Abstract
Recent studies have correlated fluctuations in jellyfish abundances with climatic changes, leading to speculation that the warming trend in the North Sea will affect the strobilation activity of Scyphozoa. The present study provides long-term data (10–22 months) on temperature effects on the species Aurelia aurita, Cyanea capillata, Cyanea lamarckii and Chrysaora hysoscella. Strobilation at current winter temperature (5°C) in the German Bight was compared to strobilation at warmer winter temperatures. Simulated winter temperature of 10°C had several positive effects on strobilation, as compared to 5°C: 1. A longer strobilation period or higher ephyra production per polyp in A. aurita, C. lamarckii and Ch. hysoscella; 2. Higher percentages of polyps strobilating in A. aurita and Ch. hysoscella; 3. More ephyrae per strobila in C. capillata and C. lamarckii; 4. A shorter strobilation duration in C. capillata and C. lamarckii. Cold winter temperatures of 5°C promoted strobilation in C. capillata, but inhibited strobilation in A. aurita and reduced ephyra production in C. lamarckii and Ch. hysoscella. These results suggest that climate warming will benefit A. aurita, but not cold-water C. capillata. The distributions of C. lamarckii and Ch. hysoscella probably could expand to the north.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reports of mass occurrences of large jellyfish (Scyphozoa) in many marine ecosystems worldwide have increased in recent decades (Purcell et al., 2007; Richardson et al., 2009). Negative impacts of such medusa blooms on ecosystems, fisheries, industries and tourism are obvious: medusae are food competitors of fish and feed on fish larvae and small fish (Barz & Hirche, 2007; Sabatés et al., 2010), the gelatinous bodies clog fishing nets and cooling systems of coastal industries, and jellyfish stinging swimmers have negative effects on the tourism industry (CIESM, 2001; Purcell et al., 2007). It is possible that consequences of anthropogenic activities, including overfishing, eutrophication, species invasions and especially climate change, have contributed to increased jellyfish abundances (Purcell et al., 2007; Richardson et al., 2009; Purcell, 2011).
Recent analyses of temperature data show a clear warming trend in global average air and ocean temperature (IPCC, 2007). A pronounced winter warming trend has been observed in the North and Baltic seas, which is predicted to continue in the future (HELCOM, 2007; Belkin, 2009). Recent rapid climate change has changed the abundances, population structures and biographical ranges of benthic and planktonic North Sea species (Mieszkowska et al., 2006; Wiltshire et al., 2010). Climate regime shifts also affect jellyfish populations. Changes in temperature, salinity, currents, predator–prey interactions and competition have measurable effects on jellyfish abundance and distribution (Purcell, 2005, 2009; Molinero et al., 2008; Lynam et al., 2010). These environmental changes will affect both the pelagic and the benthic stages of metagenetic medusae; however, the role of the polyps has been neglected in most analyses and models because of the lack of data on polyp ecology (Lynam et al., 2010).
The medusae of four semaeostome scyphozoans, Aurelia aurita (Linneaus, 1758), Cyanea capillata (Linneaus, 1758), Cyanea lamarckii Péron and Lesueur, 1809 and Chrysaora hysoscella (Linneaus, 1766), occur in the German Bight during the summer months, and mass occurrences have been documented in some years (Russell, 1970; Möller, 1980a; Hay et al., 1990; Barz & Hirche, 2007). Like most scyphozoans, the life cycle of these species includes planktonic medusa and benthic polyp generations. After settlement and metamorphosis of the planula larvae, the polyp populations increase by asexual reproduction. Each polyp seasonally produces one to several young medusae (ephyrae) in a process of transversal fission called strobilation. This process contributes to the development of jellyfish blooms because polydisc strobilation allows single polyps to produce many ephyrae (Boero et al., 2008).
Information on the environmental factors and stressors that determine the induction, timing and magnitude of the strobilation process is limited to a few species (e.g. Aurelia spp., reviewed in Purcell et al., 2012). Moreover, the benthic stage is completely undiscovered or undescribed for most scyphozoans (Tronolone et al., 2002; Jarms, 2010). Although many laboratory investigations on scyphopolyps have shown that temperature significantly affects the asexual polyp reproduction and strobilation (e.g. Holst et al., 2007; Purcell, 2007; Willcox et al., 2007; You et al., 2008; Liu et al., 2009), most of these studies were restricted to short periods of a few weeks and polyps were often exposed to extreme, abrupt changes, whereas changes in situ would be less severe (Purcell, 2005). Long-time investigations on the effects of natural seasonal temperature cycles on polyp ecology are unavailable for most species (but see Gröndahl, 1988; Brewer & Feingold, 1991; Miyake et al., 2002; Purcell et al., 2009; Di Camillo et al., 2010; Ishii & Katsukoshi, 2010). In the present study, I compare the long-term (10–22 months) effects of different temperature conditions on strobilation and ephyra production of four semaeostome jellyfish species from the North Sea to show the possible effects of increasing winter temperatures on the strobilation activity of Scyphozoa in the North Sea. Strobilation at temperature conditions similar to the current temperatures in the German Bight was compared to that at warmer winter temperatures. Additional experiments without a temperature change were conducted to determine the importance of temperature changes as inducers of strobilation in scyphozoan polyps. I tested the null hypotheses to ascertain whether the percentages of polyps strobilating and the numbers of ephyrae produced per strobila were independent of temperature; second, whether the percentages of strobilating polyps and the number of ephyrae produced per strobila were independent of polyp age; and third, whether the duration of strobilation was independent of the number of ephyrae per strobila and of temperature.
Materials and methods
Polyps of the species A. aurita, C. capillata, C. lamarckii and Ch. hysoscella were reared from planulae collected from female medusae in the summer periods of 2003 and 2004 around the island of Helgoland (details in Holst & Jarms, 2007, 2010). Watch glasses or polyethylene plates (for C. lamarckii) colonized by polyps in the laboratories of the Biologische Anstalt Helgoland were transported to the Zoological Institute of the University of Hamburg (Biocenter Grindel) 1–2 weeks after planula settlement. The substrates were transferred into 150-ml glass bowls filled with filtered North Sea water (salinity 35 ± 2). The bowls were kept in incubators at 15°C in darkness before the experiments. Only well-developed polyps with extended tentacles were used for the experiments, whereas small or contracted polyps were carefully removed from the substrates with a needle. Polyps were fed nauplii of Artemia salina for 1–2 h every 7–10 days, after which the seawater in the bowls was replaced with fresh filtered seawater of the same temperature. During strobilation, only half of the water was changed carefully, and uneaten food was removed from the bowls with a pipette to avoid disturbing the strobilation process. Polyps of each species were cultured in three different temperature groups (Table 1). In the 15°C group, the temperature was constant 15°C throughout the year. In the groups 15–10–15°C and 15–05–15°C, the temperature was 15°C until mid-October and lowered to 10°C in autumn and 5°C in winter, respectively. The temperatures were decreased in steps of 2.5°C per month (Fig. 1), and all cultures were kept in incubators in darkness. Polyps were monitored for 12 months (A. aurita from 15 September, and C. capillata from 15 October) or 10 months (C. lamarckii and Ch. hysoscella from 15 October) in the first year after settlement. Additional 10-month-long experiments (from 15 October) were conducted in the second year after settlement with A. aurita and C. capillata polyps. The polyps were counted monthly in all replicates, and all cultures were checked weekly to detect beginning strobilations. When strobilation appeared in the cultures, each strobila was monitored individually. The developing ephyrae in each strobila and the released ephyrae in the cultures were counted at least twice per week.
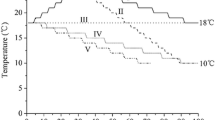
Numbers of ephyrae per polyp produced monthly (left side) and total numbers of ephyrae produced per mean numbers of polyps counted monthly during 10 months of observation (15 Oct–15 Aug, right side) in different temperature groups of A. aurita, C. capillata, C. lamarckii and Ch. hysoscella polyps. The culture temperature was constant 15°C from 15th July to 15th October in all temperature groups (not shown in the figure). Polyp numbers in different temperature groups are shown in Table 1
Calculations and statistics
The numbers of ephyrae produced monthly in each temperature group were summed and divided by the number of polyps to calculate ephyra production for each month. To account for production and mortality of polyps, the mean numbers of polyps (counted monthly) were calculated over the duration of the experiment (Table 1) and used in calculations and statistics. The total numbers of ephyrae produced per mean polyp numbers were calculated for each temperature group. The percentages of strobilating polyps in the first year were calculated for each replicate from the total numbers of strobilae divided by the mean numbers of polyps in each replicate. Percentages were arcsine square root transformed before statistical analysis to test the first null hypothesis, H01: the percentages of polyps strobilating were independent of the temperature treatments. In C. lamarckii, all polyps strobilated once in all temperature groups, therefore the percentages of polyps that strobilated twice were compared to test H01. Individually monitored strobilations in the first year were used to calculate the mean numbers of ephyrae per strobila to test H02: the numbers of ephyrae produced per strobila were independent of the temperature treatments. Strobilations from replicates 1–3 were used for analysis in all species and temperature groups; additional strobilations were included from replicates 4–6 in A. aurita 15°C, C. capillata 15–10–15°C, C. capillata 15–05–15°C and Ch. hysoscella 15–05–15°C. For H01 and H02, data with normal distributions were tested by one-way analysis of variance (ANOVA) followed by a Fisher’s least significance difference (LSD) post-hoc test. Data not normally distributed were tested by a Kruskal–Wallis ANOVA on ranks and a Student–Newman–Keuls post-hoc test. Two experimental groups were compared with a Mann–Whitney Rank Sum Test.
For comparisons among strobilations 1 and 2 years after settlement, the same three replicates of each temperature group of A. aurita and C. capillata polyps were used in both years (Table 1). The same 10-month period (15 October–15 August) were analysed in both years. The percentages of strobilating polyps and the numbers of ephyrae produced per strobila were calculated for both years, as described above, and compared by a Mann–Whitney Rank Sum Test. Two null hypotheses were tested, H03: The percentages of strobilating polyps were independent of polyp age and H04: The number of ephyrae produced per strobila was independent of the polyp age.
The durations of strobilations (days) were determined from the number of days between the start of the strobilation, when first constrictions of the polyp body appeared, until the end the strobilation, when the last ephyra detached from the strobila. Only strobilations that began and ended at the same temperature were used in the analysis. Strobilae in the first year after polyp settlement were analysed in C. lamarckii and Ch. hysoscella, and in the first and second years combined in A. aurita and C. capillata. Strobilations were analysed at 15, 10 and 5°C culture temperatures. Two null hypotheses were tested by analysis of covariance (ANCOVA), H05: The duration of strobilation was independent of the number of ephyrae per strobila and H06: The duration of strobilation was independent of the temperature treatments. ANCOVA-tests were conducted separately for each species. In all tests, duration was the dependent variable; the number of ephyrae per strobila and the culture temperature were covariates.
Results
Ephyra production and strobilation rates
A. aurita
The first A. aurita strobilations occurred in autumn in all temperature groups in the first and second years after polyp settlement. Ephyra were produced until next May in both years in the 15–10–15°C group, whereas ephyra production stopped after a temperature decrease to 5°C in January in the 15–05–15°C groups (Fig. 1, left side). Few ephyrae per polyp were produced in the temperature group with constant 15°C and the most ephyrae per polyp were produced in the 15–10–15°C group in the first and in the second year after settlement (Fig. 1, right side). Significantly higher percentages of A. aurita polyps strobilated after a temperature decrease in autumn than at constant 15°C, and H01 was rejected for both years (Figs. 2, 3; Table 2). The numbers of ephyrae produced per strobila did not differ significantly among the temperature groups in the first year or in the second year and H02 was not rejected (Fig. 2; Table 2). The percentages of strobilating polyps were higher in the second than in the first year, but the differences were not significant, and H03 was not rejected (Fig. 3; Table 3). Although the maximum numbers of ephyrae per strobila were higher in the second year than in the first, the means differed only slightly among groups, and H04 was not rejected (Fig. 3; Table 3).
Strobilating polyps (mean % ± SD) (left side) and ephyrae produced per strobila (mean number ± SD) (right side) in the first year after polyp settlement in different temperature groups of A. aurita, C. capillata, C. lamarckii and Ch. hysoscella polyps; n number of replicates (numbers of polyps as shown in Table 1). The numbers of analysed strobilae appear inside the bars. Diamond maximal number of ephyrae produced by one strobila. The observation time was 12 months for A. aurita (15 Sep–15 Sep) and C. capillata (15 Oct–15 Oct) and 10 months for C. lamarckii and Ch. hysoscella (15 Oct–15 Aug). Test statistics are shown in Table 2
Strobilating polyps (mean % ± SD) (left side) and ephyrae produced per strobila (mean number ± SD) (right side) in the first year and in the second year after polyp settlement in different temperature groups of A. aurita, C. capillata, C. lamarckii and Ch. hysoscella polyps, n number of replicates (numbers of polyps are shown in Table 1). The numbers of analysed strobilae appear inside the bars. Diamond maximal number of ephyrae produced by one strobila. In both analyses, the observation time was 10 months (15 Oct–15 Aug) in both years. Test statistics are shown in Table 3
C. capillata
C. capillata polyps never strobilated in constant 15°C during 22 months of observation. The first ephyra appeared in February–March at the cold winter temperature (5°C) in both years, whereas the first ephyra appeared at least 1 month later at the 10°C winter temperature (Fig. 1, left side). Ephyra production continued until the beginning (second year) or end of May (first year). In the first year, production of ephyrae per polyp was higher in 5°C winter temperature, whereas it was slightly higher in 10°C winter temperature in the second year (Fig. 1, right side). In the first year, the percentages of polyps strobilating were significantly higher in the 15–05–15°C group than in the 15–10–15°C and 15°C groups and H01 was rejected (Fig. 2; Table 2). Although the percentages of polyps strobilating differed only slightly for the 15–10–15°C and the 15–05–15°C groups, no strobilation occurred at 15°C and H01 was rejected in the second year as well (Fig. 3; Table 2). The numbers of ephyrae per strobila were lower in groups with 5°C than in those with 10°C winter temperature, but differences were only significant in the first year (H02 rejected, Fig. 2; Table 2). The percentages of strobilating polyps did not differ significantly in polyps of different ages and H03 was not rejected (Fig. 3; Table 3); however, the mean numbers of ephyrae per strobila were significantly higher in the second year in the 15–10–15°C group and the 15–05–15°C group (H04 rejected, Fig. 3; Table 3).
C. lamarckii
In C. lamarckii cultures, the first ephyra appeared after a temperature decrease to 10°C in groups 15–10–15°C and 15–05–15°C; however, the most ephyrae per polyp were produced in the constant 15°C group, whereas the fewest were in the coldest winter temperature (5°C; Fig. 1). All C. lamarckii polyps strobilated at least once in all temperature groups (>100%). The percentages of polyps strobilating twice were the highest in the 15–10–15°C groups, but the differences were not significant, and H01 was not rejected (Fig. 2; Table 2). The number of ephyrae produced per strobila, however, was significantly higher at constant 15°C than in treatments with temperature decreases, and H02 was rejected (Fig. 2; Table 2).
Ch. hysoscella
The monthly ephyra production per polyp was relatively low in Ch. hysoscella (Fig. 1, left side). The most ephyrae per polyp were in the 15–10–15°C group, and the fewest ephyrae per polyp were produced in the 15–05–15°C group (Fig. 1, right side). The percentages of strobilating polyps were significantly higher in the 15–10–15°C group than in the other groups, and H01 was rejected (Fig. 2; Table 2). In the 15°C and 15–05–15°C groups, maxima of three ephyrae were produced per strobila, whereas as many as six ephyrae per strobila were produced in the 15–10–15°C group (Fig. 2); however, the production of more then three ephyrae occurred only in 7% of analysed strobilae. Consequently, differences in ephyra production per strobila were not significant among temperature groups and H02 was not rejected (Fig. 2; Table 2).
Temperature change as a strobilation inducer or inhibitor
Small percentages of polyps strobilated at constant 15°C in A. aurita, demonstrating that temperature decrease was an important strobilation inducer; however, ephyra production stopped after temperature decreased further to 5°C in winter. This result demonstrated that a low winter temperature of 5°C inhibited strobilation activity from February on whereas a higher winter temperature of 10°C lead to a longer strobilation period until spring (Fig. 1). Temperature increases from 5 to 10°C in spring and then to 15°C in summer did not induce strobilation in A. aurita (Fig. 1).
Strobilation never occurred at constant 15°C in C. capillata and ephyra production was lower when temperature rose in spring and summer following strobilation at 5 or 10°C winter temperatures (Fig. 1). No ephyra was produced at temperatures exceeding 10°C in summer, suggesting strobilation inhibition at warmer temperatures in C. capillata. In C. lamarckii, ephyra production started earlier after a temperature decrease to 10°C than at constant 15°C (Fig. 1), showing a positive effect of temperature decrease on strobilation induction, although the total ephyra production was the highest at constant 15°C. In Ch. hysoscella, strobilation activity was low in all temperature treatments without clear responses to temperature changes (Fig. 1).
Temperature effects on strobilation duration and ephyrae per strobila
A significant effect of the number of ephyrae per strobila on the strobilation duration was shown by ANCOVA for all tested species (Fig. 4), and H05 was rejected. Correlations among temperature treatments and strobilation duration were not possible for A. aurita because strobilation occurred mostly at 10°C; thus, data at other temperatures were insufficient. In all other tested species, the ANCOVA confirmed a significant effect of temperature treatment on the strobilation duration (Fig. 4), and H06 was rejected.
Strobilation durations (mean days ± SD) in individually-monitored strobilae at different constant temperatures in relation to ephyrae numbers produced in the strobilae of A. aurita, C. capillata, C. lamarckii and Ch. hysoscella. The effect of ephyra numbers produced per strobila (e) on the strobilation duration and the effect of the temperature treatment (t) on the strobilation duration were tested by ANCOVA. n number of analysed strobilae. df = 1 for all performed tests. F and P values are shown in the figures
Discussion
Scyphopolyps are difficult to find and investigate in the field because of their small sizes and their preferences of colonizing the undersides of substrates and concealed habitats (Pierce, 2009; Di Camillo et al., 2010). Laboratory investigations allow quantification of polyp survival and reproduction and are therefore an important source of data (e.g. Purcell, 2007; Willcox et al., 2007; Liu et al., 2009; Sötje & Jarms, 2009; Holst & Jarms, 2010). Although the results from field observations may differ form laboratory experiments (Purcell et al., 2009), the results of this study demonstrated that simulation of natural temperature cycles in the laboratory can be used to test the effects of seasonal temperature changes on strobilation. The strobilation activity documented in two consecutive years in two species (A. aurita and C. capillata) showed that repeated temperature cycles in the laboratory experiments caused very similar responses of the polyps. The temperature cycles in the present experiments with 15°C maximum summer temperature and 5°C minimum winter temperature differed from the natural annual temperature cycles because of 2.5°C temperature changes per month. Nevertheless, the laboratory temperature conditions were similar to temperatures found recently in the German Bight (Wiltshire & Manly, 2004; www.bsh.de/en/Marine_data). The parallel experiments with warmer winter temperatures (10°C) indicated how warmer winter temperatures, as observed and predicted to progress in the North Sea (Belkin, 2009), could affect the strobilation activity of scyphopolyps in situ. In these experiments, warmer winter temperature (10°C in comparison to 5°C) positively affected strobilation in several ways: 1. A longer strobilation period or higher ephyra production per polyp (in A. aurita, C. lamarckii and Ch. hysoscella; Fig. 1); 2. Higher percentages of polyp strobilation (in A. aurita and Ch. hysoscella; Fig. 2); 3. More ephyrae per strobila (in C. capillata, C. lamarckii, see Fig. 2); 4. A shorter strobilation duration (in C. capillata and C. lamarcki, see Fig. 4). Many of the changes in species abundances, population structure and biogeographical ranges occur as a result of increased reproductive output and juvenile survival in response to increased warming (Mieszkowska et al., 2006). The results of this study suggest that this could also be true for the North Sea scyphozoans investigated.
In A. aurita, the experiments showed significantly higher percentages of strobilations after a temperature decrease in autumn compared to constant 15°C demonstrating the importance of a temperature decrease for strobilation induction in this species. Spring strobilation only occurred in experiments with 10°C winter temperature, but did not occur with rising temperature in spring after a cold winter period of 5°C. Perhaps under natural conditions, autumn strobilation in A. aurita polyps can happen only if the temperature decreases slowly and the period of moderate temperature of about 10°C lasts for several weeks, whereas a rapid temperature decrease may lead to inhibition of strobilation in autumn. In agreement with this idea, in situ autumn strobilations of A. aurita were observed in areas with moderate winter temperatures, such as in Sylt, German Bight (Thiel, 1938), Osterschelde, Netherlands (Korringa, 1953), and Gullmarfjord, western Sweden (Hernroth & Gröndahl, 1985, Gröndahl, 1988); however, in areas with a rapid temperature decrease in autumn, such as in several Baltic Sea areas, a later start of the strobilation activity in A. aurita was documented, and strobilation occurred mainly in winter and spring (Palmén, 1954; Kändler, 1961; Thiel, 1962; Rasmussen, 1973; Möller, 1980b). The ephyrae may survive the cold winter periods in deep water layers without further development, which could explain the main time of ephyra abundance in the plankton in autumn and spring (Rasmussen, 1973; Hernroth & Gröndahl, 1985; Gröndahl, 1988). In Southampton Water and Horsea Lake in southern England, ephyra production begins in December and lasts 7 months (Lucas, 1996; Lucas et al., 1997), confirming the results of this study of an extended strobilation phase in A. aurita at warmer winter temperatures (Fig. 1). Recent molecular genetic study detected that A. aurita is not a single species, but includes members of several molecular species (Dawson, 2003). A. aurita populations occurring in the North Atlantic and adjacent seas are probably adapted to the temperature regime in these areas (Dawson, 2003), and therefore, our results reflect the strobilation behaviour of polyps from this temperature regime only.
The tests of this study showed shorter strobilation duration and higher ephyra production per strobila of C. capillata polyps at warmer temperatures. On the other hand, cold winter temperatures had positive effects on strobilation in C. capillata polyps; strobilation started earlier at colder temperatures, and unlike A. aurita, ephyra production was not inhibited by the cold winter temperature (5°C). In these experiments, C. capillata was the only species without any strobilation during 22 months at the warm temperature of 15°C. Strobilation induction of C. capillata may strictly depend on a temperature decrease, which may explain why the distribution of the species is limited to northern boreal areas (Russell, 1970), whereas Cyanea medusae from the warmer U.S. Atlantic, the Gulf of Mexico and Australian waters may represent other species of Cyanea (Bayha, 2005; Dawson, 2005). In the North Sea, C. capillata medusae are rare in the southern part and do not appear in the English Channel. In the Irish Sea, their occurrence also is limited to the northern part (Russell, 1970; Hay et al., 1990; Doyle et al., 2007). The simulated annual seasons in present experiments induced ephyra production from February until June, which matched field observations on C. capillata strobilation and occurence of the ephyrae in areas with a similar temperature regime (Hartlaub, 1894; Verwey, 1942; Gröndahl, 1988). Although the polyps are obviously adapted to cold temperature conditions, they have not been reported from the southern or eastern Baltic Sea, where the C. capillata medusae appear each summer (Möller, 1980a; Barz et al. 2006). Recent studies demonstrated that C. capillata polyps are able to strobilate at a low salinity of 12 and may be more widespread in the Baltic Sea than previously thought (Holst & Jarms, 2010). In accordance with previous studies (Gröndahl, 1988), the results of this study confirm that C. capillata polyps are tolerant of cold temperatures, indicating that the low Baltic Sea winter temperatures probably do not limit their distribution. I therefore believe that the only reason why the polyps have not been found in this area to date is that there were too few efforts undertaken to find them.
The medusae of C. lamarckii have a more southern distribution in the North and Irish seas than do C. capillata medusae (Russell, 1970; Hay et al., 1990; Doyle et al., 2007). In the Baltic Sea, C. lamarckii medusae appear rarely in Danish waters (Rasmussen, 1973), whereas they occur periodically from March until June off the Swedish west coast (Gröndahl, 1988). C. lamarckii ephyrae were not found in 4 years of plankton sampling on the Swedish west coast and no strobilation was observed on settling plates in the field, leading to the conclusion that C. lamarckii polyps do not strobilate there (Gröndahl, 1988). C. lamarckii polyps have not yet been described in their natural habitat, but present laboratory studies indicate positive effects of warm temperature on strobilation: higher production of ephyrae per strobila and shorter strobilation duration. This may enable their distribution to expand to the northern North Sea with rising winter temperatures due to climate changes. From there, the polyps may also extend into the Baltic Sea because of their high tolerance of low salinity (Holst & Jarms, 2010). The present experiments demonstrated a long strobilation phase of C. lamarckii polyps from winter until the next summer in agreement with observations of abundant C. lamarckii medusae of various sizes and different developmental stages from spring until late summer in the German Bight and off the Dutch coast (Verwey, 1942; Künne, 1952).
Ch. hysoscella medusae also are distributed mainly in the southern North and Irish seas (Russell, 1970; Hay et al., 1990; Doyle et al., 2007). Verwey (1942), suggested strobilation of Ch. hysoscella with increasing temperature in spring and summer, but the polyps have not yet been found in their natural habitat in the North Sea. A mild winter in 1988 in the German Bight was followed by early appearance and high abundance of Ch. hysoscella medusae in the summer, leading to the conclusion that polyp's survival during the winter was higher at warm temperatures (Merck, 1990). These observations agree with experimental results of this study showing very low ephyra production at cold winter temperatures and shorter strobilation duration at warmer temperatures. Ch. hysoscella planulae are able to settle at salinities at least as low as 20 (Holst & Jarms, 2010), and thus Ch. hysoscella medusae and polyps may be able to spread from the North Sea into the Baltic Sea with continued climate warming.
The number of ephyrae produced by each strobila is affected by temperature, other abiotic factors, food supply and polyp size (Russell, 1970; Purcell et al., 1999). The results of this study agree with those of previous laboratory experiments on polyp cultures demonstrating that warm temperatures increase the strobilation rates of polyps and ephyra production, except at very high temperatures (Purcell et al., 1999, 2012; Purcell, 2007; Liu et al., 2009). The number of ephyrae in the strobila increases with polyp's size (Russell, 1970). This may explain why the C. capillata polyps in the present experiments were able to produce more ephyrae by the second year when they grew to a larger size. Salinity also is known to affect strobilation rates of North Sea Scyphozoa (Holst & Jarms, 2010) and other species (reviewed in Purcell et al., 2009). Purcell et al. (2009) concluded that the combined effects of temperature, salinity, light and food determined the amount and time of strobilation in situ.
Only a few recent studies have monitored polyps in situ (see Purcell et al., 2009; Di Camillo et al., 2010; Ishii & Katsukoshi, 2010). Climatic changes in the North Sea related to the North Atlantic Oscillation (NAO) affect jellyfish abundances and may also affect the strobilation of the benthic polyps in this area (Lynam et al., 2010); however, the effects of the NAO depends on the depth that macrozoobenthos animals occur, and therefore, the estimation of the NAO's effect on strobilation is not possible without knowing the locations and depths of polyp habitats (Lynam et al., 2005). The effect of the NAO on North Sea polyps is likely to be high because strobilation occurs mainly in winter and spring when the NAO's influence is the greatest in the North Sea (Lynam et al., 2010). The results of the present study support the idea that variable winter temperatures affect the strobilation activity of North Sea Scyphozoa.
Increasing winter temperature probably will affect the abundances and distributions of scyphozoan jellyfish species in the North Sea. The more southerly species, C. lamarckii and Ch. hysoscella could expand to the northern parts of the North Sea and possibly into the Baltic Sea. The adaptable species A. aurita presumably will benefit from warmer temperatures, having longer strobilation periods (present study), faster growth due to higher feeding rates (Hansson, 1997; Widmer, 2005) and higher reproduction rates of medusae (Ishii & Takagi, 2003). The cold water C. capillata might be the only North Sea scyphozoan that could suffer from warmer temperatures; however, C. capillata ephyra production also occurred at the warm winter temperature of 10°C (Fig. 1). In general, I assume that the abundances of scyphozoan jellyfish in the North and Baltic seas will increase in future years if the water temperatures continue to increase as predicted (Belkin, 2009). This assessment agrees with the opinion of other authors suggesting an increase of gelatinous predators (scyphomedusae, hydromedusae, siphonophores and ctenophores) in the North Sea due to climate changes, including increasing temperatures, the reduction of the ocean pH and probably, the increasing Atlantic inflow into the North Sea (Attrill et al., 2007; Boersma et al., 2007; Doyle et al., 2008; Lilley et al., 2009; Licandro et al., 2010). The results of this study and previous studies clearly show the linkage between physical environmental factors and ephyra production and thus forecasts of the abundance and distribution of scyphomedusae might be possible by circulation models in future (Johnson et al., 2001, 2005; Barz et al., 2006). More knowledge on the locations of polyp habitats and on the reproduction cycles of the benthic polyp stage in the field is necessary for successful monitoring and understanding the population dynamics in scyphozoan jellyfish.
References
Attrill, M. J., J. Wright & M. Edwards, 2007. Climate-related increases in jellyfish frequency suggest a more gelatinous future for the North Sea. Limnology and Oceanography 52: 480–485.
Barz, K. & H.-J. Hirche, 2007. Abundance, distribution and prey composition of scyphomedusae in the southern North Sea. Marine Biology 151: 1021–1033.
Barz, K., H.-H. Hinrichsen & H.-J. Hirche, 2006. Scyphozoa in the Bornholm Basin (central Baltic Sea) − the role of advection. Journal of Marine Systems 60: 167–176.
Bayha, K. M., 2005. The molecular systematics and population genetics of four coastal ctenophores and scyphozoan jellyfish of the United States Atlantic and Gulf of Mexico. Dissertation, University of Delaware: 283 pp.
Belkin, I. M., 2009. Rapid warming of large marine ecosystems. Progress in Oceanography 81: 207–213.
Boero, F., J. Boullion, C. Gravilli, M. P. Miglietta, T. Parsons & S. Piraino, 2008. Gelatinous plankton: irregularities rule the world (sometimes). Marine Ecology Progress Series 356: 299–310.
Boersma, M., A. M. Malzahn, W. Greve & J. Javidpour, 2007. The first occurrence of the ctenophore Mnemiopsis leidyi in the North Sea. Helgoland Marine Research 61: 153–155.
Brewer, R. H. & J. S. Feingold, 1991. The effect of temperature on the benthic stages of Cyanea (Cnidaria: Scyphozoa), and their seasonal distribution in the Niantic River estuary, Connecticut. Journal of Experimental Marine Biology and Ecology 152: 49–60.
CIESM, 2001. Gelatinous zooplankton outbreaks: theory and practice. CIESM Workshop Series 14: 112 pp.
Dawson, M. N., 2003. Macro-morphological variation among cryptic species of the moon jellyfish, Aurelia (Cnidaria: Scyphozoa). Marine Biology 143: 369–379.
Dawson, M. N., 2005. Cyanea capillata is not a cosmopolitan jellyfish: morphological and molecular evidence for C. annaskala and C. rosea (Scyphozoa: Semaeostomeae: Cyaneidae) in south-eastern Australia. Invertebrate Systematics 19: 361–370.
Di Camillo, C. G., F. Betti, M. Bo, M. Martinelli, S. Puce & G. Bavestrello, 2010. Contribution to the understanding of seasonal cycle of Aurelia aurita (Cnidaria: Scyphozoa) scyphopolyps in the northern Adriatic Sea. Journal of the Marine Biological Association of the United Kingdom 90: 1105–1110.
Doyle, T. K., J. D. R. Houghton, S. M. Buckley, G. C. Hays & J. Davenport, 2007. The broad-scale distribution of five jellyfish species across a temperate coastal environment. Hydrobiologia 579: 29–39.
Doyle, T. K., H. De Haas, D. Cotton, B. Dorschel, V. Cummins, J. D. R. Houghton, J. Davenport & G. C. Hays, 2008. Widespread occurrence of the jellyfish Pelagia noctiluca in Irish coastal and shelf waters. Journal of Plankton Research 30: 963–968.
Gröndahl, F., 1988. A comparative ecological study on the scyphozoans Aurelia aurita, Cyanea capillata and Cyanea lamarckii in the Gullmar Fjord, western Sweden. Marine Biology 97: 541–550.
Hansson, L. J., 1997. Effect of temperature on growth rate of Aurelia aurita (Cnidaria, Scyphozoa) from Gullmarsfjorden, Sweden. Marine Ecology Progress Series 161: 145–153.
Hartlaub, C., 1894. Die Coelenteraten Helgolands. Wissenschaftliche Meeresuntersuchungen Helgoland Neue Folge 1: 161–206.
Hay, S. J., J. R. G. Hislop & A. M. Shanks, 1990. North Sea scyphomedusae; summer distribution, estimated biomass and significance particularly for 0-Group gadoid fish. Netherlands Journal of Sea Research 25: 113–130.
HELCOM, 2007. Climate change in the Baltic Sea Area – HELCOM thematic assessment in 2007. Baltic Sea Environment Proceedings 111: 1–49.
Hernroth, L. & F. Gröndahl, 1985. On the biology of Aurelia aurita (L.): 2. Major factors regulating the occurrence of ephyrae and young medusae in the Gullmar Fjord, western Sweden. Bulletin of Marine Science 37: 567–576.
Holst, S. & G. Jarms, 2007. Substrate choice and settlement preferences of planula larvae of five Scyphozoa (Cnidaria) from German Bight, North Sea. Marine Biology 151: 863–871.
Holst, S. & G. Jarms, 2010. Effects of low salinity on settlement and strobilation of Scyphozoa (Cnidaria): is the lion’s mane Cyanea capillata (L.) able to reproduce in the brackish Baltic Sea? Hydrobiologia 645: 53–68.
Holst, S., I. Sötje, H. Tieman & G. Jarms, 2007. Life cycle of the rhizostome jellyfish Rhizostoma octopus (L.) (Scyphozoa, Rhizostomeae), with studies on cnidocysts and statoliths. Marine Biology 151: 1695–1710.
IPCC, 2007. Summary for policymakers. In Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor & H. L. Miller (eds), Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Asessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
Ishii, H. & K. Katsukoshi, 2010. Seasonal and vertical distribution of Aurelia aurita polyps on a pylon in the innermost part of Tokyo Bay. Journal of Oceanography 66: 329–336.
Ishii, H. & A. Takagi, 2003. Development time of planula larvae on the oral arms of the scyphomedusa Aurelia aurita. Journal of Plankton Research 25: 1447–1450.
Jarms, G., 2010. The early life history of Scyphozoa with emphasis on Coronatae a review with a list of described life cycles. Verhandlungen des Naturwissenschaftlichen Vereins Hamburg 45: 17–31.
Johnson, D. R., H. M. Perry & W. D. Burke, 2001. Developing jellyfish strategy hypotheses using circulation models. Hydrobiologia 451: 213–221.
Johnson, D. R., H. M. Perry & W. M. Graham, 2005. Using nowcast model currents to explore transport of non-indigenous jellyfish into the Gulf of Mexico. Marine Ecology Progress Series 305: 139–146.
Kändler, R., 1961. Über das Vorkommen von Fischbrut, Decapodenlarven und Medusen in der Kieler Förde. Kieler Meeresforschung 17: 48–65.
Korringa, P., 1953. The shell of Ostrea edulis as a habitat. Archives Neerlandaises de Zoologie 10: 32–59.
Künne, C., 1952. Untersuchungen über das Großplankton in der Deutschen Bucht und im Nordsylter Wattenmeer. Helgoländer Wissenschaftliche Meeresuntersuchungen 4: 1–54.
Licandro, P., D. V. P. Conway, M. N. Daly Yahia, M. L. Fernandez De Puelles, S. Gasparini, J. H. Hecq, P. Tranter & R. R. Kirby, 2010. A blooming jellyfish in the northeast Atlantic and Mediterranean. Biology Letters 6: 688–691.
Lilley, M. K. S., J. D. R. Houghton & G. C. Hays, 2009. Distribution, extent of inter-annual variability and diet of the bloom-forming jellyfish Rhizostoma in European waters. Journal of the Marine Biological Association of the United Kingdom 89: 39–48.
Liu, W.-C., W.-T. Lo, J. E. Purcell & H.-H. Chang, 2009. Effects of temperature and light intensity on asexual reproduction of the scyphozoan, Aurelia aurita (L.) in Taiwan. Hydrobiologia 616: 247–258.
Lucas, C. H., 1996. Population dynamics of Aurelia aurita (Scyphozoa) from an isolated brackish lake, with particular reference to sexual reproduction. Journal of Plankton Research 18: 987–1007.
Lucas, C. H., A. G. Hirst & J. A. Williams, 1997. Plankton dynamics and Aurelia aurita production in two contrasting ecosystems: comparison and consequences. Estuarine, Coastal and Shelf Science 45: 209–219.
Lynam, C. P., S. J. Hay & A. S. Brierley, 2005. Jellyfish abundance and climatic variation: contrasting responses in oceanographically distinct regions of the North Sea, and possible implications for fisheries. Journal of the Marine Biological Association of the United Kingdom 85: 435–450.
Lynam, C. P., M. J. Attrill & M. D. Skogen, 2010. Climatic and oceanic influences on the abundance of gelatinous zooplankton in the North Sea. Journal of the Marine Biological Association of the United Kingdom 90: 1153–1159.
Merck, T., 1990. Untersuchungen zur ökologischen Nische von Chrysaora hysoscella. Jahresbericht der Biologischen Anstalt Helgoland 1989: 53–54.
Mieszkowska, N., R. Leaper, P. Moore, M. A. Kendall, M. T. Burrows, D. Lear, E. Poloczanska, K. Hiscock, P. S. Moschella, R. C. Thompson, R. J. Herbert, D. Lafolley, J. Baxter, A. J. Southward & S. J. Hawkins, 2006. Marine biodiversity and climate change. Assessing and predicting the influence of climate change using intertidal rocky shore biota. Final report for United Kingdom funders. Marine Biological Association Occasional Publications 20: 1–53.
Miyake, H., M. Terazaki & Y. Kakinuma, 2002. On the polyps of the common jellyfish Aurelia aurita in Kagoshima Bay. Journal of Oceanography 58: 451–459.
Molinero, J. C., M. Casini & E. Buecher, 2008. The influence of the Atlantic and regional climate variability on the long-term changes in gelatinous carnivore populations in the northwestern Mediterranean. Limnology and Oceanography 53: 1456–1467.
Möller, H., 1980a. A summer survey of large zooplankton, particularly scyphomedusae, in North Sea and Baltic. Meeresforschung 28: 61–68.
Möller, H., 1980b. Population dynamics of Aurelia aurita medusae in Kiel Bight, Germany (FRG). Marine Biology 60: 123–128.
Palmén, E., 1954. Seasonal occurrence of ephyrae and subsequent instars of Aurelia aurita (L.) in the shallow waters of Tvärminne, S. Finland. Archivum Societatis Zoologicae Botanicae Fennicae Vanamo 8: 122–138.
Pierce, J., 2009. Prediction, location, collection and transport of jellyfish (Cnidaria) and their polyps. Zoo Biology 28: 163–176.
Purcell, J. E., 2005. Climate effects on formation of jellyfish and ctenophore blooms: a review. Journal of the Marine Biological Association of the United Kingdom 85: 461–476.
Purcell, J. E., 2007. Environmental effects on asexual reproduction rates of the scyphozoan Aurelia labiata. Marine Ecology Progress Series 348: 183–196.
Purcell, J. E., 2009. Extension of methods for jellyfish and ctenophore trophic ecology to large-scale research. Hydrobiologia 616: 23–50.
Purcell, J. E., 2011. Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Annual Review of Marine Science. doi:10.1146/annurev-marine-120709-142751.
Purcell, J. E., J. R. White, D. A. Nemazie & D. A. Wright, 1999. Temperature, salinity and food effects on asexual reproduction and abundance of the scyphozoan Chrysaora quinquecirrha. Marine Ecology Progress Series 180: 486–487.
Purcell, J. E., S.-I. Uye & W.-T. Lo, 2007. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: a review. Marine Ecology Progress Series 350: 153–174.
Purcell, J. E., R. A. Hoover & N. T. Schwarck, 2009. Interannual variation of strobilation by the scyphozoan Aurelia labiata in relation to polyp density, temperature, salinity, and light conditions in situ. Marine Ecology Progress Series 375: 139–149.
Purcell, J. E., D. Atienza, V. Fuentes, A. Olariaga, U. Tilves, C. Colahan, J.-M. Gili, 2012. Temperature effects on asexual reproduction rates of scyphozoan polyps from the northwest Mediterranean Sea. Hydrobiologia, this issue. doi:10.1007/s10750-012-1047-7.
Rasmussen, E., 1973. Systematic and ecology of the Isefjord marine fauna (Denmark). Ophelia 11: 1–507.
Richardson, A. J., A. Bakun, G. C. Hays & M. J. Gibbons, 2009. The jellyfish joyride: causes, consequences and management responses to a more gelatinous future. Trends in Ecology & Evolution 24: 312–322.
Russell, F. S., 1970. The medusae of the British Isles II. Pelagic Scyphozoa with a supplement to the first volume on hydromedusae. Cambridge University Press, Cambridge.
Sabatés, A., F. Pagès, D. Atienza, V. Fuentes, J. E. Purcell & J.-M. Gili, 2010. Planktonic cnidarian distribution and feeding of Pelagia noctiluca in the NW Mediterranean Sea. Hydrobiologia 645: 153–165.
Sötje, I. & G. Jarms, 2009. Derivation of the reduced life cycle of Thecoscyphus zibrowii Werner, 1984 (Cnidaria, Scyphozoa). Marine Biology 156: 2331–2341.
Thiel, M. E., 1938. Scyphomedusae. In Bronn, H. G. (ed.), Dr. H. G. Bronns Klassen und Ordnungen des Tierreichs. Akademische Verlagsgesellschaft, Leipzig.
Thiel, H., 1962. Untersuchungen über die Strobilisation von Aurelia aurita LAM. an einer Population der Kieler Förde. Kieler Meeresforschungen 18: 198–230.
Tronolone, V. B., A. C. Morandini & A. E. Bigotto, 2002. On the occurrence of scyphozoan ephyrae (Cnidaria, Scyphozoa, Semaestomeae and Rhizostomeae) in the southeastern Brazilian coast. Biota Neotropica 2: 1–18.
Verwey, J., 1942. Die Periodizität im Auftreten und die aktiven und passiven Bewegungen der Quallen. Archives Neerlandaises de Zoologie 6: 363–468.
Widmer, C. L., 2005. Effects of temperature on growth of north-east Pacific moon jellyfish ephyrae, Aurelia labiata (Cnidaria: Scyphozoa). Journal of the Marine Biological Association of the United Kingdom 85: 569–573.
Willcox, S., N. Moltschaniwskyj & C. Crawford, 2007. Asexual reproduction in scyphistomae of Aurelia sp.: effects of temperature and salinity in an experimental study. Journal of Experimental Marine Biology and Ecology 353: 107–114.
Wiltshire, K. H. & B. F. J. Manly, 2004. The warming trend at Helgoland Roads, North Sea: Phytoplankton response. Helgoland Marine Research 58: 269–273.
Wiltshire, K. H., A. Kraberg, I. Bartsch, M. Boersma, H.-D. Franke, J. Freud, C. Gebühr, G. Gerdts, K. Stockmann & A. Wichels, 2010. Helgoland Roads, North Sea: 45 years of change. Estuaries and Coasts 33: 295–310.
You, K., C. Ma, H. Gao, G. Li, M. Zhang, B. Wang & R. Wei, 2008. The effects of temperature decrease on the scyphistomae strobilation of Jellyfish, Rhopilema esculentum Kishinouye. Journal of the World Aquaculture Society 39: 706–711.
Acknowledgments
I thank PD Dr. Gerhard Jarms for his support, for imparting his knowledge on polyp culturing and for providing the research facilities. I am grateful to the Biologische Anstalt Helgoland (Alfred Wegener Institute) for providing the guest laboratory and I am thankful for the assistance of students and technicians of the Biocenter Grindel. I thank Drs. Ilka Sötje, Jennifer Purcell, and the reviewers for their helpful comments on the manuscript, and Dr. Caroline Stolter for her help in the statistics. This study is part of a Ph. D. thesis conducted at the Biocenter Grindel and Zoological Museum in Hamburg supported by EUROGEL (EUROpean GELatinous Zooplankton, European Commission Contract no. EVK-CT-2002-00074).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: J. E. Purcell, H. Mianzan & J. R. Frost / Jellyfish Blooms: Interactions with Humans and Fisheries
Rights and permissions
About this article
Cite this article
Holst, S. Effects of climate warming on strobilation and ephyra production of North Sea scyphozoan jellyfish. Hydrobiologia 690, 127–140 (2012). https://doi.org/10.1007/s10750-012-1043-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1043-y