Abstract
Aquaculture is a significant vector for the introduction of invasive species worldwide. Understanding factors influencing the proliferation and spread of invasive species from aquaculture sites to native habitats is necessary to develop management strategies aimed at mitigating their spread and subsequent impacts. This study compares population dynamics of the invasive kelp Undaria pinnatifida between mussel farms and a natural reef site in northern New Zealand (36°35′S 175°05′E) and investigates how the abundance of U. pinnatifida on mussel farms, and its presence on adjacent reefs, relates to different environmental variables and physical attributes of mussel farms. Monitoring from 2011 to 2014 found that U. pinnatifida on mussel farms were larger and more seasonally and reproductively persistent compared to populations on adjacent reefs. Region-wide surveys found U. pinnatifida at all mussel farming sites examined (n = 25) and at eight adjacent reefs. Coastal populations were most abundant in reef habitats lacking native macroalgal canopies. Abundance of U. pinnatifida on mussel farms was related to the size of mussels present, but not strongly related to wave exposure, turbidity or spatial attributes of farms. Undaria pinnatifida was found on a number of native reefs adjacent to mussel farms, but its presence on these reefs was not related to the size of farms or distance from shore. These results demonstrate how marine farms provide an optimal environment for the proliferation of invasive species, and management strategies must consider that farms of any size or position relative to shore pose a risk of introducing invasive species to native habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is one of the fastest growing sectors of the world food economy (e.g. Subasinghe et al. 2009). The rapid expansion of the industry has resulted in aquaculture becoming one of the leading vectors of introduction for aquatic invasive species worldwide (Katsanevakis et al. 2013) with introductions of seaweeds, fish, invertebrates, parasites and pathogens all linked to aquaculture activities (Naylor et al. 2001). Introductions of invasive species occur via the movement of aquaculture equipment and the transfer of farmed species amongst aquaculture sites. Once colonised, aquaculture sites can subsequently spread invasive species to native habitats (Katsanevakis et al. 2013) where it can be nearly impossible to eliminate them (Thresher and Kuris 2004; Olenin et al. 2011). Interception or removal of pathways to introduction is potentially the only effective strategy for reducing future impacts from invasive species (Carlton and Ruiz 2005; Minchin 2007; Olenin et al. 2011; Katsanevakis et al. 2013). Because aquaculture has fixed and licensed locations and operating procedures, it can be more effectively controlled than other pathways such as recreational and commercial vessel movements (Savini et al. 2010). It has been shown that compulsory regulatory controls for aquaculture practices can decrease the spread of invasive species (Katsanevakis et al. 2013). Consequently, better understanding invasion patterns and processes will allow the development of strategies to reduce the spread of invasive species via aquaculture vectors and mitigate their ecological impacts (Molnar et al. 2008; Williams et al. 2013; Ojaveer et al. 2015).
Macroalgae form a significant component of marine invasive species and pose considerable economic and environmental risks for which there are currently limited control and management options (e.g. Davidson et al. 2015). The invasive kelp Undaria pinnatifida is one of the most prolific invasive macroalgal species (Nyberg and Wallentinus 2005) and is now found throughout most temperate regions of the world (James et al. 2015). Undaria pinnatifida was first spread outside of its native Asian waters, to France, in association with Pacific oyster aquaculture (Perez et al. 1981). Following its introduction, aquaculture transfers continued to act as vectors for spread around Europe (Perez et al. 1988; Fletcher and Farrell 1999; Voisin et al. 2005), including spread to coastal reef habitats (Floc’h et al. 1991, 1996). Similarly, since the discovery of U. pinnatifida in southern New Zealand in the 1980s (Hay and Luckens 1987), its spread throughout the country has been closely associated with aquaculture activities (Hay 1990; Neill et al. 2008; Russell et al. 2008; Hunt et al. 2009; Forrest and Hopkins 2013).
Undaria pinnatifida has a limited capacity for natural dispersal (Forrest et al. 2000) but is easily spread by anthropogenic vectors. Shipping and recreational vessels commonly spread U. pinnatifida between marinas, ports and other artificial structures (e.g. Minchin and Nunn 2014). Aquaculture activities typically rely on high water quality, meaning that aquaculture transfers and practices often expose coastal areas in isolated and undeveloped areas to U. pinnatifida invasion (Inglis et al. 2000; Dodgshun et al. 2007; Hunt et al. 2009). These areas often have intact wilderness values and offer resources of economic, social and cultural importance and therefore can be regarded as high-value areas (Campbell and Hewitt 2013).
Undaria pinnatifida is an opportunistic and efficacious invasive species. It is highly fecund and has a fast growth rate and a plastic morphology (Dean and Hurd 2007; Schiel and Thompson 2012). It also has a hardy microscopic growth phase which makes monitoring and control efforts challenging (Hewitt et al. 2005). Undaria pinnatifida can change the structure of benthic ecosystems through domination of space and the alteration of species richness and composition (Hay and Luckens 1987; Hay 1990; Eno et al. 1997; Curiel et al. 2001; Neill et al. 2008; Raffo et al. 2009; Irigoyen et al. 2011a, b). Undaria pinnatifida is highly adaptable, displaying different life spans and seasonal growth patterns in different environments (see James et al. 2015) and inhabiting a broad range of both natural and artificial substrata (Floc’h et al. 1991; Dean and Hurd 2007; Russell et al. 2008; Meretta et al. 2012). However, U. pinnatifida has an affinity for artificial substrata (Hay 1990; Hay and Luckens 1987; Russell et al. 2008) and often colonises man-made structures more readily than natural reef systems (Hay 1990; Russell et al. 2008; Minchin and Nunn 2014).
The New Zealand green-lipped mussel Perna canaliculus (hereafter called mussel) provides the largest aquaculture industry in the country with more than 600 mussel farms located throughout central and northern New Zealand covering thousands of hectares of marine space (Aquaculture New Zealand 2011). The spread of U. pinnatifida around New Zealand via mussel aquaculture transfers is well recognised (e.g. Forrest and Blakemore 2006). Spread can occur via transfers of aquaculture equipment or mussel seed stock, including the overland transport of spat mussels (Minchin 2007). The subsequent spread from aquaculture structures to surrounding reef areas has been observed in southern New Zealand (e.g. Hunt et al. 2009). In northern New Zealand, U. pinnatifida is known to occur on mussel farms in the Hauraki Gulf, being first reported in 2002 (Russell et al. 2008). However, limited information exists on its prevalence on native reefs in northern New Zealand (e.g. James et al. 2014) and more generally on the factors influencing the ability of U. pinnatifida to successfully spread from mussel farms to native reef habitats.
The present study aims to compare the population ecology of U. pinnatifida on mussel farms and native reefs, identify what influences its spread from mussel farms to nearby reefs and to isolate factors which may allow the prediction and mitigation of spread within the coastal marine environment. It was hypothesised that larger aquaculture sites and those where structures are positioned closer to the coast may increase the likelihood of introducing U. pinnatifida to coastal reefs. The relationship between infestation levels and a range of environmental factors and features of mussel aquaculture sites were analysed. Reef surveys also aimed to identify which coastal habitat types are most susceptible to invasion by this species in northern New Zealand.
Materials and methods
Seasonal variation of Undaria pinnatifida on mussel farms and coastal reefs
Population monitoring was conducted at two mussel farms through monthly surveys from June 2011 until January 2014. The monitored mussel farms were positioned in 10–12 m of water within the sheltered confines of the Coromandel Harbour, fetch 167 km (Fig. 1), and annual sea surface temperatures ranged from 11.7 to 24.0 °C. Each mussel farm comprised on average 15 lines, each line is made up of two “backbone” ropes running in parallel just beneath the surface and held up with a series of buoys, and suspended from the backbone ropes are the mussel growing ropes or droppers, hanging to depths of 7–10 m. Undaria pinnatifida growing on the underside of the buoys was monitored, as opposed to that growing on the ropes, to ensure the maximum monitoring time was gained before losing the sporophytes to mussel harvesting and so a clear area (m2) of substrate could be defined. Four mussel lines were chosen for monitoring, and all U. pinnatifida found on a series of ten buoys per line were counted. Total length and sporophyll width and length were measured to the nearest 5 mm. If lines were harvested during the course of the survey, new lines were randomly selected for monitoring to ensure 40 buoys were inspected each month.
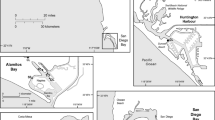
Location of mussel aquaculture areas surveyed in the Hauraki Gulf; red stars indicate smaller mussel farm sites (0.5–3 Ha), red rectangle represents the Wilson Bay Marine Farming Zone (WBMFZ; ~900 Ha), and blue star represents location of the mussel farm and coastal monitoring sites in Coromandel Harbour
Population monitoring was also conducted at a coastal reef site approximately 700 m from the mussel farm monitoring site within the Coromandel Harbour (Fig. 1). The reef site was characterised by gently sloping rocky substratum (reef, boulders and cobbles) and supported diverse stands of canopy-forming native macroalgae. The site had an U. pinnatifida population present in the shallow subtidal zone. One 50 × 2 m transect spanning the shallow subtidal zone was inspected via snorkel and all U. pinnatifida counted each month from October 2012 until January 2014. In addition to the counts, in January 2013, sporophyte measurements began. Forty randomly selected sporophytes, or all sporophytes present if numbering less than forty, were measured for total length and sporophyll length and width until January 2014 when no sporophytes remained at the site.
Distribution of Undaria pinnatifida on mussel farms and coastal reefs
Surveys of U. pinnatifida were carried out on snorkel and SCUBA at mussel aquaculture sites and adjacent reef sites around the Coromandel Peninsula and Great Barrier Island, north-eastern New Zealand (Fig. 1), between July 2011 and February 2012. In this region, each mussel aquaculture site typically comprises one to seven individual mussel farms with each individual farm between 0.6 and five hectares in surface area. These farms are positioned 50 to 300 m offshore. Fifty-three individual mussel farms are listed by Waikato Regional Council at 24 small aquaculture sites around the region. All 24 aquaculture sites were visited, and surveys were carried out on 29 individual mussel farms. In addition to the 24 aforementioned sites is the Wilson Bay Marine Farming Zone (WBMFZ) in the Firth of Thames (Fig. 1); this site comprises more than 150 mussel farms which together cover more than 900 hectares of water space approximately two kilometres offshore. Seven representative farms were surveyed within the WBMFZ.
At each mussel farm, surveyors swam the length of at least three mussel lines, on or near the surface, noting the presence and density of U. pinnatifida per fifty metre length of mussel line and the depth distribution of sporophytes on the dropper lines. Most backbone ropes along mussel lines were approximately 100 m so one mussel line comprised two transects. The number of U. pinnatifida sporophytes per 50 m transect was categorised as 0, 1–10, 10–50, 50–100, 100–250 or 250–500. A total of 304 transects were run along 159 mussel lines at 36 different mussel farms. Notes were taken on the size distribution and reproductive status of the U. pinnatifida surveyed on each line. Mussels were also measured to the nearest 5 mm (n = 5) on every line surveyed, and the average mussel size was calculated for each of these lines.
Surveys of the adjacent coastal reef were carried out concurrently with contiguous 50 × 5 m transects run along sections of coast adjacent to all mussel farming sites surveyed. A total of thirteen kilometres of coast were surveyed via SCUBA and snorkel. In New Zealand, U. pinnatifida populations that occur on native reef habitats are typically densest from the low intertidal fringe down to approximately three metres (Brown and Lamare 1993; Russell et al. 2008). Hence, sampling was concentrated on the shallow subtidal margin of the reef. The depth of the surveys ranged from mean low water to four metres (MLW) with an average depth across all transects of 2.4 m. For each transect, the habitat type, substrate type and abundance of U. pinnatifida (using the same scale as for mussel farms) were recorded. Coastal habitat types were split into four categories by dominant species or features; 1) urchin barrens, 2) large brown algal canopy (primarily including the kelp Ecklonia radiata and large fucoid species of the Carpophyllum genus), 3) coralline turf and 4) a mixed category was allocated where none of these three habitats were obviously dominant.
Environmental variables
Wave exposure at each site was estimated by calculating a topographical index of fetch for each site; this was done by summing the distance to land for each 10-degree sector of the compass rose. For open sectors of water, the radial distance was arbitrarily set to be 300 km (Gorman et al. 2003). Following Shears et al. (2008), this measurement of fetch was considered the best available estimate of local exposure to ambient swell conditions for the typically shallow coastal mussel farming sites examined in this study. Water clarity was measured using a standard 25-cm-diameter black and white Secchi disc where the reading was taken as the depth (m) of descending disappearance and ascending reappearance. Dive notes were made on visibility and observed sediment. Information on the allocated surface area (Ha) for each mussel farm was supplied by the Waikato Regional Council and confirmed using Google Earth; distances from the shore to mussel farms were calculated using Google Earth.
Statistical analysis
Distance-based linear modelling was used to investigate how both infestation levels on farms and presence on the coast related to explanatory variables using the DISTLM procedure in PRIMER v6 (Anderson et al. 2008). Explanatory variables for the analysis of infestation levels on farms included fetch, Secchi depth, distance to coast (log(x + 1)), size of individual farm and size of overall farming area (log(x + 1)). For presence–absence on coast, explanatory variables included fetch, Secchi depth, distance to coast (log(x + 1)), size of overall farming area (log(x + 1)) and mean density on the farm (log(x + 1)). For both analyses, explanatory variables were normalised and univariate analyses were based on Euclidean distance matrices. The “Best” procedure with AICc selection criterion was used to select the top models.
Results
Seasonal variation in Undaria pinnatifida populations on mussel farms and coastal reefs
Monthly monitoring revealed different seasonal patterns in abundance and reproduction between U. pinnatifida populations on the coast and those on mussel farms (Fig. 2). Undaria pinnatifida was absent from the coastal monitoring site from late summer to early winter, whereas plants were present throughout the year on mussel farm structures (Fig. 2a). The mussel farm population had consistently greater average sporophyte lengths as compared to the coastal population (Fig. 2b), and sporophytes on the mussel farm maintained reproductive capacity (measured by sporophyll presence) throughout the year (Fig. 2c).
Distribution and abundance of Undaria pinnatifida on mussel farms
Surveys of 36 individual mussel farms in the eastern Hauraki Gulf found that U. pinnatifida was present on 33 of these farms (Fig S1). At the three mussel farms where U. pinnatifida was not recorded in surveys, it was observed on neighbouring mussel farms and/or mooring lines. At all mussel farms U. pinnatifida was most prevalent in the top two metres of water and declined with depth. The maximum depth for U. pinnatifida recorded on a mussel farm was 8 m at Katherine Bay, Great Barrier Island.
The highest abundances of U. pinnatifida were recorded at a large offshore aquaculture area, Wilson Bay Marine Farming Zone (WBMFZ), and at three smaller mussel farming sites around Coromandel Harbour and Great Barrier Island (Fig. 3; Fig. S1). These sites all had farms with more than 250 sporophytes per 50 m transect on some lines, sometimes up to 500 plants per 50 m. The lowest levels of U. pinnatifida infestation were seen at Port Charles and at Kennedy Bay on the eastern side of the Coromandel Peninsula. Kennedy Bay did not have U. pinnatifida present on the mussel farms surveyed (which were newly seeded), but U. pinnatifida was identified on two mooring ropes within the bay.
Undaria pinnatifida abundance on coastal reefs and mussel farms, see Fig. 1 for locations (GBI is Great Barrier Island, Coromandel is Coromandel Harbour). Densities for mussel farms are for 50 m of mussel line; densities for coastal sites are for 50 × 5 m transects on reef. Kennedy Bay is not shown as no U. pinnatifida was found on the mussel farms or reefs at that site
Abundance of U. pinnatifida on mussel farms was not strongly related to any of the explanatory variables (Fig. 4, Table 1a). Mean abundance was significantly related to distance from shore and the overall size of the farm, but this was largely driven by the high abundances at the large offshore site (WBMFZ) (Fig. 4c, d). There was no significant relationship between U. pinnatifida abundance on mussel farms and fetch or Secchi depth (Table 1a). The “best” model based on AICc only included total farm size, but this only had r 2 = 0.19.
Relationships between U. pinnatifida abundance on mussel farms and explanatory variables. Linear regression line is shown for significant factors (Table 1). Circles represent smaller mussel farm sites, and triangles represent farms within the WBMFZ
The density of U. pinnatifida on mussel lines was positively related to the size of mussels on the lines (Fig. 5; F1,105 = 33.4, P = <0.0001). This relationship was largely driven by the fact that farms with small and newly seeded mussels (<40 mm) had little or no U. pinnatifida and farms with very large mussels (>100 mm) were highly infested.
Distribution and presence of Undaria pinnatifida on the coast
Undaria pinnatifida was found at eight coastal reef sites adjacent to mussel farming sites. Densities were generally low on coastal reefs (Fig. 3), with the highest densities recorded at Katherine Bay, Great Barrier Island (~50 plants per 125 m2 transect). Undaria pinnatifida was predominantly found in reef habitats lacking a native large brown macroalgal canopy, such as on coralline turf (Corallina officinalis) or urchin barrens (Table S1; Fig. 6). Coastal U. pinnatifida was typically found at the highest densities between depths of 0–3 m below mean low water in the shallow subtidal or very low inter-tidal zone (Table S1).
The presence of U. pinnatifida on the coast was not clearly related to any of the explanatory variables (Table 1b ; Fig. S2). DISTLM indicated that the presence of U. pinnatifida on coastal reefs was significantly related to distance to shore, farm size and the mean abundance on the farms, but not related to Secchi depth or wave exposure (Table 1b). The “best” model included overall farm size, Secchi depth and mean abundance and had an r 2 = 0.47. However, this relationship was strongly influenced by U. pinnatifida being present on the coast adjacent to the Wilsons Bay Marine Farming Zone, which was the largest aquaculture area and typically had the highest abundance (Fig. S2).
Discussion
Seasonal variation in Undaria pinnatifida populations on mussel farms and coastal reefs
Undaria pinnatifida populations on mussel farms and at the coastal monitoring site exhibited seasonal variation that is typical for this species: maximal densities occurred in late spring and the lowest densities in late summer (e.g. Koh and Shin 1990). The absence of U. pinnatifida at coastal sites in summer–autumn is consistent with global populations where summer temperatures exceed 20 °C (James et al. 2015). However, U. pinnatifida on mussel farms was more prolific (Fig. 7), with a longer annual presence and reproductive capacity and larger average sporophyte size as compared to the monitored coastal population.
A number of factors may facilitate both the longer annual presence and larger size of U. pinnatifida on mussel farm structures. On mussel farms, sporophytes are suspended in the water column at shallow depths and subsequently subject to high light levels, with no tidal variation, and enhanced water flow. Greater water motion positively influences growth by replenishing in-water nutrients (Lobban and Wynne 1981; Peteiro and Freire 2011; Shibneva and Skriptsova 2012) and preventing the build-up of fine sediments that may inhibit spore attachment and recruitment (Fletcher and Farrell 1999) as well as restrict light and nutrient penetration (Floc’h et al. 1996; Fletcher and Farrell 1999; Curiel et al. 2001). Competition from other macroalgal species and grazing pressure is also likely to be lower on farms compared to coastal reefs. In its native range, U. pinnatifida is a pioneer species and comprises an ordinary part of a successive colonisation process at open sites such as on urchin barrens (Agatsuma et al. 1997). Competitive interactions with native macroalgae can reduce success at coastal sites (e.g. South et al. 2015). Undaria pinnatifida itself is also highly palatable to grazers (Peréz et al. 1981; Sanderson 1990; Sinner et al. 2000; Thornber et al. 2004; Irigoyen et al. 2011a). Large numbers of the herbivorous mollusc Lunella smaragdus, which are not present on mussel farms, were observed on U. pinnatifida plants at the coastal monitoring site, especially during summer as the plants were senescing. The influence of such grazing pressure can greatly reduce coastal U. pinnatifida populations at certain times of year and has been seen to affect U. pinnatifida colonisation at natural reef systems when it successfully colonises nearby floating structures (Peréz et al. 1981; Castric-Fey et al. 1993; Floc’h et al. 1991, 1996; Fletcher and Farrell 1999; Thornber et al. 2004; Irigoyen et al. 2011a). Mussel farms provide an analogous environment to floating structures used to farm U. pinnatifida (see Peteiro and Freire 2011), reiterating the fact that such structures provide an optimal environment for U. pinnatifida colonisation and growth.
Distribution and abundance of Undaria pinnatifida on mussel farms
Overall, the abundance of U. pinnatifida on mussel farms was not strongly related to any of the explanatory variables investigated. However, many of the heavily infested farms were at more exposed sites surveyed, in particular the large offshore farm (WBMFZ). As with the monitored population, this is related to the enhanced water motion at such sites; U. pinnatifida growth rates, plant size and overall biomass are often higher in plants exposed to greater wave action (Castric-Fey et al. 1999; Nanba et al. 2011; Peteiro and Freire 2011; Shibneva and Skriptsova 2012). Although no significant relationship was found between Secchi depth and overall abundance, U. pinnatifida was found at greater depths where water clarity was high (Secchi depth ~8 m) compared to more turbid sites where it was restricted to shallow water (<3 m). Based on these results, it is likely that U. pinnatifida will be more prevalent at aquaculture sites with high water clarity and greater water motion.
In addition to environmental factors, cultivation practices associated with different marine farming sites may also contribute to different infestation levels. Dense populations were found on mussel lines with larger mussels (80–120 mm), including an abandoned mussel farm (Fig. 7). Lines with mussels this size have been in the water for at least a year (Aquaculture New Zealand 2011), and these lines were often heavily infested with other invasive species (e.g. the tunicate Styela clava and green seaweed Codium fragile), native seaweeds (e.g. Ecklonia radiata, Sargassum ssp.) and encrusting organisms, indicating that little or no maintenance had been carried out during this timeframe. Mussel harvesting also directly removes U. pinnatifida from the mussel lines. Ensuring that mussels are harvested before they become “oversized” means the accompanying U. pinnatifida also has a reduced growing time, potentially resulting in a reduction in U. pinnatifida plants which reach reproductive maturity. This in turn may reduce the spore supply for subsequent generations of U. pinnatifida on the mussel farms as well as nearby reef sites. Aquaculture transfers amongst farms may also drive the spread and profusion of U. pinnatifida at some sites. For example, the transfer of seed mussels (15–60 mm shell length) which are already heavily infested with U. pinnatifida gametophytes or other small life stages can influence the resulting abundance of U. pinnatifida at receiving sites (Forrest and Blakemore 2006). Mussel farms at smaller aquaculture sites, with well-maintained infrastructure, generally had a lower abundance of U. pinnatifida present. Consequently, smaller farms that are well maintained may pose less of an invasion threat to the surrounding coast than large farms.
Distribution and presence of Undaria pinnatifida on the coast
Undaria pinnatifida was found on reefs inshore from mussel farms at a number of locations, although numbers of plants were relatively low compared to the numbers recorded on farms. It was slightly more likely to find U. pinnatifida inshore from farms positioned closer to shore. However, an exception to this was the plants found on the coast adjacent to the WBMFZ, which is located approximately two kilometres offshore. This demonstrates how mechanisms exist that enable U. pinnatifida to spread over relatively large distances. While U. pinnatifida does not naturally spread long distances via spore dispersal (Forrest et al. 2000; Grulois et al. 2011; Schiel and Thompson 2012), it can spread via drifting fragments or whole plants over scales of up to 10 km (Sanderson 1997). Reproductive fragments or whole plants can be detached from mussel farms naturally or during routine mussel farming processes; these can then be transported both inshore and alongshore via winds, waves and currents. Furthermore, U. pinnatifida was found growing on mussel farm rope washed inshore from mussel farms and on mussel shells and live mussels beneath mussel farms (Fig. 7). These observations demonstrate how mussel farms facilitate the spread and establishment of U. pinnatifida through both detached farm components and by providing additional hard substrata in areas which would otherwise consist of soft sediment unsuitable for U. pinnatifida colonisation (Floc’h et al. 1991; Hewitt et al. 2005; Merreta et al. 2012).
There did not appear to be a relationship between the density of plants growing on mussel farms and the likelihood of finding U. pinnatifida on the adjacent coast. However, in addition to spore supply and dispersal of reproductive plants or plant fragments, introduction of populations to coastal sites is also dependent on appropriate receiving habitats. Undaria pinnatifida is an opportunistic species, and our finding that the highest density populations were at sites devoid of native algal canopies is consistent with results of previous experiments with U. pinnatifida, which indicates that it has a low competitive ability amongst established macroalgal populations (Floc’h et al. 1996; Johnson et al. 2004; Edgar et al. 2004; Valentine and Johnson 2003, 2004; Farrell and Fletcher 2006; Thompson and Schiel 2012). Most of the inshore reefs examined in this study are dominated by thick canopies of native large brown seaweeds, and sea urchins are rare. This is typical of relatively sheltered reefs in north-eastern New Zealand that experience high turbidity (Shears and Babcock 2004), and it is unlikely that U. pinnatifida will outcompete native macroalgal species at these sites unless additional stressors reduce native algal assemblages. For example, some of the highest densities of U. pinnatifida were recorded in urchin barrens habitat at Katherine Bay, Great Barrier Island, where native macroalgae canopies have been removed by sea urchins (Fig. 7). This site is typical of more exposed coasts in north-eastern New Zealand where sea urchins can graze down kelp forests and form urchin barrens (Shears and Babcock 2004). Given that the extent and persistence of urchin barrens are increased by overfishing of sea urchin predators (Shears and Babcock 2002), this demonstrates how other human impacts may further facilitate the spread of an invasive species onto inshore reefs (Johnson et al. 2004).
While quantitative research on invasive species which foul aquaculture structures is scarce, our findings are consistent with other studies in demonstrating that aquaculture structures provide optimal conditions for many invasive species. Ascidians, such as solitary tunicates (Styela clava and Ciona intestinalis) and colonial tunicate species (Botrylloides violaceus, Botryllus schlosseri and Didemnum sp), have been shown to be particularly well suited to colonising marine farming structures and can greatly hinder farming operations (McKindsey et al. 2007; Lutz-Collins et al. 2009; Zhan et al. 2015). In the present study, additional invasive species, including Styela clava and Didemnum sp, were found on mussel farms and on nearby coastal reefs. Like U. pinnatifida, these species are easily spread by transfers of aquaculture equipment and stock and pose risks to the surrounding environment as they subsequently spread from mussel farms to natural areas (e.g. Lutz-Collins et al. 2009).
This work on U. pinnatifida has a number of important implications for invasive species management. Aquaculture provides vectors for invasive species introductions and physical structures with optimal growing conditions for many invasive species. Based on current practices, there is no doubt that growth in aquaculture industries and expansion of marine farms into new areas, including large offshore farms, will bring a suite of invasive species that will ultimately spread to natural coastal habitats, regardless of how far they are located from shore. As a regulated activity, the placement of aquaculture sites is one pathway for invasive species introduction which can be controlled. The development of aquaculture industry at sites currently free from invasive species must be carefully considered, integrating information about the wilderness, ecological, economic, social and cultural values of coastal marine areas. Keeping pristine high-value areas free from aquaculture activities could play a vital role towards protecting them from invasive species. Furthermore, stricter controls and monitoring of aquaculture transfers and practices, especially regarding the maintenance and cleaning of farm infrastructure and measures to limit the dispersal of detached farm materials (such as rope and buoys), would aid in reducing the role that aquaculture plays in the spread of invasive species to coastal ecosystems.
References
Agatsuma Y, Matsuyama K, Nakata A, Kawai T, Nishikawa N (1997) Marine algal succession on coralline flats after removal of sea urchins in Suttsu Bay on the Japan Sea coast of Hokkaido, Japan. Nippon Suisan Gakkaishi 63(5):672–680
Anderson M, Gorley R, Clarke K (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. Primer-E, Plymouth
Aquaculture New Zealand (2011) Greenshell™ mussels—farms. http://aquaculture.org.nz/products/greenshell-mussels/farms/. Accessed 14 June 2015
Brown MT, Lamare MD (1993) The distribution of U. pinnatifida (Harvey) Suringar within Timaru harbour New Zealand. Jpn J Phycol 42:63–70
Campbell ML, Hewitt CL (2013) Protecting high-value areas from introduced marine species. Manag Biol Invasions 4(3):171–189
Carlton JT, Ruiz GM (2005) Vector science and integrated vector management in bioinvasion ecology: conceptual frameworks. In: Mooney HA, Mack RN, McNeely JA, Neville LE, Schei PE, Waage JK (eds) Invasive alien species: a new synthesis. DC Island Press, Washington, pp 36–58
Castric-Fey A, Girard A, L’Hardy-Halos MT (1993) The distribution of Undaria pinnatifida (Phaeophyceae, Laminariales) on the coast of St Malo (Brittany, France). Bot Mar 36(4):351–358
Castric-Fey A, Beaupoil C, Bouchain J, Pradier E, L’Hardy-Halos MT (1999) The introduced alga Undaria pinnatifida (Laminariales, Alariaceae) in the rocky shore ecosystem of the St Malo area: growth rate and longevity of the sporophyte. Bot Mar 42:83–96
Curiel D, Guidetti P, Bellemo G, Scattolin M, Marzocchi M (2001) The introduced alga Undaria pinnatifida (Laminariales, Alariaceae) in the Lagoon of Venice. Hydrobiologica 477:209–219
Davidson AD, Campbell ML, Hewitt CL, Schaffelke B (2015) Assessing the impacts of nonindigenous marine macroalgae: an update of current knowledge. Bot Mar 58(2):55–79
Dean PR, Hurd CL (2007) Seasonal growth, erosion rates, and nitrogen and photosynthetic ecophysiology of Undaria pinnatifida (Heterokontophyta) in southern New Zealand. J Phycol 46:1138–1148
Dodgshun TJ, Taylor MD, Forrest BM (2007) Human-mediated pathways of spread for non-indigenous marine species in New Zealand. DOC research and development series 266, Science and technical publishing, Department of Conservation, Wellington
Edgar GJ, Barrett NS, Morton AJ, Samson CR (2004) Effects of algal canopy clearance on plant, fish and macroinvertebrate communities on eastern Tasmanian reefs. J Exp Mar Biol Ecol 312:67–87
Eno NC, Clark RA, Sanderson WG (1997) Non-native marine species in British waters: a review and directory. Joint Nature Conservation Committee, Peterborough
Farrell P, Fletcher RL (2006) An investigation of dispersal of the introduced brown alga Undaria pinnatifida (Harvey) Suringar and its competition with some species on the man-made structures of Torquay Marina (Devon, UK). J Exp Mar Biol Ecol 334(2):236–243
Fletcher RL, Farrell P (1999) Introduced brown algae in the northeast Atlantic, with particular respect to Undaria pinnatifida (Harvey) Suringar. Helgol Meeresunters 52:259–275
Floc’h JY, Pajot R, Wallentinus I (1991) The Japanese brown alga Undaria pinnatifida on the coast of France and its possible establishment in European waters. J Cons Int Explor Mediterr 47:379–390
Floc’h JY, Pajot R, Mouret V (1996) Undaria pinnatifida (Laminariales, Phaeophyta) 12 years after its introduction into the Atlantic Ocean. Hydrobiologia 326(327):217–222
Forrest BM, Blakemore KA (2006) Evaluation of treatments to reduce the spread of a marine plant pest with aquaculture transfers. Aquaculture 257(1):333–345
Forrest BM, Hopkins GA (2013) Population control to mitigate the spread of marine pests: insights from management of the Asian kelp Undaria pinnatifida and colonial ascidian Didemnum vexillum. Manag Biol Invasions 4(4):317–326
Forrest BM, Brown SN, Taylor MD, Hurd CL, Hay CH (2000) The role of dispersal mechanisms in the spread of U. pinnatifida (Laminariales, Phaeophyceae). Phycologia 39(6):547–553
Gorman RM, Bryan KR, Laing AK (2003) Wave hindcast for the New Zealand region: nearshore validation and coastal wave climate. NZ J Mar Freshw Res 37:567–588
Grulois D, Leveque L, Viard F (2011) Mosaic genetic structure and sustainable establishment of the invasive kelp Undaria Pinnatifida within a bay (Bay of St-Malo, Brittany). Cah Biol Mar 52:485–498
Hay CH (1990) The dispersal of sporophytes of U. pinnatifida by coastal shipping in New Zealand, and implications for further dispersal of U. pinnatifida in France. J Br Phycol Soc 25:301–313
Hay CH, Luckens PA (1987) The Asian kelp U. pinnatifida (Phaeophyta: Laminariales) found in New Zealand harbour. NZ J Bot 25:329–332
Hewitt CL, Campbell ML, McEnnulty F, Moore KM, Murfet NB, Robertson B et al (2005) Efficacy of physical removal of a marine pest: the introduced kelp Undaria pinnatifida in a Tasmanian marine reserve. Biol Invasions 7:251–263
Hunt L, Chadderton L, Stuart M, Cooper S, Carruthers M (2009) Results of an attempt to control and eradicate Undaria pinnatifida in Southland, New Zealand, April 1997–November 2004. Department of Conservation, Invercargill
Inglis GJ, Hayden BJ, Ross AH (2000) An overview of factors affecting the carrying capacity of coastal embayments for mussel culture. Report for Ministry for the Environment No. MFE00505, Wellington
Irigoyen AJ, Trobbiani G, Sgarlatta MP, Raffo MP (2011a) Effects of the alien algae Undaria pinnatifida (Phaeophyceae, Laminariales) on the diversity and abundance of benthic macrofauna in Gulfo Nuevo (Patagonia, Argentina): potential implications for local food webs. Biol Invasions 13:1521–1532
Irigoyen AJ, Eyras C, Parma AM (2011b) Alien algae Undaria pinnatifida causes habitat loss for rocky reef fishes in north Patagonia. Biol Invasions 13:17–24
James K, Middleton I, Middleton C, Shears NT (2014) Discovery of Undaria pinnatifida (Harvey) Suringar, 1873 in northern New Zealand indicates increased invasion threat in subtropical regions. BioInvasions Rec 3(1):21–24
James K, Kibele J, Shears NT (2015) Using satellite-derived sea surface temperature to predict the potential global range and phenology of the invasive kelp Undaria pinnatifida. Biol Invasions 17(12):3393–3408
Johnson CR, Valentine JP, Pederson HG (2004) A most unusual barrens: complex interactions between lobsters, sea urchins and algae facilitates spread of an exotic kelp in eastern Tasmania. In: Heinzeller T, Nebelsick JH (eds) Echinoderms: München. Balkema, Leiden, pp 213–220
Katsanevakis S, Zenetos A, Belchior C, Cardoso AC (2013) Invading European Seas: assessing pathways of introduction of marine aliens. Ocean Coast Manag 76:64–74
Koh CH, Shin HC (1990) Growth and size distribution of some large brown algae in Ohori, east coast of Korea. Hydrobiologica 204(205):225–231
Lobban CS, Wynne MJ (1981) The biology of seaweeds. Blackwell Scientific Publications, Oxford
Lutz-Collins V, Ramsay A, Quijon PA, Davidson J (2009) Invasive tunicates fouling mussel lines: evidence of their impact on native tunicates and other epifaunal invertebrates. Aquat Invasions 4(1):213–220
Mckindsey CW, Landry T, O’beirn FX, Davies IM (2007) Bivalve aquaculture and exotic species: a review of ecological considerations and management issues. J Shellfish Res 26(2):281–294
Meretta PE, Matula VC, Casas G (2012) Occurrence of the alien kelp Undaria pinnatifida (Laminariales, Phaeophyceae) in Mar del Plata, Argentina. BioInvasions Rec 1(1):59–63
Minchin D (2007) Aquaculture and transport in a changing environment: overlap and links in the spread of alien biota. Mar Pollut Bull 55(7):302–313
Minchin D, Nunn J (2014) The invasive brown alga Undaria pinnatifida (Harvey) Suringar, 1873 (Laminariales: Alariaceae), spreads northwards in Europe. BioInvasions Rec 3(2):57–63
Molnar JL, Gamboa RL, Revenga C, Spalding MD (2008) Assessing the global threat of invasive species to marine biodiversity. Front Ecol Envirn 6(9):485–492
Nanba N, Fujiwara T, Kuwano K, Ishikawa Y, Ogawa H, Kado R (2011) Effect of water flow velocity on growth and morphology of cultured Undaria pinnatifida sporophytes (Laminariales, Phaeophyceae) in Okirai Bay on the Sanriku coast, Northeast Japan. J Appl Phycol 23(6):1023–1030
Naylor RL, Williams SL, Strong DR (2001) Aquaculture—a gateway for exotic species. Science (Washington) 294(5547):1655–1656
Neill K, Heesch S, Nelson W (2008) Diseases, pathogens and parasites of U. pinnatifida (Technical Paper No: 2009/44). Minister of Agriculture and Fisheries—Biosecurity New Zealand, Wellington
Nyberg CD, Wallentinus I (2005) Can species traits be used to predict marine macroalgal introductions? Biol Invasions 7(2):265–279
Ojaveer H, Galil BS, Campbell ML, Carlton JT, Canning-Clode J, Cook EJ et al (2015) Classification of non-indigenous species based on their impacts: considerations for application in marine management. PLoS Biol 13(4):e1002130
Olenin S, Elliott M, Bysveen I, Culverhouse PF, Daunys D, Dubelaar GB, Vandekerkhove J (2011) Recommendations on methods for the detection and control of biological pollution in marine coastal waters. Mar Pollut Bull 62(12):2598–2604
Perez R, Lee JY, Juge C (1981) Observations sur la biologie de l’algue japonaise Undaria pinnatifida (Harvey) Suringar introduite accidentellement dans l’Etang de Thau. Sci Peche 325:1–12
Perez R, Durand P, Kaas R, Barbaroux O, Barbier V, Vinot C, Bourgeau-Causse M, Leclerq M, Moigne JY (1988) Undaria pinnatifida on the French coast. Cultivation method, biochemical composition of the sporophyte and the gametophyte. In: Staedler T, Mollion J, Verdus MC, Karamanos Y, Morvan H, Christiaen D (eds) Algal biotechnology. Elsevier, London, pp 315–328
Peteiro C, Freire O (2011) Effect of water motion on the cultivation of the commercial seaweed Undaria pinnatifida in a coastal bay of Galicia, Northwest Spain. Aquaculture 314:269–276
Raffo MP, Eyras MC, Iribarne OO (2009) The invasion of Undaria pinnatifida to a Macrocystis pyrifera kelp in Patagonia (Argentina, south-west Atlantic). J Mar Biol Assoc UK 89(8):1571–1580
Russell LK, Hepburn CD, Hurd CL, Stuart MD (2008) The expanding range of Undaria pinnatifida in southern New Zealand: distribution, dispersal mechanisms and the invasion of wave-exposed environments. Biol Invasions 10:103–115
Sanderson JC (1990) A preliminary survey of the distribution of the introduced macroalga, Undaria pinnatifida (Harvey) Suringar on the East Coast of Tasmania, Australia. Bot Mar 33:153–157
Sanderson JC (1997) Survey of Undaria pinnatifida in Tasmanian coastal waters, January, February 1997. Tasmanian Department of Marine Resources, Tasmania
Savini D, Occhipinti-Ambrogi A, Marchini A, Tricarico E, Gherardi F, Olenin S, Gollasch S (2010) The top 27 animal alien species introduced into Europe for aquaculture and related activities. J Appl Ichthyol 26(Suppl. 2):1–7
Schiel DR, Thompson GA (2012) Demography and population biology of the invasive kelp Undaria pinnatifida on shallow reefs in southern New Zealand. J Exp Mar Biol Ecol 434(435):25–33
Shears NT, Babcock RC (2002) Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia 132:131–142
Shears NT, Babcock RC (2004) Community composition and structure of shallow subtidal reefs in northeastern New Zealand. Department of Conservation, Wellington
Shears NT, Babcock RC, Salomon AK (2008) Context-dependent effects of fishing: variation in trophic cascades across environmental gradients. Ecol Appl 18(8):1860–1873
Shibneva SY, Skriptsova AV (2012) Morphological Variability of Undaria pinnatifida (Harvey) Suringar, 1873 (Phaeophyceae, Laminariales) in Peter the Great Bay, Sea of Japan. Russ J Mar Biol 38(5):381–391
Sinner J, Forrest B, Taylor M (2000) A strategy for managing the Asian kelp Undaria pinnatifida: Cawthron Report No. 578, Nelson
South PM, Lilley SA, Tait LW, Alestra T, Hickford MJH, Thomsen MS, Schiel DR (2015) Transient effects of an invasive kelp on the community structure and primary productivity of an intertidal assemblage. Mar Freshw Res. doi:10.1071/MF14211
Subasinghe R, Soto D, Jia J (2009) Global aquaculture and its role in sustainable development. Rev Aquac 1(1):2–9
Thompson GA, Schiel DR (2012) Resistance and facilitation by native algal communities in the invasion success of Undaria pinnatifida. Mar Ecol Prog Ser 468:95–105
Thornber CS, Kinlan BP, Graham MH, Stachowicz JJ (2004) Population ecology of the invasive kelp Undaria pinnatifida in California: environmental and biological controls on demography. Mar Ecol Prog Ser 268:69–80
Thresher RE, Kuris AM (2004) Options for managing invasive marine species. Biol Invasions 6(3):295–300
Valentine JP, Johnson CR (2003) Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. J Exp Mar Biol Ecol 295:63–90
Valentine JP, Johnson CR (2004) Establishment of the introduced kelp Undaria pinnatifida following dieback of the native macroalga Phyllospora comosa in Tasmania, Australia. Mar Freshw Res 55:223–230
Voisin M, Engel CR, Viard F, Castilla JC (2005) Differential shuffling of native genetic diversity across introduced regions in a brown alga: aquaculture vs. maritime traffic effects. Proc Nat Acad Sci USA 102(15):5432–5437
Williams SL, Davidson IC, Pasari JR, Ashton GV, Carlton JT, Crafton RE, Fontana RE, Grosholz ED, Miller AW, Ruiz GM, Zabin CJ (2013) Managing multiple vectors for marine invasions in an increasingly connected world. Bioscience 63(12):952–966
Zhan A, Briski E, Bock DG, Ghabooli S, MacIsaac HJ (2015) Ascidians as models for studying invasion success. Mar Biol 162(12):2449–2470
Acknowledgments
The authors thank everyone who helped with field surveys and monitoring: G. Braidwood, I. James, A. Berthelson, K. Rodgers, E. Ainley, P. Caiger, J. Stanley, R. Hughes, J. Walker, B. Doak, J. James, N. White, K. Kenyon, M. James and T. James. The authors also thank Waikato Regional Council and Auckland Council for supporting this research and the reviewers of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: E. Briski.
Reviewed by F. Weinberger and an undisclosed expert.
This article is part of the Topical Collection on Invasive Species.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
James, K., Shears, N.T. Proliferation of the invasive kelp Undaria pinnatifida at aquaculture sites promotes spread to coastal reefs. Mar Biol 163, 34 (2016). https://doi.org/10.1007/s00227-015-2811-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-015-2811-9