced tropical green alga Caulerpa taxifolia in northern Mediterranean waters. J Appl Phycol 5(2):141–147
Meinesz A, Belsher T, Thibaut T, Antolic B, Mustapha KB, Boudouresque C-F, Chiaverini D, Cinelli F, Cottalorda J-M, Djellouli A, El Abed A, Orestano C, Grau AM, Ivesa L, Jaklin A, Langar H, Massuti-Pascua E, Peirano A, Tunesi A, de Vaugelas J, Zavodnik N, Zuljevic A (2001) The introduced green alga Caulerpa taxifolia continues to spread in the Mediterranean. Biol Invasions 3:201–210
Morita T, Kurashima A, Maegawa M (2003) Temperature requirements for the growth of young sporophytes of Undaria pinnatifida and Undaria undarioides (Laminariales, Phaeophyceae). Phycol Res 51(4):266–270. doi:10.1111/j.1440-1835.2003.t01-4-.x
Nelson WA (2005) Life history and growth in culture of the endemic New Zealand kelp Lessonia variegata J. Agardh in response to differing regimes of temperature, photoperiod and light. J Appl Phycol 17(1):23–28. doi:10.1007/s10811-005-5521-8
Novaczek I (1984) Response of gametophytes of Ecklonia radiata (Laminariales) to temperature in saturating light. Mar Biol 82:241–245
Occhipinti-Ambrogi A (2007) Global change and marine communities: Alien species and climate change. Mar Pollut Bull 55:342–352. doi:10.1016/j.marpolbul.2006.11.014
Ogata E, Takada H (1968) Studies on the relationship between the respiration and the changes in salinity in some marine plants in Japan. J Shimoneseki Univ Fish 16:67–88
Olischläger M, Bartsch I, Gutow L, Wiencke C (2012) Effects of ocean acidification on different life-cycle stages of the kelp Laminaria hyperborea (Phaeophyceae). Bot Mar 55(5):511–525. doi:10.1515/bot-2012-0163
Orensanz J, Schwindt E, Pastorino G (2002) No longer the pristine confines of the world ocean: a survey of exotic marine species in the southwestern Atlantic. Biol Invasions 4(1):115–143
Perez R, Lee JY, Juge C (1981) Observations sur la biologie de l’algue Undaria pinnatifida (Harvey) Suringar introduite accidentellement dans l’etang de Thau. Science et Peche 315:1–12
Perrings C, Williamson M, Barbier EB, Delfino D, Dalmazzone S, Shogren J, Simmons P, Watkinson A (2002) Biological Invasion Risks and the Public Good: an Economic Perspective. Conserv Ecol 6(1):1
Peteiro C, Sánchez N (2012) Comparing salinity tolerance in early stages of the sporophytes of a non-indigenous kelp (Undaria pinnatifida) and a native kelp (Saccharina latissima). Russ J Mar Biol 38(2):197–200. doi:10.1134/S1063074012020095
Pritchard, M, Gorman, RM, Hume, TM (2009) Tauranga Harbour Sediment Study: Hydrodynamic and Sediment Transport Modelling, NIWA Client Report
R Development Core Team (2008) R: A language and environment for statistical computing. Vienna, Austria
Raffo MP, Eyras MC, Iribarne OO (2009) The invasion of Undaria pinnatifida to a Macrocystis pyrifera kelp in Patagonia (Argentina, south-west Atlantic). J Mar Biol Assoc UK 89(08):1571–1580. doi:10.1017/S002531540900071X
Ross C, Van Alstyne KL (2007) Intraspecific variation in stress-induced hydrogen peroxide scavenging by the ulvoid macroalga Ulva lactuca. J Phycol 43:466–474. doi:10.1111/j.1529-8817.2007.00346.x
Ruiz GM, Carlton TJ, Grosholz ED, Hines HA (1997) Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent and consequences. Am Zool 37:621–632
Ruiz GM, Fofonoff P, Hines AH, Grosholz ED (1999) Non-indigenous species as stressors in estuarine and marine communities: Assessing invasion impacts and interactions. Limnol Oceanogr 44(3):950–972
Russell LK, Hepburn CD, Hurd CL, Stuart MD (2008) The expanding range of Undaria pinnatifida in southern New Zealand: distribution, dispersal mechanisms and the invasion of wave-exposed environments. Biol Invasions 10:103–115. doi:10.1007/s10530-007-9113-1
Saito Y (1975) Undaria. In: Tokida J, Hirose H (eds) Advance of Phycology in Japan. Junk Publishers, The Hague, pp 304–320
Schaffelke B, Smith JE, Hewitt CL (2007) Introduced macroalgae — A growing concern. Eighteenth International Seaweed Symposium, Dev Appl Phycol 1:303–315
Schiel DR (1990) Macroalgal assemblages in New Zealand: structure, interactions and demography. Hydrobiologia 192:59–76. doi:10.1007/BF00006227
Schiel DR, Nelson WA (1990) The harvesting of macroalgae in New Zealand. Hydrobiologia 204(205):25–33. doi:10.1007/978-94-009-2049-1_5
Schiel DR, Steinbeck JR, Foster MS (2004) Ten Years of Induced Ocean Warming Causes Comprehensive Changes in Marine Benthic Communities. Ecology 85(7):1833–1839
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. Ecol Stud 100:49–70
Sorte CJB, Williams SL, Zerebecki RA (2010) Ocean warming increases threat of invasive species in a marine fouling community. Ecology 91:2198–2204. doi:10.1890/10-0238.1
Thomsen MS, McGlathery KJ, Tyler AC (2006) Macroalgal Distribution Patterns in a Shallow, Soft-Bottom Lagoon, with Emphasis on the Nonnative Gracilaria vermiculophylla and Codium fragile. Estuaries Coasts 29(3):465–473. doi:10.1007/BF02784994
tom Dieck I (1993) Temperature tolerance and survival in darkness of kelp gametophytes (Laminariales, Phaeophyta): ecological and biogeographical implications. Mar Ecol Prog Ser 100:253–264
Valentine JP, Johnson CR (2003) Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. J Exp Mar Biol Ecol 295(1):63–90. doi:10.1016/S0022-0981(03)00272-7
Valentine JP, Johnson CR (2004) Undaria pinnatifida following dieback of the native macroalga Phyllospora comosa in Tasmania. Australia. Mar Freshwater Res 55(3):223–230
Valentine JP, Magierowski RH, Johnson CR (2007) Mechanisms of invasion: establishment, spread and persistence of introduced seaweed populations. Bot Mar 50(5/6):351–360. doi:10.1515/BOT.2007.040
Weiss RF (1974) Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar Chem 2(3):203–215. doi:10.1016/0304-4203(74)90015-2
Welcomme RL (1992) A history of international introductions of inland aquatic species. ICES Mar Sci Symp 194:3–14
Williams SL, Smith JE (2007) A Global Review of the Distribution, Taxonomy, and Impacts of Introduced Seaweeds. Annu Rev Eco Evo Syst 38:327–359
Wright SW, Jeffrey SW (1997) High resolution HPLC system for chlorophylls and carotenoids of marine phytoplankton. In: Jeffrey SW, Mantoura RFC, Wright SW (eds) Phytoplankton Pigments in Oceanography. UNESCO Publishing, Paris, pp 327–334
Zerebecki RA, Sorte CJB (2011) Temperature tolerance and stress proteins as mechanisms of invasive species success. PLoS ONE 6(4):e14806. doi:10.1371/journal.pone.0014806
Acknowledgments
This work has been conducted in the frame of the international Research Training Group INTERCOAST—Integrated Coastal Zone and Shelf-Sea Research. The authors would like to thank Dudley Bell, David Culliford and Britta Meyer-Schlosser for indispensable support and the Deutsche Forschungsgemeinschaft (DFG) for funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) and has been conducted in the frame of the International Research Training Group INTERCOAST – Integrated Coastal Zone and Shelf-Sea Research.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Responsible Editor: F. Weinberger.
Reviewed by undisclosed experts.
This article is part of the Topical Collection on Invasive Species.
Rights and permissions
About this article
Cite this article
Bollen, M., Pilditch, C.A., Battershill, C.N. et al. Salinity and temperature tolerance of the invasive alga Undaria pinnatifida and native New Zealand kelps: Implications for competition. Mar Biol 163, 194 (2016). https://doi.org/10.1007/s00227-016-2954-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2954-3