Abstract
In terrestrial systems, grazing can have lasting beneficial or damaging effects on plants based on nutrient availability, but it remains unclear how nutrients shape plant responses to grazing in marine systems, especially in resource-poor environments. We chose a phosphorus-limited subtropical turtlegrass Thalassia testudinum meadow in Bermuda to investigate short- and long-term plant responses to repeated herbivory. In a multiyear field experiment, we manipulated nitrogen (N) and phosphorus (P) availability and simulated green turtle Chelonia mydas grazing for 4 months and then tracked recovery through 2 years. In the short term, simulated grazing increased T. testudinum area-specific growth and both foliar N and P content in fertilized plots compared to the control. In unfertilized plots, simulated grazing (trimming) decreased short-term shoot-specific growth and foliar P content. By comparison, long-term data revealed sustained elevated T. testudinum growth after 2 years in fertilized plots in the absence of trimming. While trimming alone did not affect seagrass growth rates 2 years after the treatment application, these plants sustained depleted foliar P concentrations up to 1 year and displayed residual stress symptoms of fewer shoots with thinner leaves. Our data indicate the potential for N and P stoichiometry to influence “cultivation grazing” in marine carbonate environments. Seagrasses apparently responded positively to trimming when the foliar N:P balance did not exceed the “seagrass Redfield ratio” of 30:1. This P-sensitive outcome of herbivory should be evaluated at larger spatial scales and may have important implications for estimating C. mydas carrying capacity of seagrass meadows across resource gradients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cultivation grazing describes an herbivorous feeding mode that promotes plant regrowth and food nutritional quality. In terrestrial systems, whether or not cultivation grazing occurs depends on the magnitude of plant nutrient limitation (Hilbert et al. 1981; de Mazancourt et al. 1998). Cultivation grazing results from the interaction of top-down (grazing) and bottom-up (nutrients) effects on the food web. Feeding strategies that maintain an area by regularly trimming existing plants or otherwise positively altering food value have been described for a variety of marine taxa: dugongs (Preen 1995); green turtles (Bjorndal 1980); damselfish (Hata and Kato 2006); limpets (Plagányi and Branch 2000); polychaetes (Woodin 1977); and gastropods (Hunter and Russell-Hunter 1983). However, the capacity of primary producers to respond positively to grazing in nutrient-poor environments and over longer timescales still needs to be evaluated in marine ecosystems.
In seagrass meadows, the green turtle Chelonia mydas is one of the most widely distributed herbivores, inhabiting coastal areas in over 100 countries (Seminoff et al. 2015). Juvenile and adult green turtles generally commute daily from their sleeping area on fringing reefs to patches of seagrass. They selectively consume tender new growth at the base of shoots and discard older leaf material. Even Bermuda turtle hunters of the early 1900s noticed this selective feeding behavior and allegedly tracked their prey by the “quantities of this marine grass floating on the surface…” over sandy areas (Babcock 1938). Today green turtles are considered cultivation grazers because their mode of trimming shoots: (1) increases seagrass growth, foliar nitrogen (N) and protein content; (2) decreases foliar lignin concentration; and (3) minimizes ingestion of epiphytic calcium carbonate associated with taller, older leaves (Bjorndal 1980; Zieman et al. 1984).
The islands of Bermuda have experienced a recent decline of nearly 50 % (475 of 900 ha) in offshore seagrass area. The most widespread cause for global seagrass loss is nutrient over-enrichment (Orth et al. 2006); however, Bermuda’s seagrass meadows are often P-limited (N:P < 30:1) (Jensen et al. 1998; Fourqurean et al. 2015), and intense turtle grazing may have triggered local seagrass declines (Murdoch et al. 2007; Fourqurean et al. 2010; van Tussenbroek et al. 2014). Immature green turtles in Bermuda forage on seagrass year-round for up to 15 years before returning to natal beaches in Nicaragua and other Caribbean border counties or islands to breed (Bacon et al. 2006; Bermuda Turtle Project unpubl. data). Short-term, grazer-induced changes to seagrass morphology and physiology are widely recognized; however, less is known about seagrasses resilience following grazing disturbance.
The objective of this preliminary study was to evaluate the short-term and persistent effects of grazing on seagrass under varying nutrient levels through manipulating green turtle grazing and nutrient availability over 4 months and then tracking recovery after 1 and 2 years. Cultivation grazing responses were assessed through increases in foliar growth and nutrient content. Seagrass stress responses were assessed through reductions in productivity, leaf width, leaf density, shoot density and rhizome carbohydrate reserves. We hypothesized that cultivation grazing is nutrient-dependent, whereby repeated trimming would induce cultivation grazing in the high-nutrient scenario (P-sufficient) and plant stress in the low-nutrient scenario (P-deficient).
Materials and methods
Study area
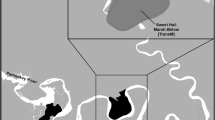
The field experiment was conducted from May 2007 to June 2009 in a continuous Thalassia testudinum-dominated seagrass bed at 1.5 m depth (mean low water) in the mesotrophic outer harbor of Bailey’s Bay, Bermuda (32°20′59″N, 64°43′30″W) (Fig. 1). This shallow bay measures approximately 0.2 km2 in area and is partially sheltered by Bay Island, Pimlico Rock, and a chain of small karst islands and patch reefs along its seaward border. The seafloor contains sandy carbonate sediments with a mosaic of dense and sparse seagrass beds separated by bare areas. The most abundant seagrass is turtlegrass T. testudinum, existing in single-species stands often of short stature (<10 cm), as well as beside manatee grass Syringodium filiforme and shoal grass Halodule wrightii in mixed-species patches. The seaweed community is dominated by Halimeda spp. and Penicillus spp. (K. Holzer unpubl. data). Primarily, productivity is generally P-limited in the outer harbor, which was confirmed by elemental ratios of seagrass leaves from our control plots (see “Results”). No evidence of natural turtle grazing was observed in experimental plots.
Experimental design
A 2 × 2 factorial design with two levels of nutrients (ambient and enriched) and two levels of simulated turtle grazing (untrimmed and trimmed) was used. Twenty 0.5 m × 0.5 m plots were established in a grid (12 m × 15 m) with 2 m separation among plots. Experimental plot size represents the lower areal limit of grazing scars observed in Bermuda, which can range from less than 0.25 m2 to well over 100 m2 (K. Holzer pers. obs.). Rhizomes around the plot borders were left intact to minimize artificial stress, permitting belowground translocation of resources between adjoined shoots. Since experimental plots may have received nutrient subsidies from neighboring shoots, we assume a more cautious assessment of plant tolerance for disturbance. Five replicates were randomly assigned to the four treatments consisting of (1) control; (2) no trimming, +nutrients; (3) +trimming, no nutrients; and (4) +trimming, +nutrients. Nutrients were mixed evenly into the top 4 cm of sediment with fingertips using time-release granular lawn fertilizer (Sta-Green®; N–P–K = 29–2–5, containing nitrogen as 97 % urea and phosphorus as diammonium phosphate). The sediment surface of unfertilized plots was also mixed in a similar fashion. Nutrients were supplied monthly from May to September 2007 at a loading rate exceeding N and P demands of T. testudinum at peak growth, calculated by multiplying maximum aboveground productivity by mean foliar N and P content. Turtle grazing was simulated with surgical scissors also from May to September 2007 using a method similar to Moran and Bjorndal (2005). Scuba divers trimmed leaves to approximately 2 cm above the sediment surface and re-trimmed them when they grew taller than 5 cm. We also maintained a 10-cm trimmed buffer around the grazing plots. A total of 10 trimmings were performed between 9- and 20-day intervals depending on plant regrowth rates. Seagrass clippings were removed from the area to mimic ingestion by turtles.
Sampling
Field data were collected by scuba divers. Before initiating the treatments (May 2007), the natural variation within the study area was quantified using 10 randomly selected plots for all response variables, except rhizome and isotope measurements. We assumed that plots were not statistically different from each other because the standard error was <10 % of the mean for all parameters except epiphyte load, which has inherently high variability. Repeated sampling occurred immediately after the 4-month treatment regime to capture short-term effects (September 2007) and after 1 (June 2008) and 2 years (June 2009) to describe residual treatment effects after the simulated grazing–fertilizer course ended.
Aboveground growth of T. testudinum was measured by the hole-punch technique of Zieman (1974) on all shoots within a 15.3-cm-diameter PVC ring (area = 0.02 m2) using a 20 G BD PrecisionGlide™ needle. From these data, mass-specific (g g−1 day−1), shoot-specific (mg shoot−1 day−1) and area-specific (g m−2 day−1) productivity were calculated. For leaf density, we counted the number of leaves per shoot for all marked shoots. The total number of shoots was tallied for seagrass density (shoot m−2) and standing stock (g m−2) measures. Width was recorded for three random leaves within each plot.
A standardized 5-cm long segment of the youngest mature leaf from five different shoots per plot was scraped clean of epiphytes using a razor blade without disrupting the epidermis of the plant. The epiphyte-free leaf was then rinsed in DIW, dried at 60 °C and ground to a fine powder (using a Wig-L-Bug® Model 002-0200 grinding mill) for calculation of N content using a NA-1500 CNS Analyzer™ (Carlo Erba). Foliar P content was determined on the same sample by a dry oxidation, hot acid digestion followed by colorimetric analysis of phosphate concentration using the technique in Fourqurean et al. (1992) minus the addition of sodium and magnesium sulfate. These reagents were omitted due to the high recovery of the P content for National Institute of Standards and Technology-certified peach leaf standard. Knowing that plants using fertilizer-derived N should contain 15δN signatures near 0 ‰ (Macko and Ostrom 1994), leaf samples from each plot were run at the University of California Davis Stable Isotope Facility to evaluate plant assimilation of the fertilizer application after 4 months and 1 year.
Epiphyte abundance was measured after 4 months on one shoot per plot. All leaves were scraped clean of fouling material in a DIW bath and then strained through a 15-cm-diameter filter with 41 μm pore size. Samples were dried to a constant weight at 60 °C and normalized by shoot surface area to obtain the epiphyte load (mg cm−2). Epiphyte organic matter was calculated on the same sample as the percent ash-free dry weight remaining after combusting 10–20 mg of dried sample at 500 °C for 4 h.
At completion of the simulated grazing–fertilizer course (4 months), rhizome samples were dried to constant weight in a 60 °C oven and ground to a fine powder for subsequent analysis of soluble carbohydrates at Florida International University Seagrass Ecosystem Research Lab (Fourqurean et al. 2010).
Statistical analyses
The main effects of nutrient enrichment, simulated turtle grazing and nutrient enrichment x simulated turtle grazing on seagrass growth and foliar nutrient composition were evaluated using two-way ANOVA followed by Tukey’s post hoc test. Data that did not meet assumptions of homogeneity or normality were transformed. For the residual treatment effects 1 and 2 years later (both measured in June), year was treated as a fixed effect. In cases when more than one value was recorded for a given response variable, they were averaged to give one datum per plot for statistical analyses. In some instances, samples were compromised in the laboratory and therefore omitted from the analysis, which is reflected in the degrees of freedom for “error.” Statistical significance was determined at p < 0.05. All analyses were run on R version 3.2.2.
Results
Effects of simulated turtle grazing and nutrient enrichment
Compensatory seagrass growth (g g−1 day−1) increased for the short-term only, in response to simulated turtle grazing regardless of nutrient status, a characteristic artifact of active trimming [F(3, 15) = 6.69, p < 0.01] (Fig. 2a). In the short term, shoot-specific growth (mg shoot−1 day−1) increased with enrichment and decreased with trimming [F(3, 15) = 9.08, p < 0.001] (Fig. 2b). After 2 years however, fertilizer plus trimming depressed shoot-specific growth for a nutrient × trimming interaction [F(7, 31) = 15.59, p < 0.0001]. In contrast, for both timeframes, area-specific growth (g m−2 day−1) increased for enriched plots only [F(3, 16) = 5.76 and F(7, 31) = 8.23, p < 0.01] (Fig. 2c). While nutrient enrichment plus trimming had a positive immediate effect on aboveground biomass (g m−2) [F(3, 15) = 15.21, p < 0.0001], only a nutrient effect persisted 2 years after the trimming–fertilizer course ended [F(7, 31) = 6.07, p < 0.001].
Separate and interactive effects of simulated turtle grazing (trimming) and nutrient enrichment (+NP) on T. testudinum aboveground productivity expressed as (a) turnover rate (b) shoot-specific growth rate and (c) area-specific growth rate, during the trimming–fertilizer course (4 months) and after the treatment ended (1 and 2 years). Letters denote significant differences among treatments within sampling period. Error bars represent ±SE, n = 5
Seagrass exhibits stress by reducing foliar growth, leaf width, leaf density and shoot density (Greenway 1974; Zieman et al. 1984). See Tables 1 and 2 for a summary of response variables, F values and significance levels. In the short term, T. testudinum showed signs of decreased leaf width in trimmed plots without nutrients added; however, in the long term, all trimmed plots grew significantly narrower leaves [F(7, 32) = 4.48, p < 0.001]. In the nutrient-enriched plots, leaf density increased in the long term [F(7, 32) = 4.50, p < 0.001] and seagrass shoot density progressively increased throughout the 2-year experiment, which was amplified by simulated grazing for a nutrient x trimming interaction [F(7, 30) = 3.39, p < 0.01]. After 2 years, trimming plus nutrient plots contained a mean of 1482 ± 151 shoots m−2. In contrast, in the low-nutrient scenario despite a short-term shoot density increase, simulated grazing without enrichment reduced shoot density in the long term for an endpoint mean of 968 ± 76 shoots m−2.
In the high-nutrient scenario, simulated turtle grazing boosted foliar N content in the short term [F(3, 15) = 7.03, p < 0.01]. However, after the interim 4-month simulated grazing–nutrient course ended, leaf N was similar for all treatments (Fig. 3a). At 4 months, the foliar δ15N values were significantly depressed to near 0 ‰ for the enrichment treatments versus 2.4 ‰ for the unfertilized treatments [F(3, 14) = 11.13, p < 0.001], confirming assimilation of the fertilizer application. However, after 1 year, the fertilizer N signal dissipated. In the short term, fertilizer produced leaves with slightly higher P content, but fertilizer plus trimming produced leaves with much higher P (25–30 %) for a nutrient × trimming interaction [F(3, 15) = 19.11, p < 0.0001]. By comparison, trimming alone depleted foliar P after 4 months, and this effect persisted for 1 year but dissipated after 2 years [F(7, 32) = 6.49, p < 0.0001] (Fig. 3b).
Separate and interactive effects of simulated turtle grazing (trimming) and nutrient enrichment (+NP) on T. testudinum leaf tissue a nitrogen, b phosphorus and c N:P ratio during the trimming–fertilizer course (4 months) and after the treatment ended (1 and 2 years). Vertical dashed line at 30:1 marks the “seagrass Redfield ratio.” Letters denote significant differences among treatments within sampling period. Error bars represent ±SE, n = 5
For seagrasses, tissue elemental N:P reflects the relative availability of these elements in the environment. In T. testudinum leaves, N:P ratio around 30:1 (i.e., seagrass Redfield ratio) represents a critical level and balance in the availability of both nutrients (Atkinson and Smith 1983; Duarte 1990; Fourqurean and Zieman 2002). In our experiment, the leaf N:P ratios showed that trimming increased plant-available P (N:P < 30:1) when fertilizer was added but depleted foliar P compared to N in the short term [F(3, 15) = 14.57, p < 0.0001]. The P-drawdown response lasted another year, but no N:P treatment effects persisted after 2 years [F(7, 32) = 7.60, p < 0.0001] (Fig. 3c).
There were no treatment patterns in epiphyte load or epiphyte organic matter after 4 months. Statistical power was low for these responses, so data were not collected for residual effects. At the 4-month sampling in September, mean epiphyte abundance for all treatments pooled was 16 ± 3 mg cm−2 and mean epiphyte organic content was 11 ± 2 %.
After 4 months, the soluble carbohydrate levels in T. testudinum rhizomes were lowest in the high-nutrient scenario with trimming (80.0 ± 6.8 mg C g−1) and greatest in the high-nutrient scenario without trimming (107.6 ± 6.8 mg C g−1) although the differences were not statistically significant. Only short-term treatment effects on carbohydrate concentration were assessed.
Discussion
Cultivation grazing response
Our experimental data support the hypothesis that sufficient nutrients are required for seagrass to respond to repeated trimming by turtles through enhanced plant regrowth and nutritional value (cultivation grazing). We demonstrate that cultivation grazing can be a function of limiting resources (in this case, P availability) in a marine environment, affirming a likely parallel between terrestrial and marine systems. This finding also provides an important qualifier for the concept of cultivation grazing by green turtles regarding nutrient availability. The prerequisite that sediments cannot be nutrient-poor may help explain observations of destructive C. mydas grazing in certain environments, i.e., Saint John, US Virgin Islands (Williams 1988); review (Heithaus et al. 2014) and offshore Bermuda (Fourqurean et al. 2010), but sustained positive effects in other locations, i.e., Bahamas (Moran and Bjorndal 2007) and Indonesia (Christianen et al. 2012). In this study, we also show empirically that residual stress symptoms of turtle grazing (thin leaf width and low shoot density) in P-deprived areas can last for at least 2 years following the initial disturbance. Therefore, we recommend taking seagrass nutrient status into consideration when calculating the long-term food supply available to green turtles.
Some limitations of the scale of our experiment warrant discussion. First, we acknowledge that due to our small plot size (0.5 m × 0.5 m), Thalassia rhizome connections could extend outside treatment areas and permit nutrient translocation, which may affect seagrass responses to trimming. However, clonal expansion of Thalassia can occur over very large spatial scales (van Dijk and van Tussenbroek 2010), and thus even larger plot sizes would be affected. Clonal integration is a key component of seagrass resilience to grazing. Second, it is possible that smaller plots may not mimic biogeochemical and hydrological processes in larger naturally grazed areas, especially particle capture and nutrient cycling. It is well known that the seagrass canopy slows water flow and promotes sediment stability and organic matter deposition (Almasi et al. 1987; Hansen and Reidenbach 2012; McGlathery et al. 2012). The surrounding (taller) canopy also likely influences water flow and sediment suspension in the experimental plots. The mechanisms influencing altered flow dynamics on adjacent areas with small versus large gaps are complex and depend on landscape configuration and patch size (Carr et al. 2015; Luhar et al. 2008). Therefore, results from plot size experiments should be applied cautiously to the ecosystem and landscape scale.
Patterns of enhanced production
Grazing had persistent effects on seagrass morphology and productivity, which depended on nutrient levels. In our experiment, area-specific production was highest in nutrient-only plots and when fertilizer was combined with simulated grazing (Fig. 2c), but through different plant growth strategies. In general, to boost production in response to changes in abiotic or biotic factors, seagrasses may increase leaf length, leaf width, leaf density or shoot density (Thayer et al. 1984). Initially, the nutrient-only plots produced plants with more leaves per shoot, but after the enrichment course ended, plants maintained enhanced area-specific production for at least 2 years through boosting leaf density (1 year) and shoot density (2 years), while retaining leaf width. By comparison, although leaves grew thinner, when nutrients were added, simulated grazing improved areal growth by progressively increasing the recruitment of new shoots until shoot density exceeded the control (30 %) and nutrient-only (20 %) plots at the 2-year sampling (Tables 1, 2). This shoot density response matches findings from a sea urchin grazing experiment also with T. testudinum (Valentine et al. 1997). The persistent increase in shoot density we detected may help explain the apparent short-term drawdown of belowground carbohydrate reserves, albeit statistically insignificant (Tables 1, 2). By comparison, in the absence of nutrients, even though trimming initially stimulated shoot recruitment, shoot density then declined significantly over the long term (Fig. 4). These unique nutrient-mediated seagrass responses (growth forms) evoke variable consequences for ecosystem function, such as habitat quality for fishes or sediment production rates.
Depiction of variation in T. testudinum growth characteristics following the simulated grazing–fertilizer course (4 months) and recovery (2 years). Nutrient-enriched plants increased leaf density in the short term and shoot density in the long term while maintaining wider leaves. Without nutrients, trimmed plants displayed persistent stress symptoms of narrower leaves and fewer shoots. Trimmed plants plus nutrients progressively increased shoot density, but grew narrower leaves
Two standard mechanisms proposed for the beneficial effect of herbivory on plant growth include: (1) nutrient replenishment from animal waste products, detritus accumulation or enhanced bacterial N-fixation, and (2) increased light reaching active underlying tissues due to a reduced canopy, i.e., light and turtles (Zieman et al. 1984); review (de Mazancourt et al. 1998), and nutrients and dugongs (Perry and Dennison 2000). Regarding the first argument, the deposit of C. mydas waste products in the foraging area is considered inconsequential because the turtles usually disperse after feeding, and the solubility and hydrological transport of any waste products dilute this potential nutrient source (Aragones et al. 2006). At natural green turtle foraging meadows in Bermuda, grazed areas typically lack leaf litter or detrital buildup (K. Holzer pers. obs.) presumably because leaf material floated away (Bjorndal 1980) or was removed through feeding. Moran and Bjorndal (2007) also observed a thinner detrital layer in trimmed versus untrimmed plots in a Bahamas simulated turtle grazing experiment. Therefore, we maintain the conclusion of Zieman et al. (1984) that T. testudinum can respond positively to grazing due to the physiological benefit of extra light reaching leaf bases. Further, we provide another example of positive seagrass responses to short-term eutrophication being contingent on preexisting nutrient limitation (Cabaço et al. 2013).
During the 4-month simulated grazing–fertilizer course, leaf turnover rates (g g−1 day−1) were higher (50 %) in trimmed plots, regardless of nutrient levels (Fig. 2a). Unlike the other productivity metrics, after the simulated grazing ended, there was no significant difference in turnover rates across treatments, suggesting that mass-specific growth measures of recently trimmed plants represent an artifact of leaf removal, rather than a physiological or ecosystem change. This result does not imply that turnover is unimportant, but other more versatile growth metrics (e.g., growth per shoot or area) can be used, while seagrasses are actively grazed or recovering from recent leaf loss to avoid overestimating the impacts of herbivory.
Patterns of relative P availability
While classic studies review the central role of N in mediating food choice (Mattson 1980; McNaughton 1984), we show that P availability can drive plant capacity for regrowth over the long term. Given the high affinity and tight adsorption of P by carbonate sediments, primary production is generally considered P-limited in subtropical and tropical seagrass environments (Fourqurean et al. 1992). The nutrient manipulations in our study may reflect field conditions with elevated P, in an otherwise P-limited environment, caused by localized percolation of human sewage from nearby cesspits or deep-sealed effluent boreholes, which in Bermuda leach nutrients laterally to the seabed adjacent to the shoreline (Jones et al. 2011). Such pulse releases of human waste to the seabed are also realistic for other coastal areas without centralized municipal sewage systems. Isotope analysis of seagrass leaves in Bermuda supports the hypothesis that the relatively high P availability at some nearshore sites comes from sewage-derived nutrients (Fourqurean et al. 2015). It is even possible that natural turtle populations in Bermuda select foraging sites where seagrasses have sufficient P to withstand repeat trimming. Furthermore, the high retention of P compared to N in the system following a nutrient enrichment pulse (Ferdie and Fourqurean 2004) may help explain long-term tolerance of certain inshore meadows for grazing.
The rapid response of T. testudinum to nutrient enrichment at our experimental site in Bailey’s Bay, Bermuda, suggests strong nutrient limitation. Plants quickly assimilated artificial N and P, producing fertilizer-derived δ15N signals at 4 months (Tables 1, 2) and P-enriched leaf tissues after 4 months that persisted for at least 2 years (Fig. 3b). Not surprisingly, chemical analyses of seagrass leaves from other locations where cultivation grazing has been described, such as Saint Croix, US Virgin Islands (Zieman et al. 1984); Bahamas (Moran and Bjorndal 2007) and Indonesia (Christianen et al. 2012), revealed N:P similar to our experimental high-nutrient scenario. Phosphorus was not limiting in our experimental high-nutrient scenario with simulated grazing (N:P = 26:1) or the abovementioned simulated turtle grazing studies (N:P = 26:1 and 29:1, respectively) (Moran and Bjorndal 2007; Christianen et al. 2012). It seems likely that these environments collectively supported a positive (cultivation) response to grazing because foliar N:P ratios were lower than the seagrass Redfield ratio of 30:1, indicating P-sufficient growth.
Sustainability of repeat turtle grazing
In the 1960s, an interest in using seagrass leaves for livestock feed due to the high protein content (~13 %) motivated the first published experimental assessments of viable harvesting procedures, using leaf length and aboveground biomass measurements before and after harvesting to measure recovery (Taylor et al. 1973). This pilot study determined that Florida T. testudinum beds could tolerate clipping twice a year at 2-month intervals during the growing season. In Jamaica, Greenway (1974) showed that T. testudinum fully recovered after four clippings at 70-day intervals. Subsequently, ecologists started estimating how long seagrass areas could tolerate continuous grazing by green turtles, which ranged from 3 months to over 1 year (Bjorndal 1980; Zieman et al. 1984). The first empirical study on how long repetitive turtle trimming could sustain production of N-rich leaves achieved at least 16 months in a P-sufficient (foliar P = 0.22 %) meadow (Moran and Bjorndal 2005). In the presence of high nutrient loading, simulated turtle grazing doubled seagrass production resulting in a positive feedback loop that could be traced back 6 years (Christianen et al. 2012). However, in extreme cases when seagrass leaf regrowth did not keep pace with consumption, C. mydas altered their feeding strategy to mine rhizomes, which led to erosion and other damaging effects (Christianen et al. 2014). These results collectively highlight why P-limited sites, such as Bermuda’s oligotrophic offshore waters, may not sustain prolonged turtle grazing.
Conclusions
Randall (1965) imagined a pre-Columbus ocean where C. mydas populations were so great that they overwhelmed their food source. Today, we revisit that scenario in which seagrass abundance can become the “limiting factor,” if seagrass habitat loss outpaces sea turtle population recovery (Chaloupka et al. 2008). In Bermuda, green turtle nesting has not been observed for centuries (Babcock 1938) until last summer (Jardine 2015), which could mark the rebound of a local rookery. Our small-scale experiment suggests that moderate turtle grazing may cultivate seagrasses when P is sufficient, but may harm seagrasses in P-deficient cases. Research on the ability of seagrasses to respond positively to herbivory across abiotic gradients of light, temperature and salinity (especially where turtle populations are increasing) over larger spatial scales would benefit resource managers. The cultivation potential of other preferred food seagrass and seaweed species in the geographic range of C. mydas requires further investigation. Finally, the consequences of grazer-mediated, P-sensitive changes to meadow architecture (e.g., shoot morphology, density) deserve additional attention.
References
Almasi MN, Hoskin CM, Reed JK, Milo J (1987) Effects of natural and artificial Thalassia on rates of sedimentation. J Sediment Res 57:901–906. doi:10.1306/212f8c9c-2b24-11d7-8648000102c1865d
Aragones LV, Lawler IR, Foley WJ, Marsh H (2006) Dugong grazing and turtle cropping: Grazing optimization in tropical seagrass systems? Oecologia 149:635–647. doi:10.1007/s00442-006-0477-1
Atkinson MJ, Smith SV (1983) C:N:P ratios of benthic marine plants. Limnol Oceanogr 28:568–574. doi:10.4319/lo.1983.28.3.0568
Babcock HL (1938) The sea-turtles of the Bermuda Islands, with a survey of the present state of the turtle fishing industry. Proc Zool Soc Lond A 107:595–602. doi:10.1111/j.1469-7998.1938.tb00015.x
Bacon JP, Gray JA, Kitson L (2006) Status and conservation of the reptiles and amphibians of the Bermuda islands. Appl Herpetol 3:323–344
Bjorndal KA (1980) Nutrition and grazing behavior of the green turtle Chelonia mydas. Mar Biol 56:147–154. doi:10.1007/BF00397131
Cabaço S, Apostolaki ET, García-Marín P, Gruber R, Hernández I, Martínez-Crego B, Mascaró O, Pérez M, Prathep A, Robinson C, Romero J, Schmidt AL, Short FT, van Tussenbroek BI, Santos R (2013) Effects of nutrient enrichment on seagrass population dynamics: evidence and synthesis from the biomass–density relationships. J Ecol 101:1552–1562. doi:10.1111/1365-2745.12134
Carr JA, D’Odorico P, McGlathery KJ, Wiberg PL (2015) Spatially explicit feedbacks between seagrass meadow structure, sediment and light: habitat suitability for seagrass growth. Adv Water Resour. doi:10.1016/j.advwatres.2015.09.001
Chaloupka M, Bjorndal KA, Balazs GH, Bolten AB, Ehrhart LM, Limpus CJ, Suganuma H, Troëng S, Yamaguchi M (2008) Encouraging outlook for recovery of a once severely exploited marine megaherbivore. Glob Ecol Biogeogr 17:297–304. doi:10.1111/j.1466-8238.2007.00367.x
Christianen MJA, Govers LL, Bouma TJ, Kiswara W, Roelofs JGM, Lamers LPM, van Katwijk MM (2012) Marine megaherbivore grazing may increase seagrass tolerance to high nutrient loads. J Ecol 100:546–560. doi:10.1111/j.1365-2745.2011.01900.x
Christianen MJA, Herman PMJ, Bouma TJ, Lamers LPM, van Katwijk MM, van der Heide T, Mumby PJ, Silliman BR, Engelhard SL, van de Kerk M, Kiswara W, van de Koppel J (2014) Habitat collapse due to overgrazing threatens turtle conservation in marine protected areas. Proc R Soc Lond B Biol 281:1–6. doi:10.1098/rspb.2013.2890
de Mazancourt C, Loreau M, Abbadie L (1998) Grazing optimization and nutrient cycling: when do herbivores enhance plant production? Ecology 79:2242–2252. doi:10.1890/0012-9658(1998)079[2242:GOANCW]2.0.CO;2
Duarte CM (1990) Seagrass nutrient content. Mar Ecol Prog Ser 67:201–207
Ferdie M, Fourqurean JW (2004) Responses of seagrass communities to fertilization along a gradient of relative availability of nitrogen and phosphorus in a carbonate environment. Limnol Oceanogr 49:2082–2094. doi:10.4319/lo.2004.49.6.2082
Fourqurean J, Zieman J (2002) Nutrient content of the seagrass Thalassia testudinum reveals regional patterns of relative availability of nitrogen and phosphorus in the Florida Keys USA. Biogeochemistry 61:229–245. doi:10.1023/A:1020293503405
Fourqurean JW, Zieman JC, Powell GV (1992) Phosphorus limitation of primary production in Florida Bay: evidence from C:N:P ratios of the dominant seagrass Thalassia testudinum. Limnol Oceanogr 37:162–171. doi:10.4319/lo.1992.37.1.0162
Fourqurean J, Manuel S, Coates K, Kenworthy W, Smith S (2010) Effects of excluding sea turtle herbivores from a seagrass bed: overgrazing may have led to loss of seagrass meadows in Bermuda. Mar Ecol Prog Ser 419:223–232. doi:10.3354/meps08853
Fourqurean JW, Manuel SA, Coates KA, Kenworthy WJ, Boyer JN (2015) Water quality, isoscapes and stoichioscapes of seagrasses indicate general P limitation and unique N cycling in shallow water benthos of Bermuda. Biogeosci Discuss 12:9751–9791. doi:10.5194/bgd-12-9751-2015
Greenway M (1974) The effects of cropping on the growth of Thalassia testudinum (König) in Jamaica. Aquaculture 4:199–206. doi:10.1016/0044-8486(74)90034-9
Hansen JCR, Reidenbach MA (2012) Wave and tidally driven flows in eelgrass beds and their effect on sediment suspension. Mar Ecol Prog Ser 448:271–287. doi:10.3354/meps09225
Hata H, Kato M (2006) A novel obligate cultivation mutualism between damselfish and Polysiphonia algae. Biol Lett 2:593–596. doi:10.1098/rsbl.2006.0528
Heithaus MR, Alcoverro T, Arthur R, Burkholder DA, Coates KA, Christianen MJA, Kelkar N, Manuel SA, Wirsing AJ, Kenworthy WJ, Fourqurean JW (2014) Seagrasses in the age of sea turtle conservation and shark overfishing. Front Mar Sci. doi:10.3389/fmars.2014.00028
Hilbert DW, Swift DM, Detling JK, Dyer MI (1981) Relative growth rates and the grazing optimization hypothesis. Oecologia 51:14–18. doi:10.1007/BF00344645
Hunter RD, Russell-Hunter WD (1983) Bioenergetic and community changes in intertidal Aufwuchs grazed by Littorina littorea. Ecology 64:761–769. doi:10.2307/1937199
Jardine H (2015) Sea turtle nest found on beach, 15 hatchlings. Bernews
Jensen HS, McGlathery KJ, Marino R, Howarth RW (1998) Forms and availability of sediment phosphorus in carbonate sand of Bermuda seagrass beds. Limnol Oceanogr 43:799–810. doi:10.2307/2839175
Jones R, Parsons R, Watkinson E, Kendell D (2011) Sewage contamination of a densely populated coral ‘atoll’ (Bermuda). Environ Monit Assess 179:309–324. doi:10.1007/s10661-010-1738-3
Luhar M, Rominger J, Nepf H (2008) Interaction between flow, transport and vegetation spatial structure. Environ Fluid Mech 8:423–439. doi:10.1007/s10652-008-9080-9
Macko SA, Ostrom NE (1994) In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science, 2nd edn. Blackwell Scientific Publications, Cambridge, pp 45–62
Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161. doi:10.1146/annurev.es.11.110180.001003
McGlathery KJ, Reynolds LK, Cole LW, Orth RJ, Marion SR, Schwarzschild A (2012) Recovery trajectories during state change from bare sediment to eelgrass dominance. Mar Ecol Prog Ser 448:209–221. doi:10.3354/meps09574
McNaughton SJ (1984) Grazing lawns: animals in herds, plant form, and coevolution. Am Nat 124:863–886. doi:10.2307/2461305
Moran KL, Bjorndal KA (2005) Simulated green turtle grazing affects structure and productivity of seagrass pastures. Mar Ecol Prog Ser 305:235–247. doi:10.3354/meps305235
Moran KL, Bjorndal KA (2007) Simulated green turtle grazing affects nutrient composition of the seagrass Thalassia testudinum. Mar Biol 150:1083–1092. doi:10.1007/s00227-006-0427-9
Murdoch TJT, Glasspool AF, Outerbridge M, Ward J, Manuel S, Gray J, Nash A, Coates KA, Pitt J, Fourqurean JW, Barnes PA, Vierros M, Holzer K, Smith SR (2007) Large-scale decline in offshore seagrass meadows in Bermuda. Mar Ecol Prog Ser 339:123–130. doi:10.3354/meps339123
Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Olyarnik S, Short FT, Waycott M, Williams SL (2006) A global crisis for seagrass ecosystems. Bioscience 56:987–996. doi:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
Perry CJ, Dennison WC (2000) Microbial nutrient cycling in seagrass sediments. J Aust Geol Geophys 17:227–231
Plagányi ÉE, Branch GM (2000) Does the limpet Patella cochlear fertilize its own algal garden? Mar Ecol Prog Ser 194:113–122. doi:10.3354/meps194113
Preen A (1995) Impacts of dugong foraging on seagrass habitats: observational and experimental evidence for cultivation grazing. Mar Ecol Prog Ser 124:201–213. doi:10.3354/meps124201
Randall JE (1965) Grazing effect on sea grasses by herbivorous reef fishes in the West Indies. Ecology 46:255–260. doi:10.2307/1936328
Seminoff JA, Allen CD, Balazs GH, Dutton PH, Eguchi T, Haas HL, Hargrove SA, Jensen MP, Klemm DL, Lauritsen AM, MacPherson SL, Opay P, Possardt EE, Pultz SL, Seney EE, Van Houtan KS, Waples RS (2015) Status review of the green turtle (Chelonia mydas) under the Endangered Species Act. NOAA Technical Memorandum NOAA-TM-NMFS-SWFSC-539: 1–571
Taylor JL, Saloman CH, Prest KW Jr (1973) Harvest and regrowth of turtle grass (Thalassia testudinum) in Tampa Bay, Florida. Fish Bull 71:145–148
Thayer GW, Bjorndal KA, Ogden JC, Williams SL, Zieman JC (1984) Role of larger herbivores in seagrass communities. Estuaries 7:351–376. doi:10.2307/1351619
Valentine JF, Heck KL, Busby J Jr, Webb D (1997) Experimental evidence that herbivory increases shoot density and productivity in a subtropical turtlegrass (Thalassia testudinum) meadow. Oecologia 112:193–200. doi:10.2307/4221763
van Dijk JK, van Tussenbroek BI (2010) Clonal diversity and structure related to habitat of the marine angiosperm Thalassia testudinum along the Atlantic coast of Mexico. Aquat Bot 92:63–69. doi:10.1016/j.aquabot.2009.10.005
van Tussenbroek BI, Cortés J, Collin R, Fonseca AC, Gayle PMH, Guzmán HM, Jácome GE, Juman R, Koltes KH, Oxenford HA, Rodríguez-Ramirez A, Samper-Villarreal J, Smith SR, Tschirky JJ, Weil E (2014) Caribbean-wide, long-term study of seagrass beds reveals local variations, shifts in community structure and occasional collapse. PLoS One 9:e90600. doi:10.1371/journal.pone.0090600
Williams SL (1988) Thalassia testudinum productivity and grazing by green turtles in a highly disturbed seagrass bed. Mar Biol 98:447–455. doi:10.1007/BF00391121
Woodin SA (1977) Algal “gardening” behavior by nereid polychaetes: effects on soft-bottom community structure. Mar Biol 44:39–42. doi:10.1007/BF00386902
Zieman JC (1974) Methods for the study of the growth and production of turtle grass, Thalassia testudinum König. Aquaculture 4:139–143. doi:10.1016/0044-8486(74)90029-5
Zieman JC, Iverson RL, Ogden JC (1984) Herbivory effects on Thalassia testudinum leaf growth and nitrogen content. Mar Ecol Prog Ser 15:151–158. doi:10.3354/meps015151
Acknowledgments
We are indebted to Rowena Day, Sarah Gosling, Nick Lewis and Russell Smith for assistance with simulated turtle grazing and other scientific diving. Mark Minton provided statistics advice. This research was funded by grants from the US Environmental Protection Agency (EPA) under the STAR Graduate Fellowship Program, the Marine Environmental Program Lab, Bermuda Institute of Ocean Sciences (BIOS) Grant-in-Aid and the Moore Endowment at University of Virginia. We dedicate this manuscript to Peter Outerbridge (1928–2014) whose keen observations returned clever insights about Bermuda’s marine environment. This is BIOS contribution 3006. The views expressed within belong to the authors and were not subject to EPA review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: F. Weinberger.
Reviewed by K. McDermid and an undisclosed expert.
Rights and permissions
About this article
Cite this article
Holzer, K.K., McGlathery, K.J. Cultivation grazing response in seagrass may depend on phosphorus availability. Mar Biol 163, 88 (2016). https://doi.org/10.1007/s00227-016-2855-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2855-5