Abstract
There is a general concern that jellyfish populations are increasing throughout marine ecosystems worldwide, mainly due to environmental (e.g., climate drivers) and anthropogenic forces (e.g., overfishing and eutrophication), or interactions among them. To identify drivers of jellyfish populations in the heavily fished northern Humboldt upwelling system (NHUS), we examined linkages between a 43-year-long annual time series (1972–2014) of the biomass of the scyphomedusae Chrysaora plocamia and several forcing factors: the Peruvian Oscillation Index, the Regime Indicator Series and commercial landings of Peruvian anchovy. We found that C. plocamia biomass fluctuated with climate drivers, but not with anchovy landings (a proxy of fishing pressure). Jellyfish biomass was high and variable during the warm El Viejo regime in the 1970s and 1980s, with peaks connected to intra-regime El Niño Southern Oscillation (ENSO) events. By contrast, no peaks occurred during warming events in the cold La Vieja regime in the late 1990s and 2000s when jellyfish biomass was very low or below detection; however, at the end of the study period, biomass rose slightly. The fishing pattern in the NHUS is just the opposite of those that previously have been attributed to removing small pelagic fish. We suggest that environmental factors and prey availability act synergistically to generate observed population size variability of this medusa in the NHUS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jellyfish (medusae and ctenophores) abundance fluctuations have been recorded in different marine ecosystems worldwide (Purcell 2005, 2012; Condon et al. 2013), giving a general impression that jellyfish blooms are becoming larger and more frequent in the global ocean. Jellyfish population sizes typically show dramatic inter-annual variability, but whether jellyfish populations have increased worldwide is less clear (Condon et al. 2012; Purcell 2012). A recent global effort suggests that populations exhibit worldwide oscillations over decadal time scales with a slight, rising trend in abundance since the 1970s (Condon et al. 2013). Variability in jellyfish population size and bloom frequency has been attributed to environmental and anthropogenic factors such as climate change, overfishing, ocean sprawl, eutrophication, biological invasions and hypoxia, as well as synergistic interactions among them (Purcell et al. 2007; Duarte et al. 2012; Miller and Graham 2012; Purcell 2012; Bayha and Graham 2014; Decker et al. 2014; Uye 2014). Climate forcing can affect ecosystem structure promoting shifts in temperature, circulation, stratification, nutrient input and oxygen contents among others (Doney et al. 2012) and, when operating along ocean basins, have been correlated with jellyfish abundance in several northern Atlantic, Pacific and Mediterranean ecosystems (Goy et al. 1989; Cargo and King 1990; Lynam et al. 2005, 2010; Purcell 2005; Purcell and Decker 2005; Brodeur et al. 2008; Molinero et al. 2009; Kogovšek et al. 2010; Robinson and Graham 2013). Climate forcing has been shown to affect ctenophore growth and reproduction (Jaspers et al. 2011; Robinson and Graham 2014) and early life-cycle processes, such as strobilation in scyphozoan medusae (Purcell et al. 1999b; Purcell 2007), through their effects of local temperature, salinity and plankton production cycles (Stenseth and Mysterud 2002).
Fisheries harvests have also been proposed to drive variability of jellyfish abundance in heavily fished ecosystems (e.g., Bakun and Weeks 2006; Richardson et al. 2009). Overfishing can produce modifications of marine ecosystems by altering biodiversity and taxa abundance across multiple trophic levels (Cury et al. 2000; Reid et al. 2000; Worm et al. 2006), sometimes leading to a process known as “fishing down the marine food web” where fisheries sequentially exploit resources at ever-decreasing trophic levels (Pauly et al. 1998). Overfishing of small pelagic planktivorous fish has been hypothesized to enhance jellyfish production by releasing zooplanktivorous jellyfish from competition for shared prey resources (Parsons and Lalli 2002; Lynam et al. 2011; Robinson et al. 2014). In some heavily fished ecosystems, the massive removal of small pelagic fishes has been linked with major jellyfish outbreaks (Purcell et al. 1999a; Bakun and Weeks 2006; Daskalov et al. 2007; Roux et al. 2013). However, evidence supporting this hypothesis is not conclusive as the paucity of long-term (>20 year), quantitative jellyfish time series, particularly in the Southern Hemisphere, has hampered the ability to identify the roles climate, and harvesting of small pelagic fish plays in regulating jellyfish bloom frequency and size in some of the most intensely fished ecosystems in the world (Brotz et al. 2012; Condon et al. 2012).
One of these ecosystems is the northern Humboldt upwelling system (NHUS) off Peru. As one of the most productive ecosystems in the world ocean, it supports one of the largest single-species fisheries (Peruvian anchovy, Engraulis ringens) (Chavez et al. 1999, 2008; Pennington et al. 2006), as well as populations of the large scyphomedusa C. plocamia (in Quiñones 2008; Quiñones et al. 2010). Biological productivity in NHUS varies on inter-annual and inter-decadal time scales, with fluctuations primarily driven by the El Niño Southern Oscillation (ENSO) and the Pacific Decadal Oscillation (PDO) (Bakun 1998; Chavez et al. 2003, 2008). Fluctuations in C. plocamia abundance over time have recently been described by Mianzan et al. (2014) who suggests that peaks occur during ENSO events within the “El Viejo” warm regime. To test the hypothesis that climate forces and fisheries harvest drive variations in C. plocamia biomass in the NHUS, we examined the relationship between jellyfish biomass, two climate indexes, and commercial landings of the forage fish, E. ringens, over the past four decades.
Materials and methods
Jellyfish time series
A 43-year time series (1972–2014) of jellyfish biomass (kg) was constructed from bycatch data taken during purse seine (N = 20) and pelagic trawl (N = 70) surveys conducted by the Instituto del Mar del Perú (IMARPE). Surveys targeted the Peruvian anchovy (Engraulis ringens) and Pacific sardine (Sardinops sagax). No cruises were performed in 1989. In all other years, cruises were performed two or three times per year, mostly during austral spring and summer. The area surveyed was along the Peruvian coast, between the borders of Ecuador (03°23′S, 80°18′W) and Chile (18°21′S, 70°22′W) and up to 100 nautical miles offshore. Only cruises conducted between September and May were used to construct the jellyfish time series because the occurrence of jellyfish starts in the austral spring and medusae are observed through late autumn (Quiñones 2008).
Trawl and purse seine nets were towed through the upper 100 m of the water column. Both gear types were operated during day and night. Chrysaora plocamia are pelagic animals that occur in the near surface layers (Mianzan et al. 2014); therefore, data from pelagic nets (both trawling and purse seines) were considered to provide representative estimates of jellyfish abundance. A total of 12,091 hauls were analyzed (Fig. 1), resulting in an average effort of 295 hauls per year. The average bell diameter of C. plocamia individuals is 50 cm (Quiñones 2008); thus, they are large enough to be retained in trawl and purse seine nets with mesh sizes of 13 mm. Jellyfish catches were weighed on board and catches <1 kg were considered as zeros.
Jellyfish biomass was standardized to the volume filtered by the net (kg 1000 m−3). Volume filtered (m3) was estimated separately for pelagic trawl and purse seine nets. For pelagic trawl nets, the mean net open area (m2), estimated by Eq. 1, was multiplied by the distance trawled (m). The width of pelagic trawl net openings ranged from 12 to 20 m, and the height of net openings ranged from 9 to 15 m. Distance trawled was estimated using the initial and final latitude and longitude coordinates of the trawl.
For purse seine nets, volume filtered was estimated, assuming that the net was a cylinder (Eq. 2). The radius (r) of the net was derived from net perimeter (m) (Eq. 3). Purse seine net perimeters ranged from 520 to 700 m, and net heights ranged from 84 to 100 m.
Jellyfish were not identified to the species level during the 1970s and 1980s surveys. To quantify jellyfish biomass for these decades, ten scientists participating as research chiefs on those cruises were interviewed. Interviews included questions about species identity using photographs of the most common jellyfish bycatch taxa in the area and the animal sizes. Ninety percent of jellyfish identified were C. plocamia.
Climate forcing
The Oceanic Niño Index (ONI) is estimated on the basis of sea surface temperature (SST) anomalies in the El Niño 3.4 region (5°–5°S, 170°–140°W), and it is commonly employed to define El Niño and La Niña episodes (Smith et al. 2008). However, the ONI does not necessarily reflect local warming events (WEs) occurring in the Peruvian coast. We employed yearly estimations of the Peruvian Oscillation Index (POI) to describe environmental variability at inter-annual scales (Montecinos et al. 2003). The POI represents the coastal SST variations in Peru and is positively correlated with the ONI and the PDO and thus reflects conditions in the eastern tropical Pacific (Montecinos et al. 2003). Because jellyfish are present from September to May, we averaged POI values along a “jellyfish biological year.” For example, the year 2012 covers from September 2011 to May 2012. The phases of ENSO episodes were taken according to the ONI (onset = −0.5, peak = 0 and offset = + 0.5). An ENSO episode is different from a WEs because an ENSO episode originates in the Central Pacific, while a WEs event could originate in the East or West Pacific, and its effect is local. WEs cannot be detected in the Central Pacific, but they have important ecological consequences in the Peruvian coast (Zuta et al. 1976).
The Regime Indicator Series (RIS3) was employed to describe environmental variability at inter-decadal scales (Chavez et al. 2003; Kamykowski 2012). This series was constructed according to alternating fluctuations in sardine and anchovy populations over their ranges in Japan, California, Peru and South Africa. RIS3 data cover the period 1972–2009 (Kamykowski 2012).
Fishing pressure
Fishing intensity was estimated using annual landings (106 tons per year; mt yr−1; FAO 2013) and the annual number of fishing trips (IMARPE) made by the industrial anchovy fishery for the years 1972 to 2012. This fleet exerts the highest fishing effort in NHUS, and its activity overlaps in space and time with C. plocamia occurrence. Landings of other small pelagic species such as sardines, jack and chub mackerels were not included since their distributions do not overlap with C. plocamia (Valdivia, pers. comm.).
Data Analysis
To explore the effects of POI, RIS3 and anchovy fisheries on jellyfish biomass, a generalized additive model (GAM; Hastie and Tibshirani 1986) approach was implemented. Data were included from 1972 to 2009, since time series data were all available in this time frame. GAMs robustly describe nonlinear correlations that may exist between fisheries and the environment (Brander 1994; Bigelow et al. 1999; Venables and Dichmont 2004) and have previously been used to model jellyfish abundance in relation to climate (Brodeur et al. 2008; Eriksen et al. 2012; Decker et al. 2013).
GAMs are relatively robust to the effects of autocorrelation (Segurado et al. 2006). However, to ensure the validity of model assumptions the presence of autocorrelation in the jellyfish time series was tested using a Durbin–Watson test. First-order temporal autocorrelation was not present (DW = 1.767, p = 0.185), and thus, we did not build models that account for autocorrelation.
The underlying probability distribution employed was selected using Akaike information criterion (AIC) following Burnham and Anderson (2002) and applied by Damalas et al. (2007). The probability distribution from the model with the highest Akaike weight was used. A constant of 1 was added to the response variable (Jellyfish Biomass) to allow transformation of the data for logarithmic models (Lo et al. 1992; Mitchell et al. 2014), and the “gamma” parameter of the GAM function was set at 1.4 following Wood (2006) to reduce overfitting. The full model examined thus takes the following form:
We employed cubic regression splines with an added shrinkage component (Wood 2006; Decker et al. 2013; Photopoulou et al. 2014) to represent the possible nonlinear effects of the predictors because of their improved stability (Venables and Dichmont 2004). Smoothness parameters were estimated by generalized cross-validation (GCV), similar to previous work (Brodeur et al. 2008; Eriksen et al. 2012; Decker et al. 2013). Given the size of the data set, the number of smoother knots (k) was limited to 4 to avoid overfitting (Brodeur et al. 2008; Decker et al. 2013). Covariates were deemed statistically insignificant at p value > 0.05.
The “mgcv” package in the free statistical software R (R Development Core Team, version 3.1.1, 2014, USA) was used for statistical analysis.
An additional comparative analysis between the strong ENSO events of 1982–1983 and 1997–1998 was carried out to test the jellyfish response during strong ENSO events within a warm and a cold regime, respectively.
Results
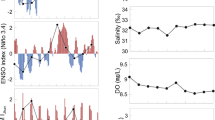
The C. plocamia time series show two periods of high abundance (Fig. 2a). During the first period (1972–1989), jellyfish show several strong oscillations ranging from zero to mean annual values higher than 12 kg 1000 m−3. A dramatic decrease took place at the beginning of the second period (ca. 1989), and jellyfishes were extremely scarce or absent for the next 20 years, though a slight abundance recovery happened at the end of the series (2009–2014). This two-stage pattern matches inter-decadal warm–cold climate fluctuations known as El Viejo and La Vieja regimes (Fig. 2d), respectively. Both regimes are clearly seen within the RIS3, which show a positive El Viejo regime in the early to mid-1970s until early 1990s and then a negative La Vieja regime between 1990s and 2000s. The same two-stage pattern is shown in anchovy landings and fishing trips (Fig. 2b), with the lesser values during the warm El Viejo regime (1970s–1980s) characterized by positive values of the RIS3 and an increase in anchovy landings during the cold La Vieja regime (1990s–2000s) characterized by negative values of the RIS3.
Time series of jellyfish (Chrysaora plocamia) abundances for the period 1972–2013 expressed as kg jellyfish 1000 m−3; abundances were log-transformed (a); Anchovy (Engraulis ringens) landings expressed in million tons, and number of fishing trips (1972–2011) (b); Peruvian Oscillation Index (POI) time series (1972–2012) (c) and Regime Indicator Series (RIS3) (1972–2009) (d)
Jellyfish biomass peaked during the strong ENSO events of 1982–1983 and 1986–1987 and warm event of 1976 during the El Viejo regime, with mean annual values of positive stations (i.e., not considering zeros) reached 5.7, 12.4 and 5.6 kg 1000 m−3, respectively. Five ENSO events can be detected in the POI series (Fig. 2c): two strong events in 1982–1983 (POI = +13.2) and 1997–1998 (POI = +10.8), and three moderate ones in 1972 (POI = +2.5), 1987 (POI = +3.4) and 1991–1993 (POI = +4.9). In addition, four WEs occurred in 1976 (POI = +2.0), 1979 (POI = +0.4), 1993 (POI = +1.1) and 2012 (POI = +0.1). Although the yearly POI value for 1976 (a year with high jellyfish abundance) was not extreme, anomalies during some months were very high (+2.6 June; +3.1 July; +3.3 August). No peaks in jellyfish biomass occurred during the ENSO events nor WEs throughout La Vieja regime (e.g., 1991–1992 or 1997–1998). At the end of the time series (2009–2014), a slight recovery of C. plocamia was noticed (Fig. 2a).
GAM modeling
We chose the gamma distribution with the inverse link function after comparing Akaike weights (wi) and evidence ratios (ER) of all candidate models (Table 2). This distribution has previously been selected to model the reproductive biology of fisheries based on the reduction in the generalized cross-validation value (GCV) (Flores et al. 2015). GAM modeling indicated anchovy fishery landings were not a significant predictor of jellyfish biomass (p value > 0.05) and were excluded from further analysis (Table 3). RIS3 and POI were both significant predictors of jellyfish biomass between 1972 and 2009 (p value < 0.05). Following diagnostics (Wood 2006; Figure S1), the model was a relatively good fit and no over or under-smoothing was detected using the “gam.check” function following the “mgcv” package literature (Pointin and Payne 2014).
GAMs were plotted and visually analyzed for trends (Fig. 3). To maintain identifiability in the model, smoothing functions were averaged to 0 (Wood and Augustin 2002). As the inverse link function interacts with the predictors (x1,…xk) such that g(µi) = 1/µi, where µ(x) is the mean of the gamma distribution, GAM smoothing of explanatory variables indicates that jellyfish biomass increases as RIS3 and POI increase and, in the case of POI, plateaus after the POI reach a value of 5. The RIS3 and POI indices are scaled such that positive (e.g., warm) and negative (e.g., cool) climate anomalies are above and below 0, respectively. Thus, jellyfish biomass increased during warming inter-decadal and inter-annual climate events (e.g., El Viejo and El Niño, respectively). These outcomes suggest that jellyfish biomass is positively correlated with the climatic variables included in the model.
Discussion
Jellyfish–climate relationships
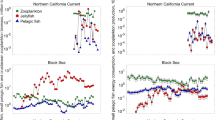
A suite of environmental conditions (e.g., SST, salinity, currents and vertical mixing) and atmospheric variability (e.g., wind patterns) may benefit jellyfish in certain circumstances (Purcell 2005; Purcell et al. 2007; Brodeur et al. 2008; Gibbons and Richardson 2009). These include Cyanaea capillata and Aurelia aurita in the Barents Sea and in the northwest region of the North and the Irish Seas (Lynam et al. 2005, 2011; Eriksen et al. 2012). As seen here in the NHUS, scyphomedusae abundances in several ecosystems were greater during warmer years. However, it is not always true that jellyfish outbreaks occur during warm years. In the Northern California Current, Chrysaora fuscescens and Aequorea sp. abundances were positively correlated with cool boreal spring–summer conditions or negative anomalies of the Pacific Decadal Oscillation (Lenarz et al. 1995; Purcell 2012; Suchman et al. 2012). During the cold La Vieja regime, jellyfish abundance was very low or below detection despite the occurrence of two ENSO events (1991–1993 and 1997–1998; Fig. 2), showing that the biophysical conditions associated with ENSO events are not adequate to trigger jellyfish biomass increase. The distribution of C. plocamia (encompassing the Pacific and Atlantic coasts of southern South America) shows that this species prefers temperate rather than subtropical or tropical waters (Mianzan et al. 2014); consequently, other factors besides temperature should be involved in producing the population size fluctuations. The information regarding the effects of environmental variability on the asexual reproduction of jellies is scarce; recent work suggests that some jellies develops multiple cohorts throughout the medusa season, suggesting the continuous production of ephyrae from polyps (Ceh et al. 2015). Favorable environmental conditions can lead to increased strobilation in many jellyfish species (Purcell 2007; Boero et al. 2008; Purcell et al. 2009; Holst 2012), leading to greater abundances of medusae. Favorable conditions driven by inter-decadal and inter-annual climate regimes most likely trigger increased production of medusae, and thus, greater biomass of C. plocamia was observed earlier in our time series.
At broader or decadal stages in the southeast Pacific Ocean, climate and productivity of coastal and open ocean ecosystems have varied over periods of about 50 years. In the mid-1970s, the Pacific changed from a cool “anchovy regime” (El Viejo) to a warm “sardine regime” (La Vieja). A return back to an “anchovy regime” occurred in the middle to late 1990s. The NHUS is the region where ENSO, and climate variability in general, is most notable (Chavez et al. 2003, 2008). Natural environmental rhythms affect several biological components of this ecosystem such as phytoplankton, zooplankton and small pelagic fish (Alheit and Niquen 2004; Ayón et al. 2008). High jellyfish abundances occurred within the El Viejo regime (warm and “sardine dominated”) since 1975 until early to mid-1990s, and the low abundances or absence of jellyfish during the La Vieja regime (cool and “anchovy dominated”) that began in the early 1990s. However, the temporal pattern of jellyfishes shows also higher frequency fluctuations during the El Viejo regime. At the end of the time series, jellyfish slightly recovers, together with the return back of sardine in artisanal fisheries of Peru.
Jellyfish populations are typically regulated by bottom-up processes (West et al. 2009; Purcell 2012) since predation on jellyfish tends to be opportunistic rather than obligatory (Purcell and Arai 2001; Arai 2005). Time series of C. plocamia abundances show that they peaked only during ENSO or warm events that occurred throughout El Viejo regime. A comparison between strong ENSO events occurring within El Viejo (ENSO 1982–83) and La Vieja (ENSO 1997–98) illustrates that both had a similar physical input (McPhaden et al. 2011) including oxygen minimum zone (OMZ), depth (Gutierrez et al. 2011) and phytoplankton composition (Ochoa et al. 1985; Sánchez et al. 2000). However, the zooplankton assemblage was dominated by different groups (Table 1). During the 1982–1983 ENSO event, small gelatinous zooplankton such as doliolids, siphonophores, hydromedusae and larvaceans dominate (Santander and Carrasco 1985; Carrasco and Santander 1987). In contrast, during 1997–1998 ENSO event, the zooplankton assemblage was dominated by copepods; small gelatinous zooplankton were present but only at reduced densities (Bonicelli 2008). It is known that other species of the genus Chrysaora may prefer gelatinous prey (Purcell 1991), C. quinchecirrha in the Chesapeake Bay is a large consumer of the ctenophore Mnemiopsis leidyi (Purcell and Decker 2005). C. fuscescens in the north Pacific (Suchman et al. 2008) and C. melanaster in the Bering Sea (Brodeur et al. 2002) consume euphausiid eggs and gelatinous zooplankton, respectively. In the NHUS, the contrast in prey fields could be the reason, at least in part, for differences in jellyfish abundances between El Viejo and La Vieja regimes (Tables 2, 3).
Since C. plocamia has a metagenetic life cycle (i.e., with benthic polyps), conditions at the sea floor need to be examined. During the 1982–1983 ENSO event, densities of benthic organisms were six times greater than during the 1997–1998 ENSO event (Tarazona et al. 1985). Favorable conditions for the benthos could have enhanced polyp abundance and ephyra production, overwhelming the effects of increased competition for space and food; indeed, there is existing evidence from other Chrysaora species showing increased asexual reproduction in warm waters (Purcell et al. 1999b; Holst 2012).
The abundance variability of jellyfish with a metagenetic life cycle cannot be fully understood if the scyphistomae ecology is not taken into consideration (Prieto et al. 2010; Lucas et al. 2012). However, the finding and study of scyphistomae in their natural environment are extremely difficult (Lucas et al. 2012). Similarly, little information is available for C.plocamia (Mianzan et al. 2014), and their polyps are only known from laboratory experiments (Riascos et al. 2013). Nevertheless, it is possible that at least part of the observed variability is related to the ecology of the benthic stage, especially considering the strong impact of the regime shifts on the benthos of the NHUS.
Fishing
Fishing pressure seems not to have the same consequences for NHUS jellyfish as those in other, heavily fished ecosystems (Fig. 2), as evidenced by GAM modeling. NHUS industrial fishery landings and annual trips were increased by a factor of three in the last two decades (1990s and 2000s), a period of almost total absence of jellyfish. Conversely, the period of the highest jellyfish abundance in the 1970s and 1980s was characterized by relatively low fishing pressure. These findings contrast links made between jellyfish proliferation and an increase in fishing pressure in other regions. Large-scale perturbations in the food web caused by fishing are suggested to increase jellyfish population growth through the reduction in competitors and top predators (Richardson et al. 2009). In the Benguela Current, it was suggested that jellyfish overtook small pelagic fish as a consequence of overfishing (Lynam et al. 2006; Roux et al. 2013). Dominant pelagic fishes like the sardine (Sardinops sagax) were replaced by the bearded goby (Sufflogobius bibarbatus) and jellyfish (Chrysaora fulgida and Aequorea forskalea) (Flynn et al. 2012). The increase in the jellyfish Aurelia aurita, Cyanea nozakii and Nemopilema nomurai in Chinese coastal waters has been attributed to overfishing, among other factors (Dong et al. 2010). Outbreaks of Aurelia aurita and Cyanea capillata in the North Sea occurred following the decline of herring stocks (Lynam et al. 2005). Trophic cascades and jellyfish outbreaks in the Black Sea have been reported as a consequence of overfishing (Daskalov and Mamedov 2007). Thus, while increases in jellyfish abundance may result from overfishing in some ecosystems (Bakun and Weeks 2006; Lynam et al. 2006; Richardson et al. 2009; Utne-Palm et al. 2010; Jensen et al. 2012), it does not appear to be the case in the NHUS, where consistent with previous research; the importance of climatic and oceanographic variation on jellyfish abundance is demonstrated in the Irish Sea (Lynam et al. 2011), Bering Sea (Decker et al. 2013) and Hawaii (Chiaverano et al. 2013), and our results suggest that climate regime, rather than fishing, driven shifts in food web structure may be a greater driver of jellyfish abundance in the NHUS.
Prey availability
Environmentally driven changes in the in the NHUS pelagic food web have been hypothesized to underlie the observed shifts in regime indicator species such as sardines and anchovies (Ayón et al. 2011). During warm episodes or regimes (e.g., ENSO and El Viejo), dinoflagellates, heterotrophic flagellates and small zooplankton dominate (Ochoa et al. 2010; Ayón et al. 2011) as a consequence of reduced nutrient inputs to the ecosystem (Bertrand et al. 2004). Conversely, the high productivity of ENSO neutral years or the cold regime promotes the dominance of large phytoplankton like diatoms and zooplankton such as euphausiids (Ochoa et al. 2010; Ayón et al. 2011). Information on gelatinous taxa in the NHUS is scarce during either regime. One study indicates that small gelatinous taxa (i.e., siphonophores, tunicates and ctenophores), which would be presumably prey for C. plocamia, were present in the NHUS during the La Vieja ENSO event of 1997 (Pagés et al. 2001). Currently, there are no diet studies of C. plocamia during an ENSO year within a warm El Viejo regime; however, during those periods pelagic gelatinous organisms and benthic macro benthos were both abundant (Table 1) and could act synergistically to trigger the jellyfish blooms due to the increase in prey availability for polyps and medusa.
Drivers responsible for jellyfish population peaks probably involve increased productivity at time scales relevant to their annual production cycles (Condon et al. 2013). Numerous jellyfish aggregations are possible a consequence of prey availability (Purcell 2003); indeed, jellyfish prey on a broad range of zooplankton, including copepods, other gelatinous taxa, meroplankton and fish eggs (Suchman et al. 2008). A recent study shows that the eggs of the Peruvian anchovy (E. ringens) and copepods constituted nearly 90 % of the diet of C. plocamia in northern Chile (Riascos et al. 2014); however, this study was performed in a cold period within a La Vieja regime (November 2010–March 2011). Consequently, these results are not representative of C. plocamia diet during peak abundance in warm El Viejo regimes.
Because fishing effects are different in the NHUS than other ecosystems, where reduction in piscivorous fish by overfishing results in a jellyfish increase (Parsons and Lalli 2002; Purcell 2012), in the NHUS other factors could probably play a role, trophic interactions between scyphomedusae and small jellies could probably explain better this situation. Trophic cascades throughout the plankton food web have been exemplified in Chesapeake Bay where Chrysaora quinquecirrha reduces ctenophores and results in more zooplankton (Purcell and Decker 2005); unfortunately, no time series of small jellies are available in the NHUS, despite the fact that we have seen several small jellies species in the gut content analysis of C. plocamia (Quiñones unpublished data); the sample size was really small and was performed only in a short period of time, and consequently, this trophic interactions remains unknown in the area; however, we cannot discard the potential importance of the trophic interactions in the jellyfish variability in the NHUS.
Conclusion
The population fluctuations of C. plocamia in the NHUS were related to ENSO and warm events that occurred during El Viejo warm regime, but not to commercial landings of anchovy. Water temperature per se does not seem enough to explain fluctuations because during strong ENSO events within the La Vieja regime, jellyfish were scarce or absent. Moreover, judging from its geographical distribution, C. plocamia seems to prefer temperate rather than warmer, subtropical or tropical waters. The NHUS is one of the ecosystems most heavily fished in the world, with pelagic fisheries targeting forage fishes. Despite fishing intensity, low C. plocamia biomass when fishing effort was high allows us to reject the hypothesis that harvests of planktivorous fishes in the NHUS enhance C. plocamia abundance. We propose an alternative hypothesis where climate-driven environmental factors and food availability act synergistically to produce the observed variability in jellyfish population size in the NHUS. Peaks in large jellyfish abundance during the El Viejo regime could be explained by the dominance of small gelatinous plankton (supposedly their preferred food), favorable conditions for their benthic polyps and a more prolonged period of warm water temperatures during ENSO events, giving more time for planula settlement and polyp growth and development. Finally, the rising trend of jellyfish abundance at the end of the series (2009–2014) could be indicative of a coming regime shift to El Viejo warm regime.
References
Alheit J, Niquen M (2004) Regime shifts in the Humboldt Current ecosystem. Prog Oceanogr 60:201–222. doi:10.1016/j.pocean.2004.02.006
Arai MN (2005) Predation on pelagic coelenterates: a review. J Mar Biol Assoc UK 85:523–536. doi:10.1017/S0025315405011458
Ayón P, Swartzman G, Bertrand A, Gutiérrez M, Bertrand S (2008) Zooplankton and forage fish species off Peru: large-scale bottom-up forcing and local-scale depletion. Prog Oceanogr 79:208–214. doi:10.1016/j.pocean.2008.10.023
Ayón P, Swartzman G, Espinoza P, Bertran A (2011) Long-term changes in zooplankton size distribution in the Peruvian Humbodt Current System: conditions favoring sardine or anchovy. Mar Ecol Prog Ser 422:211–222. doi:10.3354/meps08918
Bakun A (1998) Ocean triads and radical interdecadal variation: bane and boon to scientific fisheries management. In: Pitcher T, Haert PJ, Paula D (eds) Reinventing fisheries management. Springer, Dordrecht, pp 331–358. doi:10.1007/978-94-011-4433-9_25
Bakun A, Weeks SJ (2006) Adverse feedback sequences in exploited marine systems: are deliberate interruptive actions warranted? Fish Fish 7:316–333. doi:10.1111/j.1467-2979.2006.00229.x
Bayha MK, Graham WM (2014) Nonindigenous marine jellyfish: invasiveness, invasibility and impacts. In: Pitt KA, Lucas CH (eds) Jellyfish blooms. Springer, Dordrecht, pp 45–77. doi:10.1007/978-94-007-7015-7_1
Bertrand A, Segura M, Gutiérrez M, Vásquez L (2004) From small-scale habitat loopholes to decadal cycles: a habitat-based hypothesis explaining fluctuation in pelagic fish populations off Peru. Fish Fish 5:296–316. doi:10.1111/j.1467-2679.2004.00165.x
Bigelow KA, Boggs CH, He X (1999) Environmental effects on swordfish and blue shark catch rates in the US North Pacific longline fishery. Fish Oceanogr 8:178–198. doi:10.1046/j.1365-2419.1999.00105.x
Boero F, Bouillon J, Gravili C, Miglietta MP, Parsons T, Piraino S (2008) Gelatinous plankton: irregularities rule the world (sometimes). Mar Ecol Prog Ser 356:299–310. doi:10.3354/meps07368
Bonicelli J (2008) Distribución espacial, composición específica y abundancia del zooplancton en la costa peruana durante los años 1996 y 1998. Thesis dissertation, Universidad Nacional Agraria La Molina, Lima. Perú
Brander KM (1994) Patterns of distribution, spawning, and growth in north Atlantic cod: the utility of inter-regional comparisons. ICES Mar Sci Symp 198:406–413
Brodeur R, Sugisaki DH, Hunt GL Jr (2002) Increases in jellyfish biomass in the Bering Sea: implications for the ecosystem. Mar Ecol Prog Ser 233:89–103
Brodeur RD, Decker MB, Ciannelli L, Purcell JE, Bond NA, Stabeno PJ, Acuna E, Hunt GL Jr (2008) The rise and fall of jellyfish in the Bering Sea in relation to climate regime shifts. Prog Oceanogr 77:103–111. doi:10.1016/j.pocean.2008.03.017
Brotz L, Cheung WWL, Kleisner K, Pakhomov E, Pauly D (2012) Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia 690:3–20. doi:10.1007/s10750-012-1039-7
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information theoretic approach, 2nd edn. Springer, New York
Cargo DG, King DR (1990) Forecasting the abundance of the sea nettle, Chrysaora quinquecirrha, in the Chesapeake Bay. Estuaries 13:486–491. doi:10.2307/1351793
Carrasco S, Santander H (1987) The El Niño event and its influence on the zooplankton off Peru. J Geophys Res 92:14405–14410. doi:10.1029/JC092iC13p14405
Ceh J, Gonzalez JE, Pacheco AS, Riascos JM (2015) The elusive life cycle of scyphozoan jellyfish: metagenesis revisited. Sci Rep 5:12037. doi:10.1038/srep12037
Chavez FP, Strutton PG, Friederich GE, Feely RA, Feldman GC, Foley DG, McPhaden MJ (1999) Biological and chemical response of the Equatorial Pacific Ocean to the 1997–98 El Niño. Science 286:2126–2131. doi:10.1126/science.286.5447.2126
Chavez FP, Ryan J, Lluch-Cota SE, Niquen M (2003) From anchovies to sardines and back: multidecadal change in the Pacific Ocean. Science 299:217–221. doi:10.1126/science.1075880
Chavez FP, Bertrand A, Guevara-Carrasco R, Soler P, Csirke J (2008) The northern Humboldt current system: brief history, present status and a view towards the future. Prog Oceanogr 79:95–105. doi:10.1016/j.pocean.2008.10.012
Chiaverano LM, Holland BS, Crow GL, Blair L, Yanagihara AA (2013) Long-term fluctuations in circalunar beach aggregations of the box jellyfish Alatina moseri in Hawaii, with links to environmental variability. PLoS One. doi:10.1371/journal.pone.0077039
Condon RH, Graham WM, Duarte CM, Pitt KA, Lucas CH, Haddock SH, Madin LP (2012) Questioning the rise of gelatinous zooplankton in the world’s oceans. Bioscience 62:160–169. doi:10.1525/bio.2012.62.2.9
Condon RH, Duarte CM, Pitt KA, Robinson KL, Lucas CH, Sutherland KR, Mianzan WM, Bogeberg M, Purcell JE, Decker MB, Uye S, Madin LP, Brodeur RD, Haddock SH, Malej A, Parry GD, Eriksen E, Quiñones J, Acha M, Harvey M, Arthur JM, Graham WM (2013) Recurrent jellyfish blooms are a consequence of global oscillations. Proc Natl Acad Sci USA 110:1000–1005. doi:10.1073/pnas.1210920110
Cury P, Bakun A, Crawford RJM, Jarre A, Quiñones RA, Shannon JL, Verheye HM (2000) Small pelagics in upwelling systems: patterns of interaction and structural changes in ‘wasp-waist’ ecosystems. ICES J Mar Sci 57:603–618. doi:10.1006/jmsc.2000.0712
Damalas D, Megalofonou P, Apostolopoulou M (2007) Environmental, spatial, temporal and operational effects on swordfish (Xiphias gladius) catch rates of eastern Mediterranean Sea longline fisheries. Fish Res 84:233–246. doi:10.1016/j.fishres.2006.11.001
Daskalov G, Mamedov E (2007) Integrated fisheries assessment and possible causes for the collapse of anchovy kilka in the Caspian Sea. ICES J Mar Sci 64:503–511. doi:10.1093/icesjms/fsl047
Daskalov G, Grishin AN, Rodionov S, Mihneva V (2007) Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc Natl Acad Sci USA 104:10518–10523. doi:10.1073/pnas.0701100104
Decker MB, Liu H, Ciannelli L, Ladd C, Cheng W, Chan KS (2013) Linking changes in eastern Bering Sea jellyfish populations to environmental factors via nonlinear time series models. Mar Ecol Prog Ser 494:179–189. doi:10.3354/meps10545
Decker MB, Cieciel K, Zavolokin A, Lauth R, Brodeur RD, Coyle KO (2014) Population fluctuations of jellyfish in the Bering Sea and their ecological role in this productive shelf ecosystem. In: Pitt KA, Lucas CH (eds) Jellyfish blooms. Springer, Dordrecht, pp 153–183. doi:10.1007/978-94-007-7015-7_1
Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Plovina J, Rabalais NN, Sydeman WJ, Talley LD (2012) Climate change impacts on marine ecosystems. Mar Sci 4:11–37. doi:10.1146/annurev-marine-041911-111611
Dong Z, Liu D, Keesing JK (2010) Jellyfish blooms in China: dominant species, causes and consequences. Mar Pollut Bull 60:954–963. doi:10.1016/j.marpolbul.2010.04.022
Duarte CM, Pitt KA, Lucas CH, Purcell JE, Uye S, Robinson K, Brotz L, Decker MB, Sutherland KR, Malej A, Madin L, Mianzan H, Gili JM, Fuentes V, Atienza D, Pagés F, Breitburg D, Malek J, Graham WM, Condon RH (2012) Is global ocean sprawl a cause of jellyfish blooms? Front Ecol Environ 11:91–97. doi:10.1890/110246
Eriksen E, Prozorkevich D, Trofimov A, Howel D (2012) Biomass of scyphozoan jellyfish, and Its spatial association with 0-group fish in the Barents Sea. PLoS One 7:e33050. doi:10.1371/journal.pone.0033050
Flores A, Wiff R, Diaz E (2015) Using the gonadosomatic index to estimate the maturity ogive: application to Chilean hake (Merluccius gayi gayi). ICES J Mar Sci 72:508–514. doi:10.1093/icesjms/fsu155
Flynn BA, Richardson AJ, Brierley AS, Boyer CD, Axelsen BE, Scott L, Moroff NE, Kainge PI, Tjizoo BM, Gibbons MJ (2012) Temporal and spatial patterns in the abundance of jellyfish in the northern Benguela upwelling ecosystem and their link to thwarted pelagic fishery recovery. Afr J Mar Sci 34:131–146. doi:10.2989/1814232X.2012.675122
Gibbons MJ, Richardson AJ (2009) Patterns of pelagic cnidarian abundance in the North Atlantic. Hydrobiologia 616:51–65. doi:10.1007/s10750-008-9593-8
Goy J, Morand P, Etienne M (1989) Long-term fluctuations of Pelagia noctiluca (Cnidaria, Scyphomedusa) in the western Mediterranean Sea. Prediction by climatic variables. Deep Sea Res 36:269–279. doi:10.1016/0198-0149(89)90138-6
Gutierrez D, Bertrand A, Wosnitza-Mendo C, Dewitte B, Purca S, Peña C, Chaigneau A, Tam J, Graco M, Echevin V, Grados C, Freon P, Guevara-Carrasco R (2011) Sensibilidad del sistema de afloramiento costero del Perú al cambio climático e implicancias ecológicas Climate change sensitivity of the Peruvian upwelling system and ecological implications. Revista Peruana Geoatmosférica 3:124. Available via http://www.crh-eme.ird.fr/team/pfreon/PDF/Gutierrez_et_al_2011_Rev_Peruana_Geo-Atmosferica.pdf. Accessed 22 Jan 2014
Hastie T, Tibshirani R (1986) Generalized additive models. Stat Sci 1:297–318. doi:10.1214/ss/1177013609
Holst S (2012) Effects of climate warming on strobilation and ephyra production of North Sea scyphozoan jellyfish. Hydrobiologia 690:127–140. doi:10.1007/s10750-012-1043-y
Jaspers C, Møller LF, Kiørboe T (2011) Salinity gradient of the Baltic Sea limits the reproduction and population expansion of the newly invaded comb jelly Mnemiopsis leidyi. PLoS One 6:e24065. doi:10.1371/journal.pone.0024065
Jensen OP, Branch A, Hilborn R (2012) Marine fisheries as ecological experiments. Theor Ecol 5:3–22. doi:10.1007/s12080-011-0146-9
Kamykowski D (2012) 20th century variability of atlantic meridional overturning circulation: planetary wave influences on world ocean surface phosphate utilization and synchrony of small pelagic fisheries. Deep Sea Res Part I 65:85–99. doi:10.1016/j.dsr.2012.03.005
Kogovšek T, Bogunovic B, Malej A (2010) Recurrence of bloom-forming scyphomedusae: wavelet analysis of a 200-year time series. Hydrobiologia 645:81–96. doi:10.1007/s10750-010-0217-8
Lenarz WH, Ventresca DA, Graham WM, Schwing FB, Chavez F (1995) Explorations of El Niño events and associated biological population dynamics off central California. Calif Coop Oceanic Fish Invest Rep 36:106–119
Lo NC, Jacobson LD, Squire JL (1992) Indices of relative abundance from fish spotter data based on delta-lognormal models. Can J Fish Aquat Sci 49:2515–2526. doi:10.1139/f92-278
Lucas CH, Graham WM, Widmer C (2012) Jellyfish life histories: role of polyps in forming and maintaining scyphomedusa populations. Adv Mar Biol 63:133–196. doi:10.1016/B978-0-12-394282-1.00003-X
Lynam CP, Brierley AC, Hay SJ (2005) Jellyfish abundance and climate variation: contrasting responses in oceanographically distinct regions of the North Sea, and possible implications for fisheries. J Mar Biol Assoc UK 85:435–450
Lynam CP, Gibbons MJ, Axelsen BE, Sparks CA, Cotzee J, Heywood BG, Brierley AS (2006) Jellyfish overtake fish in a heavily fished ecosystem. Curr Biol 16:13R492. doi:10.1016/j.cub.2006.06.018
Lynam CP, Attrill JM, Skogen MD (2010) Climatic and oceanic influences on the abundance of gelatinous zooplankton in the North Sea. J Mar Biol Assoc UK 90:1153–1159
Lynam CP, Lilley MKS, Bastian T, Doyle TK, Beggs SE, Hays GC (2011) Have jellyfish in the Irish Sea benefited from climate change and overfishing? Global Change Biol 17:767–782. doi:10.1111/j.1365-2486.2010.02352.x
McPhaden MJ, Lee T, McClurg D (2011) El Niño and its relationship to changing background conditions in the tropical Pacific Ocean. Geophys Res Lett 38:L15709. doi:10.1029/2011GL048275
Mianzan H, Quiñones J, Palma S, Schiariti A, Acha M, Robinson K, Graham WM (2014) Chrysaora plocamia: a poorly understood jellyfish from South American Waters. In: Pitt KA, Lucas CH (eds) Jellyfish blooms. Springer, Dordrecht, pp 219–236. doi:10.1007/978-94-007-7015-7_1
Miller ME, Graham WM (2012) Environmental evidence that seasonal hypoxia enhances survival and success of jellyfish polyps in the northern Gulf of Mexico. J Exp Mar Biol Ecol 432:113–120. doi:10.1016/j.jembe.2012.07.015
Mitchell JD, Collins KJ, Miller PI, Suberg LA (2014) Quantifying the impact of environmental variables upon catch per unit effort of the blue shark Prionace glauca in the western English Channel. J Fish Biol 1:657–670. doi:10.1111/jfb.12448
Molinero JC, Buecher E, Lučič D, Malej A, Miloslavič M (2009) Climate and Mediteranean jellyfish: assessing the effect of temperature regimes on jellyfish outbreak dynamics. Ann Ser Hist Nat 19:11–18
Montecinos A, Purca S, Pizarro O (2003) Interannual to interdecadal sea surface temperature variability along the western coast of South America. Geophys Res Lett 30:1570. doi:10.1029/2003GL017345
Ochoa N, Rojas de Mendiola B, Gomez O (1985) Identificación del Fenómeno “El Niño” a través de los Organismos Fitoplanctónicos. In: Arntz W, Landa A, Tarazona J (eds) El Niño: Su impacto en la Fauna Marina. Boletín Instituto del Mar del Perú. Volumen Extraordinario, 23–31. Available via http://biblioimarpe.imarpe.gob.pe:8080/bitstream/handle/123456789/1156/BOL%20EXTR.%20EL%20NI%C3%91O-3.pdf?sequence=1. Accessed 01 March 2014
Ochoa N, Taylor MH, Purca S, Ramos E (2010) Intra and interannual variability of nearshore phytoplankton biovolume and community changes in the northern Humboldt Current system. J Plankton Res 32:843–855. doi:10.1093/plankt/fbq022
Pagés F, Gonzalez HE, Ramon M, Sobarzo M, Gili JM (2001) Gelatinous zooplankton assemblages associated with water masses in the Humboldt Current System, and potential predatory impact by Bassia bassensis (Siphonophora: Calycophorae). Mar Ecol Prog Ser 210:13–24. doi:10.3354/meps210013
Parsons TR, Lalli CM (2002) Jellyfish population explosions: revisiting a hypothesis of possible causes. La Mer 40:111–121
Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F (1998) Fishing down marine food webs. Science 279:860–863. doi:10.1126/science.279.5352.860
Pennington JT, Mahoney KL, Kuwahara VS, Kolber DD, Calienes R, Chavez FP (2006) Primary production in the eastern tropical Pacific: a review. Prog Oceanogr 69:285–317. doi:10.1016/j.pocean.2006.03.012
Photopoulou T, Fedak MA, Thomas L, Matthiopoulos J (2014) Spatial variation in maximum dive depth in gray seals in relation to foraging. Mar Mammal Sci 30:923–938. doi:10.1111/mms.12092
Pointin F, Payne MR (2014) A resolution to the blue whiting (Micromesistius poutassou) population paradox? PLoS One 9:e106237. doi:10.1371/journal.pone.0106237
Prieto L, Astorga D, Navarro G, Ruiz J (2010) Environmental control of phase transition and polyp survival of a massive outbreaker jellyfish. PLoS One 5:e13793. doi:10.1371/journal.pone.0013793
Purcell JE (1991) A review of cnidarians and ctenophores feeding on competitors in the plankton. In Coelenterate Biology: recent research on cnidaria and ctenophora. Hydrobiologia 216(217):335–342. doi:10.1007/978-94-011-3240-4_48
Purcell JE (2003) Predation on zooplankton by large jellyfish, Aurelia labiata, Cyanea capillata and Aequorea aequorea, in Prince William Sound, Alaska. Mar Ecol Prog Ser 246:137–152
Purcell JE (2005) Climate effects on formation of jellyfish and ctenophore blooms: a review. J Mar Biol Assoc UK 85:461–476. doi:10.1017/S0025315405011409
Purcell JE (2007) Environmental effects on asexual reproduction rates of the scyphozoan Aurelia labiata. Mar Ecol Prog Ser 48:183–196. doi:10.3354/meps07056
Purcell JE (2012) Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Annu Rev Mar Sci 4:209–235. doi:10.1146/annurev-marine-120709-142751
Purcell JE, Arai MN (2001) Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia 451:27–44. doi:10.1007/978-94-011-3240-4_48
Purcell JE, Decker MB (2005) Effects of climate on relative predation by scyphomedusae and ctenophores on copepods in Chesapeake Bay during 1987–2000. Limnol Oceanogr 50:376–387. doi:10.4319/lo.2005.50.1.0376
Purcell JE, Malej A, Benović A (1999a) Potential links of jellyfish to eutrophication and fisheries. In: Ecosystems at the land-sea margin: drainage basin to coastal sea. In: Malone TC, Malej A, Harding Jr. LW, Smodlaka N, Turner RE (eds). Coast Estuar Stud 55:241–263. doi:10.1029/CE055p0241
Purcell JE, White JR, Nemazie DA, Wright DA (1999b) Temperature, salinity and food effects on asexual reproduction and abundance of the scyphozoan Chrysaora quinquecirrha. Mar Ecol Prog Ser 180:187–196
Purcell JE, Uye S, Lo WT (2007) Anthropogenic causes of jellyfish blooms and direct consequences for humans: a review. Mar Ecol Prog Ser 350:153–174. doi:10.3354/meps07093
Purcell JE, Hoover RA, Schwarck NT (2009) Interannual variation of strobilation by the scyphozoan Aurelia labiata in relation to polyp density, temperature, salinity, and light conditions in situ. Mar Ecol Prog Ser 375:139–149. doi:10.3354/meps07785
Quiñones J (2008) Chrysaora plocamia Lesson, 1830 (Cnidaria, Scyphozoa), frente a Pisco, Perú. Informe Instituto del Mar del Perú, 35:221-230. Available via http://biblioimarpe.imarpe.gob.pe:8080/bitstream/handle/123456789/1972/INF.%2035%283%29-6.pdf?sequence=1. Accessed 17 Jan 2014
Quiñones J, Carman VG, Zeballos J, Purca S, Mianzan H (2010) Effects of El Niño-driven environmental variability on black turtle migration to Peruvian foraging grounds. Hydrobiologia 645:69–79. doi:10.1007/s10750-010-0225-8
R Development Core Team. R (2009) A language and environment for statistical computing. Vienna, Austria. Available via: http://cran.r-project.org. Accessed 02 Feb 2014
Reid PC, Battle EJV, Batten SD, Brander KM (2000) Impacts of fisheries on plankton community structure. ICES J Mar Sci 57:495–502. doi:10.1006/jmsc.2000.0740
Riascos JM, Paredes L, Gonzales K, Caceres I, Pacheco AS (2013) The larval and benthic stages of the scyphozoan medusa Chrysaora plocamia under El Niño–La Niña thermal regimes. J Exp Mar Biol Ecol 446:95–101. doi:10.1016/j.jembe.2013.05.006
Riascos JM, Villegas V, Pacheco AS (2014) Diet composition of the large scyphozoan jellyfish Chrysaora plocamia in a highly productive upwelling centre off northern Chile. Mar Biol Res 10:791–798. doi:10.1080/17451000.2013.863353
Richardson AJ, Bakun A, Hay JC, Gibbons MJ (2009) The jellyfish joyride: causes, consequences and management responses to a more gelatinous future. Trends Ecol Evol 24:312–322. doi:10.1016/j.tree.2009.01.010
Robinson KL, Graham WM (2013) Long-term change in the abundances of northern Gulf of Mexico scyphomedusae Chrysaora sp. and Aurelia sp. with links to climate variability. Limnol Oceanogr 58:235–253. doi:10.4319/lo.2013.58.1.0235
Robinson KL, Graham WM (2014) Warming of subtropical coastal waters accelerates Mnemiopsis leidyi growth and alters timing of spring ctenophore blooms. Mar Ecol Prog Ser 502:105–115. doi:10.3354/meps10739
Robinson KL, Ruzicka JJ, Decker MB, Brodeur RD, Hernandez FJ, Quiñones J, Acha EM, Uye S, Mianzan H, Graham WM (2014) Jellyfish, forage fish, and the world’s major fisheries. Oceanography 27:104–115. doi:10.5670/oceanog.2014.90
Roux JP, Van der Lingen CD, Gibbons MJ, Moroff NE, Shannon LJ, Smith AD, Cury PM (2013) Jellyfication of marine ecosystems as a likely consequence of overfishing small pelagic fishes: lessons from the Benguela. Bull Mar Sci 89:249–284. doi:10.5343/bms.2011.1145
Sánchez S (2000) Variación estacional e interanual de la biomasa fitoplanctónica y concentraciones de clorofila A, frente a la costa peruana durante 1976–2000. Boletín Instituto del Mar del Perú 19:29–44. Available via http://biblioimarpe.imarpe.gob.pe:8080/bitstream/handle/123456789/995/BOL%2019%281-2%29-5.pdf?sequence=1. Accessed 04 February 2014
Santander H, Carrasco S (1985) Cambios en el zooplancton durante El Niño 1982–1983 en el área de Chimbote. In: del Anales I (ed) Aguilar AET. Congreso Nacional de Biología Pesquera, Trujillo, pp 201–206
Segurado P, Araújo MB, Kunin WE (2006) Consequences of spatial autocorrelation for niche-based models. J Appl Ecol 43:433–444. doi:10.1111/j.1365-2664.2006.01162.x
Smith TM, Reynolds RW, Peterson CT, Lawrimore J (2008) Improvements to NOAA’s historical merged land-ocean surface temperature analysis (1880–2006). J Clim 21:2283–2296. doi:10.1175/2007JCLI2100.1
Stenseth NC, Mysterud A (2002) Climate, changing phenology, and other life history traits: nonlinearity and match–mismatch to environment. Proc Natl Acad Sci USA 99:13379–13381. doi:10.1073/pnas.212519399
Suchman CL, Daly EA, Keister JE, Peterson WT, Brodeur RD (2008) Feeding patterns and predation potential of scyphomedusae in a highly productive upwelling region. Mar Ecol Prog Ser 358:161–172. doi:10.3354/meps07313
Suchman CL, Brodeur RD, Emmett RL, Daly EA (2012) Large medusae in surface waters of the Northern California current: variability in relation to environmental conditions. Hydrobiologia 690:113–125. doi:10.1007/s10750-012-1055-7
Tarazona J, Arntz W, Canahuire E, Ayala Z, Robles A (1985) Modificaciones producidas durante “El Niño” en la infauna bentónica de áreas someras del ecosistema de afloramiento peruano. In: Arntz W, Landa A, Tarazona J (eds) El Niño: Su impacto en la fauna marina. Boletín Instituto del Mar del Perú. Volumen Extraordinario, 55–63. Available via http://biblioimarpe.imarpe.gob.pe:8080/bitstream/handle/123456789/1166/BOL%20EXTR.%20EL%20NI%C3%91O-7.pdf?sequence=1. Accessed 20 June 2014
Tarazona J, Arntz W, Valle S, Peña T (2001) Los índices de la variabilidad de El Niño y del impacto sobre las comunidades bentónicas. In: Tarazona J, Arntz W, Castillo de Maruenda E (eds) El Niño en América Latina: impactos biológicos y sociales. Consejo Nacional de Ciencia y Tecnología, Editorial Omega, Lima, pp 113–125
Utne-Palm AC, Salvanes AGV, Currie B, Kaartvedt S, Nilsson GE, Braithwaite VA, Stecyk JAW, Hundt M, Van der Bank M, Flynn B et al (2010) Trophic structure and community stability in an overfished ecosystem. Science 329:333–336. doi:10.1126/science.1190708
Uye S (2014) The giant jellyfish Nemopilema nomurai in East Asian marginal seas. In: Pitt KA, Lucas CH (eds) Jellyfish Blooms. Springer, Dordrecht, pp 185–205. doi:10.1007/978-94-007-7015-7_1
Venables WN, Dichmont CM (2004) GLMs, GAMs and GLMMs: an overview of theory for applications in fisheries research. Fish Res 70:319–337. doi:10.1016/j.fishres.2004.08.011
West JW, Pitt KA, Welsh DT, Koop K, Rissik D (2009) Top-down and bottom-up influences of jellyfish on primary productivity and planktonic assemblages. Limnol Oceanogr 54:2058–2071. doi:10.4319/lo.2009.54.6.2058
Wood S (2006) Generalized additive models: an introduction with R. Biometrics 62:392. doi:10.1111/j.1541-0420.2006.00574.x
Wood SN, Augustin NH (2002) GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecol Modell 157:157–177. doi:10.1016/S0304-3800(02)00193-X
Worm B, Barbier EB, Beaumont N, Duffy JE, Folke C, Halpern BS, Jackson JBC, Lotze HK, Micheli F, Palumbi SR, Sala E, Selkoe KA, Stachowicz JJ, Watson R (2006) Impacts of biodiversity loss on ocean ecosystem services. Science 314:787–790. doi:10.1126/science.1132294
Zuta S, Urquizo W, Rivera T, Maldonado M (1976) Informe de la exploración con bolicheras para el control costero de “El Niño” en Febrero 1976 (EBCCEN 1– 1976), Serie de Informes Especiales, Instituto del Mar del Perú, pp 1–10. Available via: http://biblioimarpe.imarpe.gob.pe:8080/bitstream/handle/123456789/1639/INF%20ESP.%20181.pdf?sequence=1. Accessed 10 Feb 2014
Acknowledgments
We thank the Instituto del Mar del Perú (IMARPE) headquarters at Callao and regional laboratory at Pisco for permission and funding in conducting this investigation. We also thank the reviewers and editors for their assistance, especially to William “Monty” Graham for the earlier version. We thank Hermes Mianzan who was an outstanding person and scientist. This work was supported by the following grants: PIP 112-201101-00892; CRN3070 from the Inter-American Institute for Global Change Research (IAI), which is supported by the US National Science Foundation (Grant GEO-1128040), and EXA 647/14 to H. Mianzan and M. Acha. KL Robinson was supported by a Lenfest Ocean Program Grant (No. 00025535) funded by the Pew Charitable Trusts to WM Graham at The University of Southern Mississippi.
Funding
This study was funded by the following grants: PIP 112–201101–00892; CRN3070 from the Inter-American Institute for Global Change Research (IAI), which was supported by the US National Science Foundation (Grant GEO-1128040), and EXA 647/14 to H. Mianzan and M. Acha.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human participants and animals consent
No human experimentation was involved in this research. The animals (jellyfish) used in this study were at low taxonomical resolution and were captured as bycatch in the pelagic scientific research cruises.
Additional information
Responsible Editor: J. Purcell.
Hermes Mianzan: Deceased.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Quiñones, J., Mianzan, H., Purca, S. et al. Climate-driven population size fluctuations of jellyfish (Chrysaora plocamia) off Peru. Mar Biol 162, 2339–2350 (2015). https://doi.org/10.1007/s00227-015-2751-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2751-4