Abstract
Subtropical fish are exposed to seasonal variations in temperature that impose a set of adaptations on their metabolism necessary for the maintenance of homeostasis. In this study, we addressed the effects of temperature variation on the metabolism of Astyanax lacustris, a species of freshwater fish common in the subtropical region of Brazil. Biomarkers of carbohydrate and protein metabolism, antioxidant defense, and oxidative damage were evaluated in the liver of A. lacustris exposed to low (15 °C) and high (31 °C) temperature thermal shock, with controls at 23 °C for 2, 6, 12, 24, 48, 72, and 96 h. A high energy demand was observed during the first 48 h of exposure to 15 °C, which is necessary for metabolic adjustment at low temperatures, with an increase in glycolysis, citric acid cycle, and amino acid catabolism. In addition, at 31 °C, glucose was exported in the first 12 h of exposure, and an increase in the citric acid cycle suggested acetyl-CoA as the pathway substrate, originating from the oxidation of lipids. The antioxidant defenses did not change at 15 °C, as opposed to 31 °C, in which there were changes in several antioxidant defense markers, indicating a response to the production of ROS. However, oxidative stress was observed at both temperatures, with oxidative damage detected by lipid peroxidation at 15 °C and protein carbonylation at 31 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature is the most important abiotic factor for aquatic ecosystems, especially for ectothermic organisms, including most fish. It directly affects their metabolic processes, subsequently influencing their growth, nutrition, reproduction, behavior, and biogeographic distribution (Golovanov 2012; Lermen et al. 2004).
The temperature range in which a fish species is able to maintain its physiological functions depends on the adaptive and evolutionary mechanisms it has developed to survive in the thermal conditions of its environment (Yamashita et al. 2010). When temperature variations exceed the minimum and maximum optimal tolerance limits, thermal stress is generated. Metabolic responses to stress are an adaptive mechanism that allows the body to maintain its homeostatic state in the face of stressors until it has adapted to the new environmental conditions (Balasch and Tort 2019; Barton 2002).
Thermal tolerance limits vary among fish species: they are broader in species from tropical and temperate regions than in species from polar regions (Peck and Conway 2000; Pörtner 2002). Marine aquatic environments, represented by large bodies of water, are generally more stable in relation to temperature changes than freshwater tributaries, in which fish fauna are more affected, experiencing greater levels of temperature variation (Balasch and Tort 2019; Hardewig et al. 2004).
Phenotypic plasticity determines the resilience of an organism in relation to environmental changes and is characterized by the manifestation of different phenotypes from the regulation of gene expression, protein activity, and epigenetics. In environments with periodic fluctuations, such as seasonal changes, reversible phenotypic plasticity is favored by natural selection. This plasticity allows individuals to acclimate via the repeated remodeling of their physiological processes to compensate for the effects of environmental change (Beaman et al. 2016).
The liver directly participates in the maintenance of homeostasis, as it controls vital physiological functions, such as nutritional metabolism, excretion, and detoxification (Polakof et al. 2012; Sun et al. 2019). The study of metabolic biomarkers in this organ can be used to determine the nutritional, homeostatic, and pathological status of an organism. The physiological responses of the liver play important roles during thermal stress, and studies have shown that temperature variations can cause oxidative damage and metabolic changes in the energy pathways (Bagnyukova et al. 2007; Rossi et al. 2017; Varis et al. 2016; Vinagre et al. 2014; Wang et al. 2019).
The south of the state of Paraná, located in southern Brazil, has a Cfb climate (Temperate Oceanic Climate-Köppen classification) (ITCG 2008), with well-defined seasons and temperatures ranging from −1 °C in winter to 34 °C in summer (EMBRAPA 2012). Lermen et al. (2004) highlighted that the water temperature in the Brazilian subtropical region can vary between a minimum of 15 °C to a maximum of 30 °C, a thermal amplitude that exerts selective pressure on the freshwater fish fauna of this region.
Due to their abundance and wide geographical distribution, Astyanax species are relatively important for the study of biogeographic, evolutionary, and biomonitoring patterns of environmental conditions and contamination (Bueno-Krawczyk et al. 2015; Carrasco-Letelier et al. 2006; Chung 2000; Loro et al. 2015; Nimet et al. 2017; Ornelas-García et al. 2008; Siqueira-Silva et al. 2015).
The yellow-tailed lambari Astyanax lacustris Garutti and Britski, 2000 (Lucena and Soares 2016) is a small fish (up to 14 cm long) with omnivorous habits (Bennemann et al. 2005; Viana et al. 2013) that is important for neotropical aquaculture, as well as used in human nutrition and as live bait in sports fishing (do Nascimento et al. 2017). In addition, its commercial production has gained prominence in recent years in the southern and southeastern regions of Brazil, as an economically and environmentally sustainable activity in rural communities and in the production of live baits for breeding carnivorous fish, with the aim of replacing Sardinella spp. to reduce fishing pressure on natural sardine stocks (Fonseca et al. 2017).
Understanding the physiological mechanisms and metabolic adjustment capacity of fish subjected to thermal stress is important to understand how these organisms have adapted to their habitats. This will enable the prediction of impacts in relation to global climate change and support commercial management, with a view to fish farming. To this end, this study investigates the effects of thermal stress on carbohydrate and protein metabolism, antioxidant defense, and oxidative damage in the liver of A. lacustris under conditions of low- and high-temperature thermal shock.
Materials and methods
Collection and acclimatization of Astyanax lacustris
An environmental license for animal collection was obtained from the Chico Mendes Institute for Biodiversity Conservation (SISBIO/ICMBio: number 63551-1). An animal experimentation license was obtained from the Ethics Committee on Animal Experimentation of the Biological Sciences Sector at the Federal University of Paraná (CEUA-BIO/UFPR: number 1228).
For the low- and high-temperature stress experiments, A. lacustris specimens (n = 280; SL: 6.17 ± 0.87 cm; average weight: 9.15 ± 3.4 g) were collected using fishing nets (multifilament fabric, 12-mm mesh between nodes) in artificial lakes at the Ildo Zago Aquaculture Research and Extension Center, located in the municipality of União da Vitória-PR, southern Brazil (26°13′12.15′ S; 51°7′51.07′ W), belonging to the Iguaçu River basin (Fig. 1), where the experiments were carried out.
Location map and sampling. The Iguaçu River basin is highlighted in gray. The municipalities of Curitiba and Foz do Iguaçu indicate the beginning and end of the Iguaçu River basin, respectively. The municipality of União da Vitória and the fish farm Ildo Zago indicate, where the animals were collected and where the experiment was performed. The table on the left shows the exposure times and the number of fish sampled in each experimental situation
The collected fish were transferred to tanks with a capacity of 830 L, with their own spring water, in continuous flow (4 L/min flow rate), at a temperature of 23 °C (Bueno-Krawczyk et al. 2015; Lermen et al. 2004), under a natural photoperiod (Bagnyukova et al. 2007; Chung 2000; Lira et al. 018; Navarro et al. 2014) of approximately 13:30 light/10:30 dark hours and constant aeration for 3 days for acclimatization (Silva and Oliveira 2018) in the following physical–chemical conditions of the water: dissolved oxygen (9.31 ± 2.17 mg/L), toxic ammonia (NH3) (0.009 ± 0.004 mg/L), pH (7.4 ± 0.31), nitrate (0.00 mg/L), nitrite (0.00 mg/L), hardness (75 ± 9 mg/L CaCO3), and without residual chlorine.
Thermal shock experiments
After acclimatization, fish were randomly separated and transferred directly to aquariums with water at temperatures of 15 ± 1 °C or 31 ± 1 °C (Lermen et al. 2004), constituting the low- and high-temperature shocks, respectively. For each experimental situation, the control group was maintained at 23 ± 1 °C. Fish were exposed to the temperatures set for 2, 6, 12, 24, 48, 72, and 96 h, totaling 10 individuals per experiment, which were taken from two different aquariums, characterizing a duplicate. The maximum density was 1.8 g of fish/L water (Chung 2000; Vieira et al. 2013).
Aquariums were used to represent static systems. They were cleaned by suction every 2 days (in experiments over 24 h), with approximately 50% of the water being renewed (Lermen et al. 2004). Submerged electronic thermostats (Aqua One®, VigoAr®, and Atman®, with 100 W power) were previously installed in the aquariums to regulate and control the temperature. Fish conditioned at 15 °C were placed in aquariums inside a horizontal refrigerator (Consul/530 L) with a digital temperature controller (TC-900E POWER/07).
The fish were fed daily with commercial fish feed (Supra® Aqua Line, with 42% protein content) in a proportion of 1% animal weight (Chung 2000; Lermen et al. 2004). Feeding was performed simultaneously in both the control and experimental groups. The first day of acclimatization was the first day of food supply. The final meal was provided between 22 and 24 h before euthanasia (Madeira et al. 2016; Vinagre et al. 2014) to avoid nutritional status influencing the results (Hemre and Kahrs 1997; Souza et al. 2018). During feeding, observations were made for the mobility and food intake of the fish (Donatti and Fanta 2002). At the end of each experiment, the fish were anesthetized with 20 mg L−1 benzocaine (from a 0.1% stock solution (w v−1) in 95% ethanol) and euthanized by spinal section and immediately dissected. The liver was collected on ice and stored in liquid nitrogen.
Analytical methods
Analyses were performed in triplicate, with optimum pH conditions, saturating substrate concentration, and at a room temperature of 22 °C (Robinson 2008) in a microplate spectrophotometer (Epoch Microplate Spectrophotometer; BioTek, Winooski, VT, USA). The concentration of total proteins was determined by the method reported by Bradford (1976) using Coomassie Brilliant Blue G-250 and bovine serum albumin (BSA) as a standard, with absorbance measured at 595 nm. The levels of enzyme activity were expressed in international units (U), with 01 U equal to 01 µmol substrate converted into product per minute. The specific enzymatic activity was expressed in units per mg of protein (U mg−1).
Determination of enzyme activity in carbohydrate and protein metabolism
Liver samples were weighed and homogenized in 50 mM Tris–HCl buffer (pH 7.4) at a ratio of 1: 5 (w v−1) in an ice bath, sonicated for 15 s (maximum 18 J) for disruption of subcellular structures, and centrifuged at 14,000×g at 4 °C for 10 min. The supernatant was used to determine the enzyme activity levels.
The activity of glycogen phosphorylase (GP) (EC 2.4.1.1) was determined according to Milligan (2003), with the modifications proposed by Chang et al. (2007). The reaction system was prepared in 45 mM potassium phosphate buffer (pH 7.0) containing 0.34 mM NADP+, 4 µM glucose-1,6-bisphosphate, 0.1 mM EDTA, 15 mM MgCl2, 1.6 U/mL phosphoglucomutase, 12 U/mL glucose-6-phosphate dehydrogenase, 1.6 mM 5′ AMP, and 0.2 mg/mL glycogen. The reduction of NADP+ to NADPH was monitored at an absorbance of 340 nm.
Glucose-6-phosphatase activity (G6Pase) (EC 3.1.3.9) was measured according to the methodology established by Fathi et al. (2002). The reaction medium was prepared with 50 mM imidazole buffer (pH 7.4), 0.9 mM EDTA, 13.25 mM glucose-6-phosphate, and 17.5 mM NaOH. The reaction was quenched with the addition of malachite green solution (containing ammonia molybdate, malachite green, and Tween), and the inorganic phosphate formed was measured at an absorbance of 600 nm.
Hexokinase activity (HK) (EC 2.7.1.1) was measured according to Baldwin et al. (2007), monitoring the reduction of NAPD+ in the presence of glucose-6-phosphate dehydrogenase (G6PDH) in a reaction system containing 50 mM imidazole buffer (pH 7.4), 2 mM glucose, 2 mM ATP, 10 µM MgCl2, 0.4 mM NADP+, 1 mM dithiothreitol, 2 mM KCl, and 0.3 U/ml G6PDH at a wavelength of 340 nm.
Phosphofructokinase activity (PFK) (EC 2.7.1.11) was assessed following the methodology reported by Baldwin et al. (2007) in a reaction system with 50 mM Tris–HCl buffer (pH 8.2), 10 mM MgCl2, 1 mM ATP, 0.15 mM NAPH, 0.15 mM NADH, 2 mM AMP, 250 mM KCl, 1 U/mL glycerol-3-phosphate dehydrogenase (GPDH), 1.2 U/mL aldolase (ALD), 10 U/mL triosephosphate isomerase (TPI), and 5 mM fructose-6-phosphate at a wavelength of 340 nm.
Pyruvate kinase activity (PK) (EC 2.7.1.40) was determined from the oxidation of NADH to NAD+ during the formation of lactate from pyruvate generated by phosphoenolpyruvate dephosphorylation (Levesque et al. 2002) in a reaction medium with 50 mM imidazole buffer (pH 7.4), 10 mM MgCl2, 25 mM KCl, 150 µM NADH, 5 mM ADP, 5 mM phosphoenolpyruvate (PEP), and 9 U/ml lactate dehydrogenase (LDH). The absorbance was measured at 340 nm.
Lactate dehydrogenase activity (LDH) (EC 1.1.1.27) was measured from the oxidation of NADH, resulting from the conversion of pyruvate to lactate (Thuesen et al. 2005) in a reaction system containing 50 mM Tris HCl buffer (pH 7.4), 1 mM sodium pyruvate, 100 mM KCl, and 0.25 mM NADH. The absorbance was measured at 340 nm.
Citrate synthase activity (CS) (EC 4.1.3.7) was determined from the complex of CoA-SH with 5.5′-dithio-bis (2-nitrobenzoic acid) (DTNB) (Saborowski and Buchholz 2002). The reaction medium was formed with 50 mM Tris HCl buffer (pH 7.4), 100 mM KCl, 1 mM EDTA, 200 µM DTNB, 200 µM acetyl-SCoA, and 500 µM oxaloacetate, with the absorbance measured at 412 nm.
Malate dehydrogenase activity (MDH) (EC 1.1.1) was verified by the conversion of malate to oxaloacetate, with the measurement of NADH oxidation (Childress and Somero 1979) in reaction medium with 50 mM Tris–HCl buffer ( pH 7.4), 0.4 mM oxaloacetate, 20 mM MgCl2, and 150 µM NADH, with the absorbance measured at 340 nm.
Glucose-6-phosphate dehydrogenase activity (G6PDH) (EC 1.1.1.49) was measured according to Ciardiello et al. (1995) with the reduction of NADP+ with the oxidation of glucose-6-phosphate. The reaction system consisted of 100 mM Tris–HCl buffer (pH 7.4), 0.2 mM NADP+, and 1 mM glucose-6-phosphate with the absorbance measured at 340 nm.
The activity of glutamate dehydrogenase (GLDH, EC 1.4.1.3) was determined by the formation of NADPH from the oxidation of NADP+, according to Ciardiello et al. (2000), in a reaction system containing 100 mM Tris HCl buffer (pH 7.4), 2 mM NADP+, 0.8 mM ADP, and 40 mM l-glutamate. The absorbance was measured at 340 nm.
The activity of aspartate aminotransferase/glutamic-oxaloacetic transaminase (AST/TGO) (EC 2.6.1.1) was determined using a commercial kit (MS 80022230149, reference no. 352; Gold Analisa Diagnóstica Ltd), with the formation of glutamate and oxaloacetate. The absorbance was measured at 505 nm.
The activity of alanine aminotransferase/glutamic pyruvic transaminase (ALT/TGP) (EC 2.6.1.2) was measured using a commercial kit (MS 80022230150, reference no. 353; Gold Analisa Diagnóstica Ltd.), with the formation of glutamate and pyruvate. The absorbance was measured at 505 nm.
Determination of enzyme activity of the antioxidant defense system
Liver samples were weighed and homogenized in 50 mM Tris–HCl buffer (pH 7.4) at a ratio of 1:5 (w v−1) in an ice bath and centrifuged at 12,000×g at 4 °C for 20 min. The supernatant was used to determine the levels of the oxidative stress markers.
The activity of superoxide dismutase (SOD) (EC 1.15.1.1) was determined by the method reported by Crouch et al. (1981) for its ability to inhibit the reduction of nitrotetrazolium blue chloride (NBT) to formazan blue by superoxide (O2−), which is generated by hydroxylamine in alkaline solution. The reaction system was prepared with 0.05 mM EDTA, 100 µM NBT, and 36.85 mM hydroxylamine chloride in 91 mM sodium carbonate buffer (pH 10.2), and the absorbance was measured at 560 nm.
Catalase activity (CAT) (EC 1.11.1.6) was determined by the degradation of hydrogen peroxide (H2O2) in oxygen and water in a reaction medium containing 50 mM Tris–HCl buffer (pH 8.0) and 30 mM H2O2. The reduction in the absorbance of H2O2 was monitored at 240 nm (Aebi 1984).
The activity of glutathione peroxidase (GPx) (EC 1.11.1.9) was measured by NADPH oxidation. The enzyme uses reduced glutathione (GSH) to reduce organic peroxide, giving rise to disulfide glutathione (GSSG). The latter is reduced by the enzyme glutathione reductase (GR) using electrons donated by NADPH (Wendel 1981). The reaction medium contained 0.1 M sodium phosphate buffer (pH 7.0), 2 mM sodium azide, 0.2 mM NADPH, 2 mM GSH, 1 U/mL GR, and 0.5 mM H2O2, with the absorbance measured at 340 nm.
Glutathione reductase activity (GR) (EC 1.8.1.7) was measured according to Sies et al. (1979) in the conversion of GSSG to GSH, in 0.1 M potassium phosphate buffer (pH 7.6) in 5 mM EDTA, 0.5 mM NADPH, and 5 mM GSSG. The absorbance was measured at 340 nm.
The activity of glutathione S-transferase (GST) (EC 2.5.1.18) was evaluated after the conjugation of the thiol group of GSH to the substrate of 1-chloro-2,4-dinitrobenzene (CDNB), which is formed as a product of the reaction, the GS-DNB conjugate, according to Keen et al. (1976). The reaction medium consisted of 0.1 M potassium phosphate buffer (pH 6.5), 1.5 mM GSH, and 2 mM CDNB. The absorbance was measured at 340 nm.
Determination of the reduced glutathione (GSH) concentration and oxidative damage markers
The levels of reduced glutathione and other thiols were determined using the methodology reported by Sedlak and Lindsay (1968). The method is based on protein precipitation and the subsequent reaction of non-protein thiols with 0.16 mM 5,5′-dithiobis-2-nitrobenzoic acid (DTNB), generating a product that absorbs light at 415 nm. Values are expressed in nanomoles per milligram of protein.
For the determination of lipid peroxidation (LPO), the thiobarbituric acid reactive substances (TBARS) method was used as described by Federici et al. (2007) with some modifications. The method consists of measuring one of the final products of lipid peroxidation, malondialdehyde (MDA), whose reaction with thiobarbituric acid (TBA) can be read at 535 nm. Values are expressed in µmol of MDA per mg protein.
Oxidative damage in proteins (PCO) was determined by the method reported by Levine et al. (1994), which consists of the reaction of carbonylated proteins with 2,4-dinitrophenylhydrazine (DNPH), forming dinitrophenyl hydrazones, which was measured at 360 nm absorbance. The results were expressed in nanomoles of carbonyls per mg of protein.
Statistical analysis
The data of the biomarkers evaluated were standardized (z-transformed) and centralized with a mean of 0 and a standard deviation of 1 (Schielzeth 2010). For the statistical comparison of the effect of the exposure time variable or the possible interactions between the exposure time and temperature, the data were subjected to two-way analysis of variance (ANOVA) at a level of 5% significance. In case of evidence of a significant effect of time or interaction between time and temperature, Tukey’s test was applied as a post-hoc test for each response variable at a level of 5% significance.
Results
There were 11 deaths (3.9%) of fish during the tests, which occurred in the control (eight fish) and treatment at 15 °C (three fish). The ANOVA results (Tables 1, 2) indicate the markers with significant changes between the temperature and time (control versus treatment over the periods of exposure) and only over time (control and treatment varied together). The levels of the enzymatic activity of all the evaluated markers are shown in Supplementary Tables 1, 2, 3, and 4.
While the fish kept at 15 °C showed low swimming activity and little food intake, the fish at 31 °C showed higher swimming activity and ate immediately after food supply, consuming it completely.
Low- and high-temperature effects on carbohydrate and protein metabolism
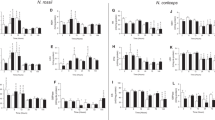
The levels of enzymatic activity of the carbohydrate and protein metabolism markers at 15 and 23 °C are listed in Supplementary Table 1. Reducing the temperature to 15 C did not affect the activity of the enzymes GP, HK, PK, LDH, MDH, and G6PDH (Fig. 2A, C, E, F, H, and I, respectively) in the liver of the animals compared with the activities observed in the control groups. However, there was an increase in the G6Pase activity at 72 h, and a decrease at 12 and 24 h (Fig. 2B), while PFK showed an increase in activity at 2 and 48 h (Fig. 2D). Similarly, CS showed an increase in activity after 12 and 24 h of exposure to low temperature (Fig. 2G). Regarding amino acid metabolism, while there was no influence of the temperature of 15 °C on AST activity (Fig. 2K), an increase in the GLDH activity in 2 h (Fig. 2J) and ALT at 6, 12, and 24 h was observed, with a decrease of 72 h, in the groups at 15 °C compared to the control groups (Fig. 2L).
Activity of enzymes used as markers of the effect of thermal stress on the metabolism of carbohydrates and proteins in the liver of Astyanax lacustris exposed to thermal shock at 15 °C during 2, 6, 12, 24, 48, 72 and 96 h. Markers: GP, glycogen phosphorylase; G6Pase, glucose 6-phosphatase; HK, hexokinase; PFK, phosphofructokinase; PK, pyruvate kinase; LDH, lactate dehydrogenase; CS, citrate synthase; MDH, malate dehydrogenase; G6PDH, glucose-6-phosphate dehydrogenase; GLDH, glutamate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase. Control groups are shown with white bars and experimental groups are shown with black bars. Letters indicate differences in the mean values between the exposure times. Asterisks (*) indicate significant differences (p ≤ 0.05) between fish at 23 °C (control group) and 15 °C (treatment groups). Data are presented as the mean ± standard error of the mean (SEM)
Regarding the variations in enzymatic activity over time in the low-temperature experiments, the activity of GP increased within 6 h at 15 and 23 °C when compared to other exposure times (Fig. 2A). G6Pase increased the activity within 72 and 96 h in the groups at 23 °C compared to 2 and 24 h and 2, 12, and 48 h, respectively. In the groups at 15 °C, activity decreased in 12 h compared to 2, 6, 72, and 96 h and increased at 72 and 96 h compared to 2, 12, 24, and 48 h (Fig. 2B). HK activity did not change over time (Fig. 2C), while increased PFK activity was observed in the group at 15 °C after 2 h of exposure compared to other hours, which decreased in the group at 15 °C after 12 h compared to 96 h (Fig. 2D). PK decreased activity in the group at 15 and 23 °C after 6 h compared to all other treatments (Fig. 2E). LDH increased activity at 2 and 6 h at 15 and 23 °C compared to all other treatments (Fig. 2F). Increased CS activity was observed at 6 and 12 h in the group at 23 °C compared to 48 and 72 h, respectively, while in the group at 15 °C, the activity increased at 6, 12, and 24 h compared to 48, 72, and 9 h, 2, 48, 72, and 96 h, and 48 and 96 h, respectively (Fig. 2G). Increased MDH activity was observed within 2 h and 6 h at 15 °C and 23 °C compared to 12, 24, 48, 72, and 96 h (Fig. 2H). G6PDH activity increased at 12 and 72 h at 15 and 23 °C compared to 2, 6, 24, 48, and 72 h and 6, 48, and 96 h, respectively (Fig. 3I). GLDH increased the activity in 2 h at 23 °C compared to 6 and 48 h, and at 15 °C compared to 6, 12, 24, 48, 72, and 96 h (Fig. 2J). AST decreased activity at 15 °C and 23 °C at 2, 6, 12, and 96 h compared to 24, 48, and 72 h, 24, 48, and 72 h, 24 and 48 h, and 24 h, respectively (Fig. 2K). Decreased ALT activity was observed at 6, 12, and 24 h at 23 °C compared to 72 and 96 h, 72, and 72 h, respectively, and increased activity at 15 °C in 12 h compared to 2 h and 72 h (Fig. 2L).
Activity of enzymes used as markers of the effect of thermal stress on the metabolism of carbohydrates and proteins in the liver of Astyanax lacustris exposed to thermal shock at 31 °C during 2, 6, 12, 24, 48, 72 and 96 h. Markers: GP, glycogen phosphorylase; G6Pase, glucose 6-phosphatase; HK, hexokinase; PFK, phosphofructokinase; PK, pyruvate kinase; LDH, lactate dehydrogenase; CS, citrate synthase; MDH, malate dehydrogenase; G6PDH, glucose-6-phosphate dehydrogenase; GLDH, glutamate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase. Control groups are shown with white bars and experimental groups are shown with black bars. Letters indicate differences in the mean values between the exposure times. Asterisks (*) indicate significant differences (p ≤ 0.05) between fish at 23 °C (control group) and 15 °C (treatment groups). Data are presented as the mean ± SEM
The levels of the enzymatic activity of the energy metabolism markers at 31 °C and 23 °C are presented in Supplementary Table 2. Increasing the temperature to 31 °C did not alter the activity of the enzymes GP, HK, PFK, PK, MDH, G6PDH, GLDH, AST, or ALT (Fig. 3A, C, D, E, H,–L, respectively) when comparing the groups at 31 °C with their respective controls. However, an increase in G6Pase activity was verified at 12 h and a decrease at 24 h (Fig. 3B), a decrease in LDH activity in 48 h (Fig. 3F), and an increase in CS at 2 and 12 h in the groups subjected to 31 °C (Fig. 2G).
Regarding the variations in enzymatic activity over time in the high-temperature experiments, the GP activity increased within 6 h at 31 °C and 23 °C compared to 48 h and 72 h (Fig. 3A). G6Pase activity decreased in 24 h at 31°Ccompared to 6, 12, 72, and 96 h, and decreased at 48 h in the group at 23 °C compared to 72 h and 96 h (Fig. 3B). HK activity decreased at 72 h and 96 h at 31 °C and 23 °C compared to that at 2 h and 48 h (Fig. 3C). PFK activity increased by 2, 72, and 96 h at 31 °C and 23 °C compared to 2, 24, and 48 h, 12 and 24 h, and 6, 12, 24, 48, and 72 h, respectively (Fig. 3D). PK activity decreased at 2 h and 6 h at 31 °C and 23 °C compared to 12, 24, 48, 72, and 96 h, respectively, and increased at 12 h at 31 °C and 23 °C compared to 24, 72, and 96 h (Fig. 3E). LDH activity increased in 6 h at 31 °C and 23 °C compared to the other hours, and increased at 23 °C in 2 h and 48 h compared to 12, 24, and 72 h (Fig. 3F). CS activity increased in 6 h at 31 °C and 23 °C compared to 2, 12, 24, 48, 72, and 96 h, decreased at 48 h compared to 12 and 96 h, and increased at 2 h and 12 h at 31 °C compared to 12 h and 48 h and 24, 48, 72, and 96 h, respectively (Fig. 3G). MDH activity increased at 2, 6, and 96 h at 31 °C and 23 °C compared to 6, 12, 24, 48, and 72 h, 12, 24, 48, 72, and 96 h, and 12, 24, 48, and 72 h, respectively (Fig. 3H). G6PDH activity increased in 12 h at 31 °C and 23 °C compared to the other hours (Fig. 3I). GLDH activity increased at 12, 24, and 72 h at 31 °C and 23 °C, respectively, compared to 2, 6, 48, and 96 h (Fig. 3J). AST activity decreased at 2, 6, and 12 h at 31 °C and 23 °C compared to 12, 24, and 72 h, 24 and 72 h, and 24 h, and increased at 24 h and 72 h at 31 °C and 23 °C, respectively, compared to 48 h and 96 h, and 96 h, respectively (Fig. 3K). ALT activity increased at 24 h and 72 h at 31 °C and 23 °C, respectively, compared to 2 h and 6 h, and 6 h, respectively (Fig. 3L).
Effects of low- and high-temperature on the antioxidant defense system and oxidative damage
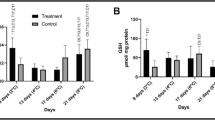
The levels of the antioxidant defense markers of enzymatic activity, GSH concentration, and oxidative damage in the groups subjected to 15 and 23 °C are shown in Supplementary Table 3. Reducing the temperature to 15 °C did not affect the activity of SOD, CAT, GPx, GR, or GST (Fig. 4A–E, respectively), nor the levels of GSH (Fig. 4F) when comparing the experimental groups with their respective controls. Considering the molecular damage caused by oxidative stress, there was no variation in PCO (Fig. 4H). However, a decrease in LPO was observed at 48 h and an increase after 72 h of exposure to 15 °C (Fig. 4G).
Enzymatic activity, levels of non-protein thiols, and oxidative damage used as markers of the effect of thermal stress on the antioxidant defense system of the Astyanax lacustris liver exposed to 15 °C thermal shock during 2, 6, 12, 24, 48, 72 and 96 h. Markers: SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; GR, glutathione reductase; GST, glutathione-S-transferase; GSH, reduced glutathione; LPO, lipid peroxidation; PCO, protein carbonylation. Control groups are shown with white bars and experimental groups are shown with black bars. Letters indicate differences in the mean values between the exposure times. Asterisks (*) indicate significant differences (p ≤ 0.05) between fish at 23 °C (control group) and 15 °C (treatment group). Data are presented as the mean ± SEM
Regarding the variations in the markers over time in the low-temperature experiments, the SOD activity decreased in 24 h at 15 °C and 23 °C compared to 12 h and 96 h (Fig. 4A). CAT activity increased at 2, 6, 12, and 24 h at 15 °C and 23 °C compared to 24, 48, and 72 h, 24, 48, and 72 h, 24 h and 48 h, and 96 h, respectively (Fig. 4B). GPx activity increased at 48 h and 96 h at 15 °C and 23 °C, respectively, compared to all other treatments (Fig. 4C). GR activity decreased in 12 h at 15 °C and 23 °C compared to 24 and 48 h, and increased in 24 h at 15 °C and 23 °C compared to 72 and 96 h (Fig. 4D). GST activity increased at 6 h and 72 h at 15 °C and 23 °C compared to 2, 12, 24, and 48 h, and 12 and 48 h, respectively (Fig. 4E). The GSH levels increased at 2 and 6 h at 15 and 23 °C compared to 6, 12, 24, 48, 72, and 96 h, and 24 and 72 h, respectively (Fig. 4F). The LPO levels increased after 48 h at 23 °C compared to 6, 24, and 72 h (Fig. 4G). The PCO levels were not altered with exposure (Fig. 4H).
The levels of the antioxidant defense markers of enzymatic activity, GSH concentration, and oxidative damage in the groups subjected to 31 and 23 °C are listed in Supplementary Table 4. The increase in temperature to 31 °C did not change the activity of the GPx enzyme (Fig. 5C), while the SOD activity increased at 96 h (Fig. 5A) and CAT activity decreased in 12 h and increased in 72 h (Fig. 5B). The GR activity decreased in 24 h (Fig. 5D) and GST activity increased at 48 h and 72 h (Fig. 5E). The GSH levels increased at 24 and 72 h (Fig. 5F). Regarding oxidative damage, LPO was not affected by high temperature (Fig. 5G), but an increase in the PCO levels was observed at 96 h (Fig. 5H).
Enzymatic activity, levels of non-protein thiols, and oxidative damage used as markers of the effect of thermal stress on the antioxidant defense system of the Astyanax lacustris liver exposed to thermal shock at 31 °C during 2, 6, 12, 24, 48, 72, and 96 h. Markers: SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; GR, glutathione reductase; GST, glutathione-S-transferase; GSH, reduced glutathione; LPO, lipid peroxidation; PCO, protein carbonylation. Control groups are shown with white bars and experimental groups are shown with black bars. Letters indicate differences in the mean values between the exposure times. Asterisks (*) indicate significant differences (p ≤ 0.05) between fish at 23 °C (control group) and 15 °C (treatment group). Data are presented as the mean ± SEM
Regarding the changes in the markers over time in the high-temperature experiments, the SOD activity increased in 96 h at 31 °C in relation to the other timepoints (Fig. 5A). The CAT activity did not change over time (Fig. 5B), while GPx activity increased at 96 h at 31 °C and 23 °C compared to the other timepoints (Fig. 5C). The GR levels increased in 12 h at 31 °C compared to 96 h and increased in 24 h at 23 °C compared to 2, 6, 48, 72, and 96 h (Fig. 5D). GST activity decreased in 12 h at 31 °C compared to 6, 48, 72, and 96 h and increased in 72 h at 31 °C compared to 2 h and 24 h (Fig. 5E). The GSH levels decreased at 24, 48, and 72 h at 23 °C compared to 6 h and 12 h, 12 h, and 2, 6, 12, and 96 h, respectively (Fig. 5F). LPO decreased in 72 h at 31 °C and 23 °C in relation to 24 h (Fig. 5G) and PCO increased in 96 h at 31 °C in relation to 2, 6, 24, 48, and 72 h (Fig. 5H).
Discussion
The mortality of 3.9% fish observed during the tests was considered low and was possibly caused by individual physiological conditions. This mortality was not related to experimental conditions, as the water quality parameters (dissolved oxygen, ammonia, and pH) were adequate in all situations tested. Almeida et al. (2015) considered a mortality rate below 10% acceptable for the validation of bioassays.
In this study, changes in the physiological markers that occurred only in relation to the time of exposure were considered to be due to the circadian cycle, widely identified as a factor of variation in the physiological parameters of organisms ((Balasch and Tort 2019) and, therefore, were not related to the effects of temperature variations.
The circadian system is responsible for the temporal organization of physiological functions that involve cycles of hormonal activity, such as melatonin and cortisol, especially every 24 h (Isorna et al. 2017), whose signaling of metabolic pathways precedes the natural events of environmental change, such as lighting, temperature, and food availability (Zhdanova and Reebs 2005). In addition to participating in circadian clocks, melatonin neutralizes free radicals and increases the activity of antioxidant enzymes, such as GPx, SOD, and CAT (Albarrán et al. 2001; Prokkola and Nikinmaa 2018; Sánchez-Vázquez et al. 2019; Tomás-Zapico and Coto-Montes 2005), and decreases the secretion of cortisol (Sánchez-Vázquez et al. 2019), which also affects the pathways of energy metabolism throughout the circadian cycle (Isorna et al. 2017).
During the experiments, fish subjected to thermal shock at 15 °C showed low swimming activity and little food intake compared to those subjected to 31 °C. This behavioral difference has also been observed in other studies and has been attributed to reduced metabolic rates at low temperatures (Beyan et al. 2015; Costa et al. 2013; Fernandes and McMeans 2019; Inoue et al. 2008; Rossi et al. 2017). During thermal stress, fish can adjust their metabolism to compensate for temperature changes until they are acclimatized (Lermen et al. 2004), and in our study, metabolic responses at 15 °C were different from those at 31 °C, indicating that different adaptive mechanisms act during exposure to low and high temperatures in these animals.
Effects of low- and high-temperature stress on hepatic carbohydrate and protein metabolism
Metabolic adjustments to maintain homeostasis in the face of thermal variations can alter energy demands (Wen et al. 2017). In this study, there was an increase in energy demand in liver cells as early as 2 h of exposure to cold, as evidenced by the stimulus of the glycolytic pathway and an increase in the degradation of amino acids by the oxidative deamination reaction catalyzed by GLDH. This enzyme is essential for the interconnection between carbohydrate and protein metabolism. An increase in the ADP/ATP ratio is an important positive modulator of GLDH activity (Plaitakis et al. 2017). This stimulus in GLDH activity generates an increase in the production of α-keto acids, which are used as substrates in the citric acid cycle, as evidenced by the high activity of CS at 12 and 24 h, indicating an increase in aerobic metabolism.
The use of amino acids as sources of carbonic skeletons for the synthesis of intermediates in the citric acid cycle can also be observed by stimulating ALT activity at 6, 12, and 24 h. This high energy demand in hepatocytes may be associated with metabolic adaptation to low-temperature stress (Kammer et al. 2011; Pol et al. 2017; White et al. 2012). However, the use of glycogenic amino acids as energy substrates during cold adaptation is a species-dependent response. As reported by Lermen et al. (2004), when exposing Rhamdia quelen to 15 °C and 31 °C for 12 h and 21 days, a decrease in proteins in the liver was observed only at 31 °C and after 21 days of exposure.
The high energy demand in the liver at 15 °C seems to have been met by stimulating the glycolytic pathway and amino acid deamination reactions up to 48 h of exposure. This can be seen by inhibiting glucose exports, with a reduction in G6Pase activity at 12 and 24 h due to low levels of ATP. Nevertheless, the high activity of G6Pase within 72 h at 15 °C indicates that the liver acts to maintain glycemic levels at the end of the adaptation period. However, it was not possible to determine by the studied markers whether the origin of the exported glucose was from gluconeogenesis or glycogenolysis. Studies suggest that hepatic glycogen stores are maintained during the cold as a survival strategy for situations of greater energy demand, such as escape (Hemre et al. 2002; Rossi et al. 2017; Speers-Roesch et al. 2018) and, due to amino acid catabolism, we believe that gluconeogenesis is more likely to occur in 72 h.
Increasing the temperature to 31 °C caused several changes in the hepatic metabolism of carbohydrates and proteins compared to that at 15 °C. There was a stimulation of aerobic metabolism, represented by an increase in CS activity at 2 and 12 h. In addition, the need to export glucose to regulate blood glucose is evidenced by the increased activity of G6Pase after 12 h of exposure. As there was no change in the activity of glycolysis and amino acid catabolism enzymes, and there was inhibition of the carbohydrate fermentation pathway in 48 h, it can be inferred that fatty acids are being used as an energy source for the citric acid cycle in 2 and 12 h. Triglycerides are used as an energy source during stress, with ATP production in the aerobic pathway from the introduction of fatty acids in the citric acid cycle, such as acetyl-CoA (Rui 2014).
These metabolic differences in energy metabolism that were observed after exposure to cold or heat stress indicate that A. lacustris is more tolerant to heat than to cold, since at 31 °C there was no evidence of changes in anaerobic respiration, amino acid catabolism, or glycolysis. Therefore, the baseline levels of enzyme activity, with the exception of CS and G6Pase, were sufficient to meet the energy demand. The absence of changes in the markers studied after 72 h in fish subjected to cold and after 48 h in fish exposed to heat indicates that these animals have adapted to changes in temperature, and the quickest response to adjusting homeostasis was to heat.
Effects of low- and high-temperature stress on the hepatic antioxidant defense system
In situations of physiological stress, the induction of antioxidant defenses is important for the effect of reactive oxygen species (ROS) on cell structures (Souza et al. 2018). However, when the optimal thermal tolerance limits of a species are exceeded, oxidative damage can occur (Vinagre et al. 2014). Several studies have shown that the responses of the antioxidant defense system to thermal stress are specific to the organ, species, temperature, and duration (Abele and Puntarulo 2004; Birnie-Gauvin et al. 2017; Grim et al. 2013; He et al. 2015; Kammer et al. 2011; Lushchak and Bagnyukova 2006; Madeira et al. 2013, 2016; Souza et al. 2018).
Thermal stress at low temperatures causes different responses to the antioxidant defenses of the liver compared to tropical and subtropical fish species. In tilapia (Oreochromis niloticus) exposed to 13 °C for 120 h, there was an increase in the activity of SOD, GPx, CAT, and GSH in the liver (He et al. 2015). Brazilian flounder (Paralichthys orbignyanus) exposed to 17.1 °C for up to 72 h showed an increase in liver GST and CAT levels, with an increase in LPO (Garcia et al. 2015). In the liver of Gasterosteus aculeatus exposed to 8 °C, there was an increase in the levels of total glutathione and SOD (Kammer et al. 2011). On the other hand, in the study by Rossi et al. (2017), the liver of Hoplosternum littorale exposed to 10 °C for 1 day showed inhibition of the activity of the enzymes GST and GR, and in 21 days, there was a decrease in CAT activity, with an increase in LPO.
In this study, at 15 °C, there was no change in the activity of the antioxidant enzymes evaluated or in the levels of GSH. This condition may be due to low metabolic rates, which are characteristic of fish at cold temperatures (Grim et al. 2013; Radovanovic et al. 2010; Rossi et al. 2017). This inactivation of antioxidant defenses indicates that A. lacustris was not able to contain the effects of ROS after 72 h of exposure to 15 °C, resulting in LPO. An increase in ROS production may be related to the increase in aerobic cell respiration to meet energy demands during the adaptation period, as evidenced by the increase in the activity of CS at 12 and 24 h.
In addition, at low temperatures, the fluidity of fish cell membranes is maintained by increasing polyunsaturated fatty acids (PUFAs) in their composition (Abele and Puntarulo 2004; Birnie-Gauvin et al. 2017; Crockett 2008; Farkas et al. 2001; Vinagre et al. 2012). On the one hand, this condition is associated with an increase in mitochondrial density (Abele and Puntarulo 2004; O’Brien 2011), allowing for the adaptation of freshwater, marine, and Antarctic fish to the cold (Birnie-Gauvin et al. 2017; Farkas et al. 2001; Liu et al. 2019), and increasing their aerobic capacity. On the other hand, the membranes are more susceptible to reactions with ROS, leading to the formation and spread of lipid hydroperoxides (LOOH) and oxidative damage to membranes (Crockett 2008; Ibarz et al. 2010).
In contrast to what was observed in cold exposure, the antioxidant defense system showed several changes at 31 °C. The first changes occurred after 12 h of exposure, with a reduction in CAT activity, indicating that liver cells were more susceptible to the harmful action of peroxides, especially hydrogen peroxide. The most important changes in the antioxidant defense system were observed after 72 h of exposure to heat. An increase in the enzymatic activity of CAT and GST and in the levels of hepatic GSH was observed, indicating that the cellular antioxidant defense system was able to respond to a possible increase in the formation of ROS, caused by heat. These results are similar to those of Madeira et al. (2016), who exposed Sparus aurata to heat stress and observed an increase in the activity of CAT, SOD, and GST in the liver. The increase in temperature is related to the increase in cellular metabolic rates and, consequently, increases in ROS production (Birnie-Gauvin et al. 2017; Lushchak 2011; Madeira et al. 2013), which can activate the antioxidant defense system to prevent oxidative cell damage (Rossi et al. 2017).
The study of the role of the transcription factor Nrf2 in the induction of antioxidant responses indicated that the presence of ROS increased the translocation and accumulation of this transcription factor in the nucleus, inducing the expression of CAT, SOD, GR, GPx, and GST genes, as well as enzymes related to the synthesis and renewal of GSH (Costa-Silva et al. 2015; Dinkova-Kostova et al. 2018; Mukaigasa et al. 2012; Wang and Zhu 2019). Therefore, the induction of antioxidant defense can occur even in the absence of oxidative damage. Our results indicate that the high-temperature thermal shock induced an increase in the production of ROS and consequently the response of the antioxidant defense, but that this response was not able to maintain protection against oxidative damage in 96 h, as demonstrated by the increase in damage to proteins (PCO) in liver cells.
Regarding oxidative damage to lipids, LPO was not found at 31 °C, most likely due to the increase in CAT activity in 72 h and SOD in 96 h. A similar condition was reported by Rossi et al. (2017) in the liver of Hoplosternum littorale exposed to 33 °C for 1 day and 21 days, with the activation of CAT and GR, without an increase in LPO. The increase in GST activity at 48 and 72 h in fish at 31 °C may also have been responsible for the absence of LPO at high temperatures. GSTs are a family of dimeric enzymes that detoxify pollutants, drugs, and endogenous reactive compounds involved in cellular metabolism (Rossi et al. 2017). Regoli et al. (2011) associated the activity of GST isoforms in the reduction of LOOH to alcohol while oxidizing GSH into GSSG. In this study, damage to lipids was assessed using an indirect marker of lipid peroxidation, detected by the thiobarbituric acid reactive substances (TBARS) method, which mainly quantifies MDA, one of the final products of lipid peroxidation. Therefore, there may have been the formation of LOOH at 31 °C, with neutralization from the activity of GST, which did not produce significant amounts of TBARS. In the study by Bagnyukova et al. (2007), the kidney and brain of Carassius auratus showed high levels of LOOH with increasing temperature, but the MDA levels were not significantly changed.
Long periods of exposure to high temperatures require an increase in the activity of glutathione-dependent enzymes to maintain stable levels of lipid peroxidation and protein carbonylation stable (Bagnyukova et al. 2007). The glutathione-dependent antioxidant defense system was activated at 31 °C, with high GST activity at 48 and 72 h, and high levels of GSH at 24 and 72 h. Glutathione is fundamental in the cellular defense against oxidative stress, and its reduced form (GSH) acts on the conversion of electrophilic compounds under physiological conditions, spontaneously or catalyzed by GST and, in a reaction of degradation of hydrogen peroxide catalyzed by the enzyme GPx, with regeneration of oxidized glutathione (GSSG). The levels of reduced glutathione in the cell are dependent on its synthesis or regeneration from GSSG by the GR enzyme in the presence of NADPH, maintaining the GSH levels always above GSSG (Deponte 2013). In A. lacustris subjected to high temperatures, an increase in the GSH levels observed at 24 and 72 h does not seem to be related to GR activity, but possibly to the induction of synthesis by the transcription factor Nrf2, which enhances the gene expression of enzymes glutamate cysteine ligase and glutathione synthetase (Mukaigasa et al. 2012).
In this study, oxidative stress was observed in the livers of animals subjected to low and high temperatures, with the occurrence of cell damage. At 15 °C, there was no change in antioxidant defenses, with an increase in LPO; in turn, at 31 °C, the antioxidant defenses were increased after 24 h of exposure, with increased activity of the enzymes CAT, SOD, and GST and in the levels of GSH. However, they were not able to maintain the balance of the antioxidant system after 96 h of exposure, when oxidative damage to proteins was observed.
Conclusion
Regarding the metabolism of carbohydrates and proteins, low temperatures caused more changes in the metabolism of A. lacustris compared to high temperatures. Even with low metabolic rates, there was a high energy demand in the first 48 h of exposure and the liver used, in addition to glycolysis, the amino acid catalysis to supply energetic substrates for the aerobic pathway. At 31 °C, the normal activity rates of the addressed enzymes, with the exception of CS and G6Pase, were sufficient to meet the energy demands of physiological adjustment to temperature changes. Nonetheless, in relation to antioxidant defenses, the temperature of 31 °C affected the metabolism of this species. However, despite the activation of antioxidant defenses, protein carbonylation was observed at 96 h. At 15 °C, despite the inactivation of antioxidant defenses and the occurrence of LPO, A. lacustris managed to recover lipid damage at 96 h.
References
Abele D, Puntarulo S (2004) Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp Biochem Physiol A 138(4):405–415. https://doi.org/10.1016/j.cbpb.2004.05.013
Aebi H (1984) Catalase in vitro. In: Methods in enzymology, vol 105. Elsevier, Amsterdam, pp 121–126. https://linkinghub.elsevier.com/retrieve/pii/S0076687984050163
Albarrán MT, López-Burillo S, Pablos MI, Reiter RJ, Agapito MT (2001) Endogenous rhythms of melatonin, total antioxidant status and superoxide dismutase activity in several tissues of chick and their inhibition by light: melatonin, total antioxidant status and superoxide dismutase activity rhytms. J Pineal Res 30(4):227–233. https://doi.org/10.1034/j.1600-079X.2001.300406.x
Almeida JR, Gravato C, Guilhermino L (2015) Effects of temperature in juvenile Seabass (Dicentrarchus labrax L.) biomarker responses and behaviour: implications for environmental monitoring. Estuaries Coasts 38(1):45–55. https://doi.org/10.1007/s12237-014-9792-7
Bagnyukova TV, Lushchak OV, Storey KB, Lushchak VI (2007) Oxidative stress and antioxidant defense responses by goldfish tissues to acute change of temperature from 3 to 23 °C. J Therm Biol 32(4):227–234. https://doi.org/10.1016/j.jtherbio.2007.01.004
Balasch JC, Tort L (2019) Netting the stress responses in fish. Front Endocrinol 10:1–12. https://doi.org/10.3389/fendo.2019.00062
Baldwin J, Elias JP, Wells RMG, Donovan DA (2007) Energy metabolism in the tropical abalone, Haliotis asinina Linné: comparisons with temperate abalone species. J Exp Mar Biol Ecol 342(2):213–225. https://doi.org/10.1016/j.jembe.2006.09.005
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42(3):517–525. https://doi.org/10.1093/icb/42.3.517
Beaman JE, White CR, Seebacher F (2016) Evolution of plasticity: mechanistic link between development and reversible acclimation. Trends Ecol Evol 31(3):237–249. https://doi.org/10.1016/j.tree.2016.01.004
Bennemann ST, Gealh AM, Orsi ML, de Souza LM (2005) Occurrence and trophic ecology of four species of Astyanax (Characidae) in different rivers of the Tibagi River Basin, Paraná. Braz Iheringia Série Zool 95(3):247–254. https://doi.org/10.1590/S0073-47212005000300004
Beyan C, Boom BJ, Liefhebber JMP, Shao K-T, Fisher RB (2015) Natural swimming speed of Dascyllus reticulatus increases with water temperature. ICES J Mar Sci 72(8):2506–2511. https://doi.org/10.1093/icesjms/fsv104
Birnie-Gauvin K, Costantini D, Cooke SJ, Willmore WG (2017) A comparative and evolutionary approach to oxidative stress in fish: a review. Fish Fish 18(5):928–942. https://doi.org/10.1111/faf.12215
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bueno-Krawczyk ACD, Guiloski IC, Piancini LDS, Azevedo JC, Ramsdorf WA, Ide AH, Guimarães ATB, Cestari MM, Silva de Assis HC (2015) Multibiomarker in fish to evaluate a river used to water public supply. Chemosphere 135:257–264. https://doi.org/10.1016/j.chemosphere.2015.04.064
Carrasco-Letelier L, Eguren G, Mello FT, Groves PA (2006) Preliminary field study of hepatic porphyrin profiles of Astyanax fasciatus (Teleostei, Characiformes) to define anthropogenic pollution. Chemosphere 62(8):1245–1252. https://doi.org/10.1016/j.chemosphere.2005.07.005
Chang JC-H, Wu S-M, Tseng Y-C, Lee Y-C, Baba O, Hwang P-P (2007) Regulation of glycogen metabolism in gills and liver of the euryhaline tilapia (Oreochromis mossambicus) during acclimation to seawater. J Exp Biol 210(19):3494–3504. https://doi.org/10.1242/jeb.007146
Childress JJ, Somero GN (1979) Depth-related enzymic activities in muscle, brain and heart of deep-living pelagic marine teleosts. Mar Biol 52(3):273–283. https://doi.org/10.1007/BF00398141
Chung KS (2000) Heat resistance and thermal acclimation rate in tropical tetra Astyanax bimaculatus of Venezuela. Environ Biol Fish 57(4):459–463. https://doi.org/10.1023/A:1007696027169
Ciardiello MA, Camardella L, di Prisco G (1995) Glucose-6-phosphate dehydrogenase from the blood cells of two Antarctic teleosts: Correlation with cold adaptation. Biochimica et Biophysica Acta (BBA). Protein Struct Mol Enzymol 1250(1):76–82. https://doi.org/10.1016/0167-4838(95)00046-W
Ciardiello MA, Camardella L, Carratore V, di Prisco G (2000) l-Glutamate dehydrogenase from the Antarctic fish Chaenocephalus aceratus. Primary structure, function and thermodynamic characterisation: relationship with cold adaptation. Biochim Biophys Acta 1543(1):11–23. https://doi.org/10.1016/S0167-4838(00)00186-2
Costa IASF, Driedzic WR, Gamperl AK (2013) Metabolic and cardiac responses of cunner Tautogolabrus adspersus to seasonal and acute changes in temperature. Physiol Biochem Zool 86(2):233–244. https://doi.org/10.1086/669538
Costa-Silva DG, Nunes MEM, Wallau GL, Martins IK, Zemolin APP, Cruz LC, Rodrigues NR, Lopes AR, Posser T, Franco JL (2015) Oxidative stress markers in fish (Astyanax sp. and Danio rerio) exposed to urban and agricultural effluents in the Brazilian Pampa biome. Environ Sci Pollut Res 22(20):15526–15535. https://doi.org/10.1007/s11356-015-4737-7
Crockett EL (2008) The cold but not hard fats in ectotherms: consequences of lipid restructuring on susceptibility of biological membranes to peroxidation, a review. J Comp Physiol B 178(7):795–809. https://doi.org/10.1007/s00360-008-0275-7
Crouch RK, Gandy SE, Kimsey G, Galbraith RA, Galbraith GMP, Buse MG (1981) The inhibition of islet superoxide dismutase by diabetogenic drugs. Diabetes 30(3):235–241. https://doi.org/10.2337/diab.30.3.235
da Silva EMP, de Oliveira RHF (2018) Portable point-of-care device as alternative tool for monitoring blood glucose in lambari Astyanax altiparanae: stress and sex-specific effects. Bol Inst Pesca 43(4):557–568. https://doi.org/10.2095/1678-2305.2017v43n4p557
de Garcia LO, Okamoto MH, Riffel APK, Saccol EM, Pavanato MA, Sampaio LAN (2015) Oxidative stress parameters in juvenile Brazilian flounder Paralichthys orbignyanus (Valenciennes, 1839) (Pleuronectiformes: Paralichthyidae) exposed to cold and heat shocks. Neotrop Ichthyol 13(3):607–612. https://doi.org/10.1590/1982-0224-20140148
de Siqueira-Silva DH, dos Silva AP, S., Silveira, A. N., & Silveira, R. V. (2015) The effects of temperature and busulfan (Myleran) on the yellowtail tetra Astyanax altiparanae (Pisces, Characiformes) spermatogenesis. Theriogenology 84(6):1033–1042. https://doi.org/10.1016/j.theriogenology.2015.06.004
de Souza MRDP, Herrerias T, Zaleski T, Forgati M, Kandalski PK, Machado C, Silva DT, Piechnik CA, Moura MO, Donatti L (2018) Heat stress in the heart and muscle of the Antarctic fishes Notothenia rossii and Notothenia coriiceps: carbohydrate metabolism and antioxidant defence. Biochimie 146:43–55. https://doi.org/10.1016/j.biochi.2017.11.010
De Lucena CAS, Soares HG (2016) Review of species of the Astyanax bimaculatus “caudal peduncle spot” subgroup sensu Garutti & Langeani (Characiformes, Characidae) from the rio La Plata and rio São Francisco drainages and coastal systems of southern Brazil and Uruguay. Zootaxa 4072(1):101. https://doi.org/10.11646/zootaxa.4072.1.5
Deponte M (2013) Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta 1830(5):3217–3266. https://doi.org/10.1016/j.bbagen.2012.09.018
Dinkova-Kostova AT, Kostov RV, Kazantsev AG (2018) The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J 285(19):3576–3590. https://doi.org/10.1111/febs.14379
do Nascimento NF, Pereira-Santos M, Piva LH, Manzini B, Fujimoto T, Senhorini JA, Yasui GS, Nakaghi LSO (2017) Growth, fatty acid composition, and reproductive parameters of diploid and triploid yellowtail tetra Astyanax altiparanae. Aquaculture 471:163–171. https://doi.org/10.1016/j.aquaculture.2017.01.007
Donatti L, Fanta E (2002) Influence of photoperiod on visual prey detection in the Antarctic fish Notothenia neglecta. Antarct Sci 14(2):146–150. https://doi.org/10.1017/S0954102002000706
EMBRAPA (2012) Atlas climático da região sul do Brasil. J Chem Inf Model 53(9):1689–1699
Farkas T, Fodor E, Kitajka K, Halver JE (2001) Response of fish membranes to environmental temperature: fish membranes and temperature. Aquac Res 32(8):645–655. https://doi.org/10.1046/j.1365-2109.2001.00600.x
Fathi A-R, Krautheim A, Lucke S, Becker K, Juergen Steinfelder H (2002) Nonradioactive technique to measure protein phosphatase 2A-like activity and its inhibition by drugs in cell extracts. Anal Biochem 310(2):208–214. https://doi.org/10.1016/S0003-2697(02)00377-9
Federici G, Shaw B, Handy R (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84(4):415–430. https://doi.org/10.1016/j.aquatox.2007.07.009
Fernandes T, McMeans BC (2019) Coping with the cold: energy storage strategies for surviving winter in freshwater fish. Ecography 42(12):2037–2052. https://doi.org/10.1111/ecog.04386
Fonseca T, Costa-Pierce BA, Valenti WC (2017) Lambari aquaculture as a means for the sustainable development of rural communities in Brazil. Rev Fish Sci Aquac 25(4):316–330. https://doi.org/10.1080/23308249.2017.1320647
Golovanov VK (2012) Influence of various factors on upper lethal temperature (review). Inland Water Biol 5(1):105–112. https://doi.org/10.1134/S1995082911040079
Grim JM, Simonik EA, Semones MC, Kuhn DE, Crockett EL (2013) The glutathione-dependent system of antioxidant defense is not modulated by temperature acclimation in muscle tissues from striped bass, Morone saxatilis. Comp Biochem Physiol A 164(2):383–390. https://doi.org/10.1016/j.cbpa.2012.11.018
Hardewig I, Pörtner HO, Dijk PV (2004) How does the cold stenothermal gadoid Lota lota survive high water temperatures during summer? J Comp Physiol B 174(2):149–156. https://doi.org/10.1007/s00360-003-0399-8
He J, Qiang J, Yang H, Xu P, Zhu ZX, Yang RQ (2015) Changes in the fatty acid composition and regulation of antioxidant enzymes and physiology of juvenile genetically improved farmed tilapia Oreochromis niloticus (L.), subjected to short-term low temperature stress. J Therm Biol 53:90–97. https://doi.org/10.1016/j.jtherbio.2015.08.010
Hemre G-I, Mommsen TP, Krogdahl Å (2002) Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes: carbohydrates in fish nutrition. Aquac Nutr 8(3):175–194. https://doi.org/10.1046/j.1365-2095.2002.00200.x
Hemre, & Kahrs, F. (1997) 14 C-glucose injection in Atlantic cod, Gadus morhua, metabolic responses and excretion via the gill membrane. Aquac Nutr 3(1):3–8. https://doi.org/10.1046/j.1365-2095.1997.00052.x
Ibarz A, Martín-Pérez M, Blasco J, Bellido D, de Oliveira E, Fernández-Borràs J (2010) Gilthead sea bream liver proteome altered at low temperatures by oxidative stress. Proteomics 10(5):963–975. https://doi.org/10.1002/pmic.200900528
Inoue LAKA, Moraes G, Iwama GK, Afonso LOB (2008) Physiological stress responses in the warm-water fish matrinxã (Brycon amazonicus) subjected to a sudden cold shock. Acta Amazon 38(4):603–609. https://doi.org/10.1590/S0044-59672008000400002
Isorna E, de Pedro N, Valenciano AI, Alonso-Gómez ÁL, Delgado MJ (2017) Interplay between the endocrine and circadian systems in fishes. J Endocrinol 232(3):R141–R159. https://doi.org/10.1530/JOE-16-0330
ITCG (2008) Clima - estado do Paraná. Instituto de Terras, Cartografia e Geociências Do Paraná, 54
Kammer O, J. I., & O’Brien, K. M. (2011) Oxidative stress is transient and tissue specific during cold acclimation of threespine stickleback. J Exp Biol 214(8):1248–1256. https://doi.org/10.1242/jeb.053207
Keen JH, Habig WH, Jakoby WB (1976) Mechanism for the several activities of the glutathione S-transferases. J Biol Chem 251(20):6183–6188
Lermen CL, Lappe R, Crestani M, Vieira VP, Gioda CR, Schetinger MRC, Baldisserotto B, Moraes G, Morsch VM (2004) Effect of different temperature regimes on metabolic and blood parameters of silver catfish Rhamdia quelen. Aquaculture 239(1–4):497–507. https://doi.org/10.1016/j.aquaculture.2004.06.021
Levesque HM, Moon TW, Campbell PGC, Hontela A (2002) Seasonal variation in carbohydrate and lipid metabolism of yellow perch (Perca flavescens) chronically exposed to metals in the field. Aquat Toxicol 60(3–4):257–267. https://doi.org/10.1016/S0166-445X(02)00012-7
Levine RL, Williams JA, Stadtman EP, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. In: Methods in enzymology, vol 233, pp 346–357. Elsevier, Amsterdam. https://linkinghub.elsevier.com/retrieve/pii/S0076687994330409
Lira LVG, Kuradomi RY, de Souza TG, Hainfellner P, Batlouni SR (2018) Astyanax altiparanae ovarian maturation after spawning in water recycling systems. Bol Inst Pesca 44(4):438–455. https://doi.org/10.2095/1678-2305.2018.44.4.207
Liu C, Dong S, Zhou Y, Shi K, Pan Z, Sun D, Gao Q (2019) Temperature-dependent fatty acid composition change of phospholipid in steelhead trout (Oncorhynchus mykiss) tissues. J Ocean Univ China 18(2):519–527. https://doi.org/10.1007/s11802-019-3775-z
Loro VL, Murussi C, Menezes C, Leitemperger J, Severo E, Guerra L, Costa M, Perazzo GX, Zanella R (2015) Spatial and temporal biomarkers responses of Astyanax jacuhiensis (Cope, 1894) (Characiformes: Characidae) from the middle rio Uruguai. Brazil Neotrop Ichthyol 13(3):569–578. https://doi.org/10.1590/1982-0224-20140146
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101(1):13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
Lushchak VI, Bagnyukova TV (2006) Temperature increase results in oxidative stress in goldfish tissues. 1. Indices of oxidative stress. Comp Biochem Physiol C 143(1):30–35. https://doi.org/10.1016/j.cbpc.2005.11.017
Madeira D, Narciso L, Cabral HN, Vinagre C, Diniz MS (2013) Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp Biochem Physiol A 166(2):237–243. https://doi.org/10.1016/j.cbpa.2013.06.008
Madeira VC, Diniz NS (2016) Are fish in hot water? Effects of warming on oxidative stress metabolism in the commercial species Sparus aurata. Ecol Ind 63:324–331. https://doi.org/10.1016/j.ecolind.2015.12.008
Milligan CL (2003) A regulatory role for cortisol in muscle glycogen metabolism in rainbow trout Oncorhynchus mykiss Walbaum. J Exp Biol 206(18):3167–3173. https://doi.org/10.1242/jeb.00538
Mukaigasa K, Nguyen LTP, Li L, Nakajima H, Yamamoto M, Kobayashi M (2012) Genetic evidence of an evolutionarily conserved role for Nrf2 in the protection against oxidative stress. Mol Cell Biol 32(21):4455–4461. https://doi.org/10.1128/MCB.00481-12
Navarro FKSP, Navarro RD, Murgas LDS, de Felizardo VO (2014) Effect of photoperiod stress assessment and locomotor activity of female lambari (Astyanax bimaculatus). Ciência e Agrotecnologia 38(2):173–180. https://doi.org/10.1590/S1413-70542014000200008
Nimet J, Guimarães ATB, Delariva RL (2017) Use of muscular cholinesterase of Astyanax bifasciatus (Teleostei, Characidae) as a biomarker in biomonitoring of rural streams. Bull Environ Contam Toxicol 99(2):232–238. https://doi.org/10.1007/s00128-017-2111-9
O’Brien KM (2011) Mitochondrial biogenesis in cold-bodied fishes. J Exp Biol 214(2):275–285. https://doi.org/10.1242/jeb.046854
Ornelas-García C, Domínguez-Domínguez O, Doadrio I (2008) Evolutionary history of the fish genus Astyanax Baird & Girard (1854) (Actinopterygii, Characidae) in Mesoamerica reveals multiple morphological homoplasies. BMC Evol Biol 8(1):340. https://doi.org/10.1186/1471-2148-8-340
Peck LS, Conway LZ (2000) The myth of metabolic cold adaptation: oxygen consumption in stenothermal Antarctic bivalves. Geol Soc Lond 177(1):441–450. https://doi.org/10.1144/GSL.SP.2000.177.01.29
Plaitakis A, Kalef-Ezra E, Kotzamani D, Zaganas I, Spanaki C (2017) The glutamate dehydrogenase pathway and its roles in cell and tissue biology in health and disease. Biology 6(4):11. https://doi.org/10.3390/biology6010011
Polakof S, Panserat S, Soengas JL, Moon TW (2012) Glucose metabolism in fish: a review. J Comp Physiol B 182(8):1015–1045. https://doi.org/10.1007/s00360-012-0658-7
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol 132(4):739–761. https://doi.org/10.1016/S1095-6433(02)00045-4
Prokkola JM, Nikinmaa M (2018) Circadian rhythms and environmental disturbances–underexplored interactions. J Exp Biol. https://doi.org/10.1242/jeb.179267
Radovanovic T, Borkovic-Mitic S, Perendija B, Despotovic S, Pavlovic SZ, Cakic PD, Saicic Z (2010) Superoxide dismutase and catalase activities in the liver and muscle of barbel (Barbus barbus) and its intestinal parasite (Pomphoryinchus laevis) from the Danube river, Serbia. Arch Biol Sci 62(1):97–105. https://doi.org/10.2298/ABS1001097R
Regoli F, Giuliani ME, Benedetti M, Arukwe A (2011) Molecular and biochemical biomarkers in environmental monitoring: a comparison of biotransformation and antioxidant defense systems in multiple tissues. Aquat Toxicol 105(3–4):56–66. https://doi.org/10.1016/j.aquatox.2011.06.014
Robinson E (2008) Antarctic fish: thermal specialists or adaptable generalists? In: University of Canterbury, Christchurch, New Zealand. https://www.semanticscholar.org/paper/Antarctic-Fish%3A-Thermal-Specialists-or-Adaptable-Robinson/df4fa3215a036388bda4819c858641dd99210a2a
Rossi A, Bacchetta C, Cazenave J (2017) Effect of thermal stress on metabolic and oxidative stress biomarkers of Hoplosternum littorale (Teleostei, Callichthyidae). Ecol Ind 79:361–370. https://doi.org/10.1016/j.ecolind.2017.04.042
Rui L (2014) Energy metabolism in the liver. In: Terjung R (ed) Comprehensive physiology. John Wiley & Sons, New York, pp 177–197. https://doi.org/10.1002/cphy.c130024
Saborowski R, Buchholz F (2002) Metabolic properties of Northern krill, Meganyctiphanes norvegica, from different climatic zones. II. Enzyme characteristics and activities. Mar Biol 140(3):557–565. https://doi.org/10.1007/s00227-001-0734-0
Sánchez-Vázquez FJ, López-Olmeda JF, Vera LM, Migaud H, López-Patiño MA, Míguez JM (2019) Environmental cycles, melatonin, and circadian control of stress response in fish. Front Endocrinol 10:279. https://doi.org/10.3389/fendo.2019.00279
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients: Interpretation of regression coefficients. Methods Ecol Evol 1(2):103–113. https://doi.org/10.1111/j.2041-210X.2010.00012.x
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Sies H, Koch OR, Martino E, Boveris A (1979) Increased biliary glutathione disulfide release in chronically ethanol-treated rats. FEBS Lett 103(2):287–290. https://doi.org/10.1016/0014-5793(79)81346-0
Speers-Roesch B, Norin T, Driedzic WR (2018) The benefit of being still: energy savings during winter dormancy in fish come from inactivity and the cold, not from metabolic rate depression. Proc R Soc B 285(1886):20181593. https://doi.org/10.1098/rspb.2018.1593
Sun S, Wu Y, Yu H, Su Y, Ren M, Zhu J, Ge X (2019) Serum biochemistry, liver histology and transcriptome profiling of bighead carp Aristichthys nobilis following different dietary protein levels. Fish Shellfish Immunol 86:832–839. https://doi.org/10.1016/j.fsi.2018.12.028
Thuesen EV, McCullough KD, Childress JJ (2005) Metabolic enzyme activities in swimming muscle of medusae: is the scaling of glycolytic activity related to oxygen availability? J Mar Biol Assoc UK 85(3):603–611. https://doi.org/10.1017/S0025315405011537
Tomás-Zapico C, Coto-Montes A (2005) A proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymes: mechanism against oxidative stress. J Pineal Res 39(2):99–104. https://doi.org/10.1111/j.1600-079X.2005.00248.x
van de Pol I, Flik G, Gorissen M (2017) Comparative physiology of energy metabolism: fishing for endocrine signals in the early vertebrate pool. Front Endocrinol. https://doi.org/10.3389/fendo.2017.00036
Varis J, Haverinen J, Vornanen M (2016) Lowering temperature is the trigger for glycogen build-up and winter fasting in Crucian carp (Carassius carassius). Zool Sci 33(1):83–91. https://doi.org/10.2108/zs150072
Viana LF, Suarez YR, Lima-Junior SE (2013) Influence of environmental integrity on the feeding biology of Astyanax altiparanae (Garutti & Britski, 2000) in the Ivinhema river basin. Acta Sci Biol Sci 35(4):541–548. https://doi.org/10.4025/actascibiolsci.v35i4.19497
Vieira VARO, Correia TG, Moreira RG (2013) Effects of aluminum on the energetic substrates in neotropical freshwater Astyanax bimaculatus (Teleostei: Characidae) females. Comp Biochem Physiol C 157(1):1–8. https://doi.org/10.1016/j.cbpc.2012.08.003
Vinagre C, Madeira D, Narciso L, Cabral HN, Diniz M (2012) Effect of temperature on oxidative stress in fish: lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol Ind 23:274–279. https://doi.org/10.1016/j.ecolind.2012.04.009
Vinagre C, Madeira D, Mendonça V, Dias M, Roma J, Diniz MS (2014) Effect of increasing temperature in the differential activity of oxidative stress biomarkers in various tissues of the Rock goby, Gobius paganellus. Mar Environ Res 97:10–14. https://doi.org/10.1016/j.marenvres.2014.01.007
Wang Y, Li C, Pan C, Liu E, Zhao X, Ling Q (2019) Alterations to transcriptomic profile, histopathology, and oxidative stress in liver of pikeperch (Sander lucioperca) under heat stress. Fish Shellfish Immunol 95:659–669. https://doi.org/10.1016/j.fsi.2019.11.014
Wang M, Zhu Z (2019) Nrf2 is involved in osmoregulation, antioxidation and immunopotentiation in Coilia nasus under salinity stress. Biotechnol Biotechnol Equip 33(1):1453–1463. https://doi.org/10.1080/13102818.2019.1673671
Wen B, Jin S-R, Chen Z-Z, Gao J-Z, Wang L, Liu Y, Liu H-P (2017) Plasticity of energy reserves and metabolic performance of discus fish (Symphysodon aequifasciatus) exposed to low-temperature stress. Aquaculture 481:169–176. https://doi.org/10.1016/j.aquaculture.2017.09.002
Wendel A (1981) Glutathione peroxidase. In: Methods in enzymology, vol 77. Elsevier, Amsterdam, pp 325–333. https://linkinghub.elsevier.com/retrieve/pii/S0076687981770460
White CR, Alton LA, Frappell PB (2012) Metabolic cold adaptation in fishes occurs at the level of whole animal, mitochondria and enzyme. Proc R Soc B 279(1734):1740–1747. https://doi.org/10.1098/rspb.2011.2060
Yamashita M, Yabu T, Ojima N (2010) Stress protein HSP70 in fish. Aqua Biosci Monogr 3(4):111–141. https://doi.org/10.5047/absm.2010.00304.0111
Zhdanova IV, Reebs SG (2005) Circadian rhythms in fish. In: Fish physiology, vol 24, pp 197–238. Elsevier, Amsterdam. https://linkinghub.elsevier.com/retrieve/pii/S1546509805240062
Acknowledgements
We thank the Ildo Zago Aquaculture Research and Extension Center (Paraná State University) for their help and support during the execution of the experiments in the present work. This study was financed in part by the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) (Finance Code 001) and by the National Council for the Development of Scientific and Technological Research (CNPq) (process 407658/2018-1 and 307452/2019-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Pelster.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schleger, I.C., Pereira, D.M.C., Resende, A.C. et al. Cold and warm waters: energy metabolism and antioxidant defenses of the freshwater fish Astyanax lacustris (Characiformes: Characidae) under thermal stress. J Comp Physiol B 192, 77–94 (2022). https://doi.org/10.1007/s00360-021-01409-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-021-01409-2