Abstract
The Senegalese sole, Solea senegalensis, originates from subtropical waters and displays great adaptability to environmental factors such as temperature. A comprehensive study on the effect of long-term temperature acclimation on xenobiotic metabolism, along with the assessment of other parameters related to physiological status, was designed to characterize the response of this species to temperature fluctuations within a realistic range. S. senegalensis juveniles were acclimated for a period of 60 days to two different ambient temperatures, 15 and 20 °C. Several hepatic, gill, muscular and plasmatic parameters were measured over time at the two temperatures. The lower temperature triggered, over time, the synthesis of hepatic microsomal cytochrome P450-related enzymes (e.g. 7-ethoxyresorufin O-deethylase (EROD), carboxylesterases, and the conjugating enzyme uridine diphosphate glucuronosyltransferase and, more significantly, EROD activity in gills. The antioxidant enzyme activities: catalase and glutathione reductase in liver were positively correlated to temperature. Plasmatic parameters (glucose, lactate, triglycerides and osmolality) were consistent with a good physiological status of the experimental fish. The expression of heat shock proteins in muscle did not significantly change in the two temperature groups. The results evidenced that the subtropical species S. senegalensis also uses the temperature compensation strategy to different degrees for most biotransformation enzymes; this response was more intense and faster in gills than in liver. This compensatory strategy did not apply to antioxidant enzymes and GST. The present findings highlight the need to consider the thermal history of the fish when using S. senegalensis as a sentinel in a biomarker-based pollution monitoring study. The fish plasticity on its strategy of physiological adaptation to temperature changes could contribute to explain the success in the geographical expansion of this species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature is a crucial abiotic factor that has a major impact on the physiological processes of ectothermic species, including fish. Although adaptation in their metabolism and physiology to compensate for environmental temperature fluctuations has been long described, it has become especially relevant in terms of climate change (Pörtner 2002; Pörtner et al. 2005; Somero 2010, 2012). Temperature-driven changes trigger many regulatory processes in order to maintain homoeostasis. One of the best known mechanisms involved in biochemical adaptation to lower temperatures is the enhancement in protein synthesis. Several studies have already demonstrated that under a fixed assay temperature, organisms acclimated to lower temperatures display higher enzymatic activities to compensate for lower reaction rates occurring as a consequence of the Arrhenius’ law. This is achieved by increasing enzyme synthesis, and it also embraces enzymes involved in xenobiotic metabolism (Carpenter et al. 1990; Fitzsimmons et al. 2007; West et al. 1999 and references within).
Senegalese sole (Solea senegalensis, Kaup 1858) displays a great environmental adaptability as it originates from subtropical waters but has expanded to more temperate northern latitudes and into the Mediterranean (Zenetos et al. 2012). In addition to temperature adaptation, it also tolerates wide salinity variations as it inhabits marine and estuarine areas (Vinagre et al. 2006, 2013). All these characteristics and its high economic value favour its use for aquaculture purposes in Southern Europe (Dinis et al. 1999). Moreover, it has recently been used as a sentinel for pollution assessment in laboratory experiments and field monitoring in the Iberian Peninsula (Costa et al. 2012; Fonseca et al. 2011a, b; Gonçalves et al. 2013; López-Galindo et al. 2010a, b; Oliva et al. 2010, 2012; Siscar et al. 2013). To date, most studies on temperature acclimation have been carried out in fish from higher latitudes where ambient temperatures are usually low. Only recently, S. senegalensis has been used as a model subtropical species to study (1) the influence of rearing temperature, together with diet composition, on oxidative stress parameters (Castro et al. 2012), (2) the influence of acclimation temperature alone on the osmoregulatory capacity and thyroidal system (Arjona et al. 2010) and (3) the effect of temperature on plasmatic metabolites and amino acid composition (Costas et al. 2012).
As a subtropical species, S. senegalensis is expected to display an ample temperature window in which it can operate at high performance. Thermal tolerance encompasses adjustments at several biological levels from systemic to molecular (Pörtner 2002; Pörtner et al. 2005). At the systemic level, plasmatic metabolite values such as glucose, lactate, triglycerides and lactate dehydrogenase activity (LDH) are indicative of energetic metabolism (Bonga 1997) and osmolality values are indicative of osmoregulation capacity (McCormick 2001). Changes in water temperature can also generate modifications at the cellular and molecular level that can be assessed by the measurement of biochemical responses. They include hepatic enzymes involved in the phase I step of xenobiotic metabolism such as activities of 7-ethoxyresorufin O-deethylase (EROD), 3-cyano-7-ethoxycoumarin O-deethylase (CECOD) and carboxylesterase (CbE), the phase II conjugation step of uridine diphosphate glucuronosyltransferase (UDPGT) and glutathione S-transferase (GST) activities (van der Oost et al. 2003). Although most enzymes involved in xenobiotic metabolism are more represented in liver than in gills, in some cases, the magnitude of the change is similar or higher in gills (López-Galindo et al. 2010a, b; Regoli et al. 2011).
Additionally, the expression of heat shock proteins (HSP) is associated with temperature variations. HSP70 are ubiquitous and highly conserved, and they are involved in the protection and repair of the cells in response to stress and harmful conditions to ensure the proper folding and cell compartimentation of proteins (Tomanek and Somero 1999).
The aim of the study was to evaluate in a model subtropical species, S. senegalensis, the effect of long-term temperature acclimation (15 and 20 °C) on a comprehensive set of parameters involved either in xenobiotic biotransformation, neural transmission, antioxidant defences and other metabolic processes, in order to characterize the response of this species to temperature fluctuations within a realistic range. The promptness and magnitude of the changes are of particular interest as they can be indicative of biochemical and physiological plasticity which, in turn, can be of relevance for predicting the performance of this species in a changing anthropogenic environment, as well as for explaining its geographical expansion.
Materials and methods
Fish obtaining and maintenance
Senegalese sole juveniles were hatched and reared under aquaculture conditions by Stolt Sea Farm SA (La Coruña, Spain). Specimens of about 150–200 g wet weight were transported and maintained in the University of Valencia facilities. To ensure adaptation after transport and to the new laboratory conditions, the temperature acclimation experiment started 2 months after arrival. Twenty-four fish at each temperature regime either 15 or 20 °C were distributed into 4 round tanks of 3 m3 each. Physical water conditions were checked daily and maintained at the targeted temperatures. Ambient temperature was 20 °C, and the desired 15 °C was reached by lowering the temperature at a rate of about 1 °C/day. The water parameters from the start of the experiment (t 0) and for a period up to 60 days (t 60) for the 15 °C group were as follows: Tª 15.8 ± 1.8 °C; salinity 29.0 ± 0.9 psu and O2 98.3 ± 1.7 % and for the 20 °C group: Tª 19.1 ± 0.6 °C; salinity 29.6 ± 1.2 psu and O2 97.8 ± 1.3 %. An intermediate sampling was conducted on day 30 (t 30). The light regime was 12L:12D, and fish were fed daily ad libitum by hand with commercial Le-5-Elite trout pellet (Skretting, UK), but fasted for 24 h prior to the sampling of eight fish (2 from each 4 replicate tanks) for each time and temperature condition. No lower temperatures (<15 °C) were attempted as S. senegalensis stopped feeding at about 12 °C (Arjona et al. 2010), and at higher temperatures (>20 °C), there was higher risk of bacterial infection under long-term confinement conditions.
Fish sampling
Before killing, biometrics such as total length and weight was recorded and blood was withdrawn from the caudal vein using a 1-mL heparinized syringe. Plasma was obtained after 3,000g × 15 min centrifugation at 4 °C. Fish were killed by spinal cord severing, following which the fish were dissected and their organs flash frozen in liquid nitrogen. Livers and gonads were also weighed to calculate gross morphometric indexes. Tissues were stored at −80 °C until analysis. Handling of the fish was done according to national and institutional regulations of the Spanish Council for Scientific Research (CSIC) and the European Directive 2010/63/EU.
Hepatic, gill and muscle S10 and liver microsomes preparation
Livers (≈1 g) or gills (≈0.3 g) were homogenized in ice-cold buffer phosphate (100 mM pH 7.4) containing 150 mM KCl, 1 mM dithiothreitol (DTT), 0.1 mM phenanthroline, 0.1 mg/mL trypsin inhibitor and 1 mM ethylenediaminetetraacetic acid (EDTA) in a 1:4 (w:v) ratio using a polytron® blender. A portion of muscle (≈0.3 g) was homogenized in ice-cold buffer phosphate (50 mM pH 7.4) alone. The homogenates obtained were centrifuged at 10,000g × 30 min (S10). In the case of liver, to further obtain microsomes, another step of 100,000g × 60 min at 4 °C was required. The microsomal pellet obtained was dissolved in the above homogenization buffer, which also contained 20 % glycerol in a 2:1 (w:v) ratio (Koenig et al. 2013).
Liver, gill and muscle biomarkers
Assay conditions were as individually described, and only the sample volume was changed in order to maintain linearity in the enzymatic measurements. All assays were carried out in triplicate at 25 or 30 °C in 96-well plates using a TECAN Infinite M200 microplate reader, as individually indicated.
7-ethoxyresorufin (EROD) and 3-cyano-ethoxycoumarin (CECOD) O-deethylase activities. Fifty microlitres (µL) of undiluted liver or gill S10 samples were used for EROD measurements and 10 µL of microsomes for EROD and CECOD measures. All samples were incubated at 30 °C with a reaction mixture containing: 0.2 mM NADPH, 3.3 μM 7-ethoxyresorufin (ER) or 10 μM 3-cyano-7-ethoxycoumarin (CEC) in 100 mM phosphate buffer pH 7.4 (Smith and Wilson 2010). The reaction was followed over resorufin or 3-cyano-7-hydroxy-4-methylcoumarin (metabolites) formation for 10 min with a 96-well plate using the fluorescence mode. EROD was measured at 537 nm EX/583 nm EM and CECOD at 408 nm EX/455 nm EM. In both cases, a six-point standard of the respective metabolites (0–160 nM) was used to relate activity in pmol/min/mg protein.
Carboxylesterase activity was measured in gill (5-fold diluted), liver S10 (20-fold diluted) and microsomes (40-fold diluted). Briefly, 25 µL of sample and 200 µL of αNA as substrate (250 µM final concentration in well) were measured during 5 min at 235 nm as described elsewhere (Mastropaolo and Yourno1981). Activity was expressed in nmol/min/mg protein.
Uridine diphosphate glucuronosyltransferase determination was performed in the microsomal fraction according to the published method (Collier et al. 2000). Assay conditions were 10 µL of microsomes and 0.1 mM 4-methyl umbelliferone (MU) in 100 mM Tris buffer pH 7.4 (containing 5 mM MgCl2 and 0.05 % BSA) and 2 mM 5′-diphospho-glucuronic acid (UDPGA). The decrease in fluorescence was recorded over 10 min at 30 °C, and an eight point MU standard (0–16 µM) was used to relate the activity. Fluorimeter conditions were 355 nm EX/460 nm EM. Results were expressed in nmol/min/mg prot.
Glutathione S-transferase activity was measured using 25 µL of 5-fold diluted gill and 20-fold diluted liver S10 using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate. The final reaction mixture contained 1 mM CDNB and 1 mM reduced glutathione (GSH). The activity rate was measured for 5 min at 340 nm (Habig et al. 1974) and expressed as nmol/min/mg protein.
Antioxidant enzyme activities: catalase (CAT), total glutathione peroxidase (t-GPX) and glutathione reductase (GR) were determined in the S10 fraction of the liver according to the methodology described previously (Koenig and Solé 2012). CAT was also measured in gills (S10) and quantified as a decrease in absorbance at 240 nm using H2O2 (50 mM) as substrate, t-GPX and GR as decreases at 340 nM using cumene hydroperoxide (CHP; 0.625 mM) and oxidized glutathione (GSSG; 0.9 mM) as respective substrates and NADPH as cofactor in both assays. Activity was expressed as µmol/min/mg prot (CAT) or nmol/min/mg prot (t-GPX and GR).
Lactate dehydrogenase activity was measured in liver S10 according to the method developed by Vassault (1983), modified to 96-well microplate format. A 25-µL aliquot of S10 was mixed with 150 µL NADH (300 µM) and 50 µL pyruvate (4.5 mM) and the decrease in absorbance measured over 3 min at 340 nm. Activity was expressed as nmol/min/mg prot.
Acetylcholinesterase (AChE) activity was measured in undiluted S10 muscle and gills tissue. In each microplate well, 25 μL of sample was mixed with 150 μL of 5,5′-dithio-bis-2-nitrobenzoat (DTNB; 270 μM), and after 2 min of preincubation, the reaction was started by adding 50 μL of the substrate acetylthiocholine (ASCh; 1 mM final concentration). Reading was performed in kinetic mode at 405 nm for 5 min following the Ellman et al. (1961) protocol. Activity was expressed in nmol/min/mg protein.
Total protein content of the samples for all enzymatic assay calculations was determined by the Bradford method (1976) adapted to microplate, using Bradford Bio-Rad Protein Assay reagent and bovine serum albumin (BSA) as standard. Absorbance was read at 595 nm.
Protein yield (PY) was expressed as the total protein content per gram of wet weight tissue.
Lipid peroxidation (LP) was determined using 200 μL of muscle S10 and mixed with 650 μL of 1-methyl-2-phenylindole in methanol:acetonitrile (1:3) and 150 μL of 37 % HCl. Incubation was performed at 45 °C for 40 min, and the reaction was stopped in ice and further centrifuged at 13,000g × 10 min to precipitate proteins. Absorbance was read at 586 nm versus a standard solution of 1,1,3,3-tetramethoxypropane (MDA) treated in the same manner. LP content was expressed as nmol MDA/g w.w.
Heat shock proteins (HSP70) were measured in muscle. Tissues were homogenized, using a polytron blender, in 1:10 (w:v) cold calcium–magnesium-free saline buffer containing 20 mM Hepes, 500 mM NaCl, 12.5 mM KCl (pH = 7.3), freshly complemented with 1 mM DTT, 1 mM phenymethysulfonyfluoride (PMSF), Igepal (1 %) and 1 % protease inhibitor cocktail (Complete-Mini, EDTA-free ROCHE). Samples were centrifuged at 15,000g for 15 min (4 °C), and the supernatant was kept at −80 °C. Total soluble protein contents were determined using Lowry Bio-Rad DC-Protein Assay kit with BSA as standard, and absorbance was read at 750 nm. Equal amounts of protein (21 µg) from each sample were separated by 1D-SDS-PAGE using a Mini-Protean Tetra cell system (Bio-Rad), for 50 min at 200 V, transferred to a PVDF membrane at 1.3 A for 40 min in a Trans-Blot R Turbo TM Blotting System (Bio-Rad). Immunodetection was performed using HSP70 mouse monoclonal antibody (Sigma, H5147), and anti-mouse IgG secondary antibody conjugated with peroxidase (Sigma, A4416). Blots were visualized on a VERSADOC Imaging system (Bio-Rad) using ELC-PRIME reagent (Amersham) and quantified by densitometry using the Quantity One software (Bio-Rad). The density of each band was normalized to the density of the HSP70 band of a commercial standard (Sigma, H9776) in each blot, and HSP70 levels were expressed as arbitrary units HSP70/ng protein (Varó et al. 2007).
Plasmatic parameters
Glucose and lactate content in plasma were analysed using the SPINREACT commercial kits ref: 41011 and 1001330, respectively, following manufacturer instructions and using 2.5 µL of plasma in a final volume in well of 250 µL. Absorbance was measured at 550 nm using a TECAN plate reader SpectraFluor model. Plasma triglycerides were quantified using the commercial kit SPINREACT ref: 1001312. Sample volume and final volume were as for glucose and lactate but reading was at 490 nm in the Victor2 V plate reader. All results were reported in mmol/L.
Osmolality was measured using the Fiske®, 210 Micro-Sample Osmometer. Instrument calibration was done using a standard solution at a concentration of 290 mOsm/kg H2O.
Statistical analysis
Data were tested for normality (Kolmogorov–Smirnov´s test) and homogeneous variance (Levene´s test) and were log10 (X)-transformed when suitable to comply with normality and homoscedasticity assumptions. Sex or size was not seen as variables to influence the enzymatic activities or HSP70 levels (p > 0.05).
In search of differences among acclimation time groups (0, 30 and 60 days), one-way ANOVA followed by Student–Newman–Keuls (SNK) multi-comparison test was applied. Student´s t test was used to contrast temperature groups (15 and 20 °C). Plasma parameters, except triglycerides, were analysed by one-way ANOVA with the Welch transformation, followed by the Games-Howel´s test for post hoc multi-comparison, in the case of variance heteroscedasticity.
Results were presented as mean ± SEM (standard error). The Pearson product moment correlation was used to test pairwise associations between parameters (n = 48). All statistical analyses were carried out using SPSS Systems (SPSS Inc., v19), and the significance level was always set at α = 0.05.
Results
Biological and gross morphometric parameters
Fish gained in length/weight at a similar rate under both temperature regimes during the experience. The condition factor (CF) was maintained during the 2 months but the hepatosomatic index (HSI) gradually and significantly decreased in the fish group kept at 20 °C, whereas it transitorily increased in the 15 °C acclimated group. The gonadosomatic index (GSI) of males and females differed, as expected, but it was not related to the temperature of acclimation. Biological traits and gross morphometric markers are presented in Table 1.
Liver, gill, muscle and plasma responses
7-ethoxyresorufin O-deethylase activities in liver S10 and in liver microsomal fractions and in gill S10 are presented in Fig. 1. Liver UDPGT, CECOD and CbE activities are shown in Fig. 2, while the remaining enzyme activities determined in the S10 fraction of liver, gill, muscle and in plasma are displayed in Fig. 3.
7-ethoxyresorufin O-deethylase activity in gill S10 and in liver S10 and microsomal fractions of the juvenile S. senegalensis acclimated to two temperatures (15 and 20 °C) over 2 months. Contrasts between the six groups were made after one-way ANOVA with SNK post hoc test and differences indicated by different letters (significance set at p < 0.05). Data are expressed as mean ± SEM
Microsomal UDPGT, CECOD and CbE activities of the liver in juvenile S. senegalensis acclimated to two temperatures (15 and 20 °C) over 2 months. Contrasts between the six groups were made after one-way ANOVA with SNK post hoc test and differences indicated by different letters (significance set at p < 0.05). Data are expressed as mean ± SEM
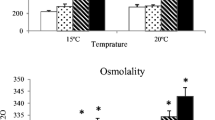
Enzyme activities in the S10 fraction of liver, gill, muscle and plasma. Different letters denote statistical differences (p < 0.05) between groups after one-way ANOVA and SNK post hoc test for each parameter (except glucose, lactate and osmolality done with the Games-Howell post hoc test) between the 6 groups presented in two independent figures of 15 and 20 °C. Beside the legend of the parameter measured, the units are indicated in brackets: (1) nmol/min/mg prot, (2) µmol/min/mg prot, (3) nmol malondialdehide (MDA)/g w.w, (4) arbitrary units/ng prot, (5) mmol/l and (6) mOsm/kg H2O). Data are expressed as mean ± SEM
In Table 2, the two-way ANOVA results (F values) for the above-mentioned parameters to test the effect of temperature, acclimation time and their interaction are presented. In the S10 fraction of the liver, EROD and CbE are significantly affected by temperature, so that lower temperature resulted in an increase of enzyme activities (t test; p < 0.01). The activities of CAT and GR were also influenced by temperature but in the opposite way. Time of acclimation also had an effect on enzymatic activities: GST and CAT increased while t-GPX and EROD decreased over time (one-way ANOVA; p < 0.05). LDH was not dependent on either factor. In the microsomal fraction, EROD, CECOD and UDPGT were affected by temperature and UDPGT by temperature but also by the acclimation time. Microsomal CbE activity differed in relation to temperature only after 60 days acclimation. Of the five parameters measured in gill, EROD and GST activities were dependent on temperature while CAT varied over time. Muscular AChE varied over time being higher at the end of the experiment. It should also be noted that a positive correlation between AChE and fish size was found (r = 0.459; p = 0.001). Neither LP nor HSP70 levels varied depending on the rearing temperature or exposure time.
7-ethoxyresorufin O-deethylase activity was highly responsive to temperature but it also changed over time, and this trend was observed in the two liver subcellular fractions analysed (S10 and microsomes) and in the S10 of gills (Fig. 1). In the microsomal fraction, UDPGT, CECOD and CbE activities were also influenced by temperature (Fig. 2) and this was already evidenced after 30 days in UDPGT while for CECOD and CbE this was not evidenced until day 60. In all cases, activities at 15 °C were higher than at 20 °C. The temporal variation of the remaining parameters analysed in relation to the two temperatures is given in Fig. 3.
As opposed to most hepatic and gill parameters, plasmatic markers were mostly dependent on time of acclimation (Table 2). Glucose and lactate evolved similarly at both temperature regimes (r = 0.458; p = 0.001) reaching a stable condition after 30 days, whereas osmolality showed a sustained decrease over time. Glucose and lactate were lower after 30 days while triglycerides increased at the intermediate time period (p < 0.05). Osmolality decreased significantly (p < 0.05) over time (Fig. 3). Moreover, glucose and osmolality followed an opposite trend (r = −0.296; p = 0.041).
Correlations between hepatic and gill parameters
Table 3 presents the correlation coefficients between the different parameters tested in the two liver fractions and gills (n = 48). There was a good correlation between hepatic S10 and microsomal parameters representative of phase I reactions (from r = 0.350; p < 0.05 to r = 0.767; p < 0.001), between microsomal EROD, CECOD and CbE (r = 0.547–0.826; p < 0.001) and also between phase I parameters and phase II UDPGT activity (r = 0.392–0.505; p < 0.01). On the contrary, the liver antioxidant enzyme CAT was negatively related to EROD (r = −0.502; p < 0.001); but hepatic CAT was also negatively correlated to EROD and GST activities in gill (r = −0.428; p < 0.01 and r = −0.369; p < 0.05). Similarly, the antioxidant GR also was negatively related to most of phase I and II enzymatic activities (r value from −0.362 to −0.556; p < 0.05). Conversely, LDH was positively related to GR and GPX (r = 0.412–0.577; p < 0.05).
Discussion
The similar growth rates observed under the two temperature regimes and the maintenance of a stable CF throughout the experience suggest fish were in good physiological condition. Confinement conditions alone are a stressing factor that can be evaluated by measuring plasmatic metabolites such as glucose and lactate levels and osmolality. The steady levels reached after 30 days of acclimation for glucose and lactate were not mirrored in osmolality, as it continued decreasing at day 60. Nonetheless, in fish, the natural influence of hormonal factors during fish growth and maturation on the physiological osmoregulatory process (McCormick 2001) cannot be disregarded. All the above parameters are suggestive of a good adaptation process in S. senegalensis. In addition, plasmatic metabolite levels (glucose, lactate and triglycerides) and the gross morphometric marker HSI fall within those reported in similar experiences studying temperature effects on juveniles of S. senegalensis (Arjona et al. 2010; Costas et al. 2012). Similarly, LDH activity in liver and HSP70 levels in muscle remained similar throughout the experience, suggesting that S. senegalensis was well adapted to temperatures ranging between 15 and 20 °C in the Mediterranean (Salat and Pascual 2002). Previous work showed that, in fish, threshold temperatures inducing HSP70 expression depended on acclimation temperatures (Feidantsis et al. 2009, 2013). In Sparus aurata, laboratory studies found that expression of HSP70 was induced below 13 °C (Kyprianou et al. 2010) and over 22–24 °C (Feidantsis et al. 2009). It is likely that the response in HSP70 to temperature in this experience had already occurred during the time taken to reach the targeted 15 °C temperature, before the onset of the experiment (t 0). Although HSP production in animals preferentially responds to short-term temperature challenges (Pörtner 2002), variations in HSP levels were also found after long-term exposures but when exposed to greater temperature gradients (Feidantsis et al. 2009).
Temperature had a clear effect on enzymes involved in phase I metabolism. This was more significant in gills and liver EROD activity, with temperature-driven differences up to fivefold after 60-day acclimation. A similar trend, although with more moderate differences, was seen in CECOD and CbE activities in liver. As far as we are aware, no comparable data of similar acclimation studies are available for S. senegalensis or any other subtropical species. Previous studies, focussed on temperate fish, also reported higher basal hepatic EROD activity in those acclimated to lower temperatures (Lange et al. 1998; Jorgensen and Wolkers 1999; Sleiderink et al. 1995) as well as in gill EROD (Abrahamson et al. 2008). All these findings in fish agree with the accepted strategy of enzymatic activity compensation during temperature acclimation that also embraces biotransformation enzymes (Fitzsimmons et al. 2007). The main mechanism proposed for the above-mentioned responses is an increase in protein synthesis at lower temperatures. Recent transcriptional studies with Atlantic salmon give support to an upregulation on genes encoding for CYP1A as temperature decreases (Olsvik et al. 2013). The fivefold increase in gill EROD activity as a consequence of an acclimatation to 5 °C temperature decrease was unexpected, as it would account for a full temperature compensation of an activity with a Q10 of 25, while the Q10 reported for most enzymatic activities is about 2. Other mechanisms, such as temperature-driven membrane fluidity changes in membrane-bound enzymes (homeoviscous adaptation) during temperature acclimation, such as CYP1A-linked EROD activity, cannot be ruled out (Jorgensen and Wolkers 1999).
Despite the well-known effect of temperature on EROD activity, less is known on its modulation of other phase I enzymes or phase II biotransformation reactions in fish. To the best of our knowledge, this is the first study on fish to show a temperature-driven effect on CECOD and CbE activities. It is likely that in Solea spp., the substrates ER (CYP1A1) and CEC (representing CYP1A2 and CYP2C9 and 2C19 in mammals) are metabolized by the same isoform. This is because the CYP1A2 isoform seems to be absent in these species (Koenig et al. 2013) and the CYP2 family in fish greatly differs from that in mammals (Arellano-Aguilar et al. 2009). In any case, the magnitude of change after temperature acclimation when using CEC as substrate was lower. In this occasion, the existence of other unknown CYP isoforms seems possible, which are also responsible for CEC metabolism, but less influenced by temperature. CbE activity measured using the αNA as substrate showed that temperature compensation also affects enzymes involved in pesticide protection in fish (Wheelock et al. 2008) including S. senegalensis (Solé et al. 2012). In relation to phase II enzymes, microsomal UDPGT activity also showed temperature acclimation, reaching a twofold increase after 60 days at the lowest temperature that would account for temperature compensation. GST activity showed a tissue-dependent response pattern. Gill GST also showed temperature compensation, whereas the hepatic form did not. Neither was GST activity in liver altered in rainbow trout reared at the physiological temperatures of 10 and 18 °C for 28 days despite microsomal-related activity (aryl hydrocarbon hydroxylase) being higher at the lowest acclimation temperature (Carpenter et al. 1990).
Antioxidant enzyme (GR and CAT) activities in liver also responded to temperature of acclimation but, in this case, enzyme activities were lower in the fish acclimated to the lowest temperature. In a Portuguese field study with S. senegalensis, CAT activity was also positively related to environmental temperature (Fonseca et al. 2011b). On the contrary, Castro et al. (2012) reported contradictory responses in S. senegalensis in the antioxidant enzymes response to temperature (12 and 18 °C) over a 2 month period; however, in this latter study, where diet was also considered, this was a potential confounding factor. In our laboratory acclimation experience, food administration and the abiotic factors (O2, pressure and salinity) were constant and only temperature was variable; therefore, the present experimental conditions allow for unequivocally testing the influence of temperature alone. It is well known that reactive oxygen species (ROS) production raises as a consequence of increased aerobic metabolism in ectotherms subjected to higher temperatures. Although the temperature regime selected for this study was within the physiological range for this species in the Atlantic and the Mediterranean waters of the Iberian Peninsula (Vinagre et al. 2013; Salat and Pascual 2002), the response observed in the antioxidant enzymes was indicative of their role in preventing the occurrence of oxidative stress damage. In fact, no variation in LP levels was observed as a consequence of temperature variations.
AChE activity in muscle increased, regardless of temperature, during the longest period of acclimation. The influence of temperature acclimation on AChE activity is controversial. While some fish studies have reported a positive effect (Durieux et al. 2011), in others, it was negative (Botte et al. 2013) or not significant (Beauvais et al. 2002). The increase in AChE activity at the end of the experience, when the fish are bigger, contradicts the general trend of lower AChE activity in bigger specimens, also previously described for S. senegalensis (Solé et al. 2012), although the size range in the reported study was wider.
According to correlation analysis, membrane-bound enzyme activities EROD, CECOD, UDPGT and CbE were all positively correlated among themselves in liver microsomes. Also, as expected, microsomal phase I and phase II activities evolved similarly as they represent consecutive steps in biotransformation processes in liver. In a S. senegalensis field survey in the Tejo estuary of Portugal, a good agreement between phase I (EROD) and phase II (GST) was also seen (Fonseca et al. 2011b). Also worth noting is the good correlation in gills between EROD and GST and between EROD and CbE, as it is a target tissue not so widely considered. Furthermore, hepatic EROD activity in the S10 fraction and in microsomes was well correlated (r = 0.767; p < 0.001), which further supports the use of the S10 fraction as a good estimate of EROD activity in this species as previously suggested (Sánchez-Nogué et al. 2013; Solé et al. 2012). The opposite influence of temperature acclimation on the antioxidant enzymes (CAT and GR) and EROD activity (in liver and gills), expressed as a negative correlation between the two groups of enzymes, suggests separate regulatory pathways for these two groups of enzymes in which ROS and hypoxia may have a role (Regoli and Giuliani 2014).
The magnitude of the changes after long-term acclimation to a 5 °C decrease in temperature in S. senegalensis supports the great adaptability of this species to moderate temperature fluctuations. The variations mainly affected enzymes implied in biotransformation but also entailed modifications in antioxidant defences in order to maintain homoeostasis. In fact, the extent of the variations in xenobiotic metabolism enzymatic activities in S. senegalensis in this study is, in general, higher than those described under field situations, where seasonal and pollution-driven fluctuations acted simultaneously. That is, enzyme activities usually experienced changes between two- and threefolds (Fonseca et al. 2011a, b; Oliva et al. 2010, 2012; Gonçalves et al. 2013). Nonetheless, the biological characteristics of the fish, such as age, and the combination of variable physical and chemical factors have to be taken into consideration. The significance of our findings could contribute to explain the success of this species in its biogeographical expansion (Zenetos et al. 2012). There is no data on the relative abundance of S. senegalensis and other sympatric, but more temperate soles, in the NW Mediterranean. However, a study conducted in a warm-adapted species Sardinella aurita in the Mediterranean reports on the expansion of this species to more northern latitudes due to climate change (Sabatés et al. 2006). Unfortunately, no data on species abundance are available for S. senegalensis to conduct a similar type of study, but we believe a similar phenomenon would be likely to occur. Due to the increasing use of this species as a sentinel in marine pollution monitoring, our findings could be of significance to ecotoxicologists. Moreover, in aquaculture, the ability of this species to cope with the predicted temperature variations due to climate change could be of some economic interest.
References
Abrahamson A, Brandt I, Brunstrom B, Sundt RC, Jorgensen EH (2008) Monitoring contaminants from oil production at sea by measuring gill EROD activity in Atlantic cod (Gadus morhua). Environ Pollut 153:169–175
Arellano-Aguilar O, Montero Montoya R, Macías García C (2009) Endogenous functions and expression of cytochrome P450 enzymes in teleost fish: a review. Rev Fish Sci 17:541–556
Arjona FJ, Ruiz-Jarabo I, Vargas-Chacoff L, del Rio MPM, Flik G, Mancera JM, Klaren PHM (2010) Acclimation of Solea senegalensis to different ambient temperatures: implications for thyroidal status and osmoregulation. Mar Biol 157:1325–1335
Beauvais SL, Cole KJ, Atchison GJ, Coffey M (2002) Factors affecting brain cholinesterase activity in bluegill (Lepomis macrochirus). Water Air Soil Pollut 135:249–264
Bonga SEW (1997) The stress response in fish. Physiol Rev 77:591–625
Botte ES, Smith-Keune C, Jerry DR (2013) Temperature: a prolonged confounding factor on cholinesterase activity in the tropical reef fish Acanthochromis polyacanthus. Aquat Toxicol 140:337–339
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carpenter HM, Fredrickson LS, Williams DE, Buhler DR, Curtis LR (1990) The effect of thermal-acclimation on the activity of arylhydrocarbon hydroxylase in rainbow-trout (Oncorhynchus mykiss). Comp Biochem Physiol C Comp Pharmacol 97:127–132
Castro C, Perez-Jimenez A, Guerreiro I, Peres H, Castro-Cunha M, Oliva-Teles A (2012) Effects of temperature and dietary protein level on hepatic oxidative status of Senegalese sole juveniles (Solea senegalensis). Comp Biochem Physiol A Mol Integr Physiol 163:372–378
Collier AC, Tingle MD, Keelan JA, Paxton JW, Mitchell MD (2000) A highly sensitive fluorescent microplate method for the determination of UDP-glucuronosyl transferase activity in tissues and placental cell lines. Drug Metab Dispos 28:1184–1186
Costa PM, Caeiro S, Vale C, DelValls TA, Costa MH (2012) Can the integration of multiple biomarkers and sediment geochemistry aid solving the complexity of sediment risk assessment? A case study with a benthic fish. Environ Pollut 161:107–120
Costas B, Aragao C, Ruiz-Jarabo I, Vargas-Chacoff L, Arjona FJ, Mancera JM, Dinis MT, Conceicao LEC (2012) Different environmental temperatures affect amino acid metabolism in the eurytherm teleost Senegalese sole (Solea senegalensis Kaup, 1858) as indicated by changes in plasma metabolites. Amino Acids 43:327–335
Dinis MT, Ribeiro L, Soares F, Sarasquete C (1999) A review on the cultivation potential of Solea senegalensis in Spain and in Portugal. Aquaculture 176:27–38
Durieux EDH, Farver TB, Fitzgerald PS, Eder KJ, Ostrach DJ (2011) Natural factors to consider when using acetylcholinesterase activity as neurotoxicity biomarker in young-of-year striped bass (Morone saxatilis). Fish Physiol Biochem 37:21–29
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Feidantsis K, Pörtner HO, Lazou A, Kostoglou B, Michaelidis B (2009) Metabolic and molecular stress responses of the gilthead seabream Sparus aurata during long-term exposure to increasing temperatures. Mar Biol 156:797–809
Feidantsis K, Antonopoulou E, Lazou A, Portner HO, Michaelidis B (2013) Seasonal variations of cellular stress response of the gilthead sea bream (Sparus aurata). J Comp Physiol B 183:625–639
Fitzsimmons PN, Lien GJ, Nichols JW (2007) A compilation of in vitro rate and affinity values for xenobiotic biotransformation in fish, measured under physiological conditions. Comp Biochem Physiol C Toxicol Pharmacol 145:485–506
Fonseca VF, Franca S, Serafim A, Company R, Lopes B, Bebianno MJ, Cabral HN (2011a) Multi-biomarker responses to estuarine habitat contamination in three fish species: Dicentrarchus labrax, Solea senegalensis and Pomatoschistus microps. Aquat Toxicol 102:216–227
Fonseca VF, Franca S, Vasconcelos RP, Serafim A, Company R, Lopes B, Bebianno MJ, Cabral HN (2011b) Short-term variability of multiple biomarker response in fish from estuaries: influence of environmental dynamics. Mar Environ Res 72:172–178
Gonçalves C, Martins M, Costa MH, Caeiro S, Costa PM (2013) Ecological risk assessment of impacted estuarine areas: integrating histological and biochemical endpoints in wild Senegalese sole. Ecotoxicol Environ Saf 95:202–211
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-Transferases. J Biol Chem 249:7130–7139
Jorgensen EH, Wolkers J (1999) Effect of temperature on the P4501A response in winter- and summer-acclimated Arctic char (Salvelinus alpinus) after oral benzo a pyrene exposure. Can J Fish Aquat Sci 56:1370–1375
Koenig S, Solé M (2012) Natural variability of hepatic biomarkers in Mediterranean deep-sea organisms. Mar Environ Res 79:122–131
Koenig S, Guillen K, Solé M (2013) Comparative xenobiotic metabolism capacities and pesticide sensitivity in adults of Solea solea and Solea senegalensis. Comp Biochem Physiol C Toxicol Pharmacol 157:329–336
Kyprianou T-D, Pörtner H, Anestis A, Kostoglou B, Feidantsis K, Michaelidis B (2010) Metabolic and molecular stress responses of gilthead seam bream Sparus aurata during exposure to low ambient temperature: an analysis of mechanisms underlying the winter syndrome. J Comp Physiol B 180:1005–1018
Lange U, Saborowski R, Siebers D, Buchholz F, Karbe L (1998) Temperature as a key factor determining the regional variability of the xenobiotic-inducible ethoxyresorufin-O-deethylase activity in the liver of dab (Limanda limanda). Can J Fish Aquat Sci 55:328–338
López-Galindo C, Vargas-Chacoff L, Nebot E, Casanueva JF, Rubio D, Solé M, Mancera JM (2010a) Biomarker responses in Solea senegalensis exposed to sodium hypochlorite used as antifouling. Chemosphere 78:885–893
López-Galindo C, Vargas-Chacoff L, Nebot E, Casanueva JF, Rubio D, Solé M, Mancera JM (2010b) Sublethal effects of the organic antifoulant Mexel (R) 432 on osmoregulation and xenobiotic detoxification in the flatfish Solea senegalensis. Chemosphere 79:78–85
Mastropaolo W, Yourno J (1981) An ultraviolet spectrophotometric assay for alpha-naphthyl acetate and alpha-naphthyl butyrate esterases. Anal Biochem 115:188–193
McCormick SD (2001) Endocrine control of osmoregulation in teleost fish. Am Zool 41:781–794
Oliva M, de Canales MLG, Gravato C, Guilhermino L, Perales JA (2010) Biochemical effects and polycyclic aromatic hydrocarbons (PAHs) in senegal sole (Solea senegalensis) from a Huelva estuary (SW Spain). Ecotoxicol Environ Saf 73:1842–1851
Oliva M, Vicente JJ, Gravato C, Guilhermino L, Galindo-Riano MD (2012) Oxidative stress biomarkers in Senegal sole, Solea senegalensis, to assess the impact of heavy metal pollution in a Huelva estuary (SW Spain): seasonal and spatial variation. Ecotoxicol Environ Saf 75:151–162
Olsvik PA, Vikesa V, Lie KK, Hevroy EM (2013) Transcriptional responses to temperature and low oxygen stress in Atlantic salmon studied with next-generation sequencing technology. BMC Genomics 14(1):817
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A Mol Integr Physiol 132:739–761
Pörtner HO, Lucassen M, Storch D (2005) Metabolic biochemistry: its role in thermal tolerance and in the capacities of physiological and ecological function. In: Farrell AP, Steffensen JF (eds) The physiology of polar fishes. Fish physiology, Vol 22. Elsevier Inc, Amsterdam, p 79. doi:10.1016/S1546-5098(04)22003-9
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93:106–117
Regoli F, Giuliani ME, Benedetti M, Arukwe A (2011) Molecular and biochemical biomarkers in environmental monitoring: a comparison of biotransformation and antioxidant defense systems in multiple tissues. Aquat Toxicol 105:56–66
Sabatés A, Martin P, Lloret J, Raya V (2006) Sea warming and fish distribution: the case of the small pelagic fish, Sardinella aurita, in the western Mediterranean. Glob Chang Biol 12:2209–2219
Salat J, Pascual J (2002) The oceanographic and meteorological station at l’Estartit (NW Mediterranean). In: Tracking long-term hydrological change in the Mediterranean Sea. CIESM Workshop Series, 16:29–32. Monaco
Sánchez-Nogué B, Varó I, Solé M (2013) Comparative analysis of selected biomarkers and pesticide sensitivity in juveniles of Solea solea and Solea senegalensis. Environ Sci Pollut Res 20:3480–3488
Siscar R, Palanques A, Torreblanca A, Solé M (2013) Metal concentrations and detoxification mechanisms in Solea solea and Solea senegalensis from NW mediterranean fishing grounds. Mar Pollut Bull 77:90–99
Sleiderink HM, Beyer J, Scholtens E, Goksoyr A, Nieuwenhuize J, Vanliere JM, Everaarts JM, Boon JP (1995) Influence of temperature and polyaromatic contaminants on CYP1A levels in north-sea dab (Limandalimanda). Aquat Toxicol 32:189–209
Smith EM, Wilson JY (2010) Assessment of cytochrome P450 fluorometric substrates with rainbow trout and killifish exposed to dexamethasone, pregnenolone-16 alpha-carbonitrile, rifampicin, and beta-naphthoflavone. Aquat Toxicol 97:324–333
Solé M, Vega S, Varó I (2012) Characterization of type “B” esterases and hepatic CYP450 isoenzymes in Senegalese sole for their further application in monitoring studies. Ecotoxicol Environ Saf 78:72–79
Somero GN (2010) The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol 213:912–920
Somero GN (2012) The physiology of global change: linking patterns to mechanisms. Annu Rev Mar Sci 4:39–61
Tomanek L, Somero GN (1999) Evolutionary and acclimation-induced variation in the heat-shock responses of congeneric marine snails (genus Tegula) from different thermal habitats: implications for limits of thermotolerance and biogeography. J Exp Biol 202:2925–2936
van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Varó I, Navarro JC, Nunes B, Guilhermino L (2007) Effects of dichlorvos aquaculture treatments on selected biomarkers of gilthead sea bream (Sparus aurata L.) fingerlings. Aquaculture 266:87–96
Vassault A (1983) Lactate dehydrogenase. In: Bergmeyer MO (ed) Methods of enzymatic analysis, enzymes: oxidoreductases, transferases. Academic Press, New York, pp 118–126
Vinagre C, Fonseca V, Cabral H, Costa MJ (2006) Habitat suitability index models for the juvenile soles, Solea solea and Solea senegalensis, in the Tagus estuary: defining variables for species management. Fish Res 82:140–149
Vinagre C, Narciso L, Pimentel M, Cabral HN, Costa MJ, Rosa R (2013) Contrasting impacts of climate change across seasons: effects on flatfish cohorts. Reg Environ Change 13:853–859
West JL, Bailey JR, Almeida-Val VMF, Val AL, Sidell BD, Driedzic WR (1999) Activity levels of enzymes of energy metabolism in heart and red muscle are higher in north-temperate-zone than in Amazonian teleosts. Can J Zool 77:690–696
Wheelock CE, Phillips BM, Anderson BS, Miller JL, Miller MJ, Hammock BD (2008) Applications of carboxylesterase activity in environmental monitoring and toxicity identification evaluations (TIEs). Rev Environ Contam Toxicol 195:117–178
Zenetos A, Gofas S, Morri C, Rosso A et al (2012) Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr Mar Sci 13:328–352
Acknowledgments
This work was financed by the Ministry of Science and Innovation of Spain (ref: CTM2010-16611). S. Piñeiro and L. Cabrera from the SCSIE University of Valencia are acknowledged for the fish maintenance and the students K. Guillén, S. Brau and A. Martínez for their help in sample processing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Wallace.
Rights and permissions
About this article
Cite this article
Solé, M., Varó, I., González-Mira, A. et al. Xenobiotic metabolism modulation after long-term temperature acclimation in juveniles of Solea senegalensis . Mar Biol 162, 401–412 (2015). https://doi.org/10.1007/s00227-014-2588-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2588-2