Abstract
The goal of this study was to quantify growth and metabolic responses of oysters to increased temperatures like those that will occur due to global warming. Impact of temperature on eastern oyster (Crassostrea virginica) shell growth and metabolism was investigated by sampling 24 sites along the eastern North American seaboard ranging from New Brunswick, Canada, to Florida, USA, in March and August 2013. There was a positive correlation between oyster shell thickness and site temperature. At southern sites, shells were up to 65 % thicker than at the northernmost site, likely due to higher precipitation of CaCO3 in warmer water. This was supported by laboratory experiments showing that thicker shells were produced in response to temperatures 2, 4, and 6 °C above ambient seawater temperatures (8–14 °C) in Connecticut, USA. Field experiments with oyster respiration were conducted during winter and summer at 13 sites to compare responses to thermal stress with latitude. Respiration rates were much higher during summer than winter, but the combination of summer and winter data fell along the same exponential curve with respect to temperature. At all sites, temperature-specific metabolic rates at elevated temperatures were lower than predicted, indicating significant seasonal acclimatization by C. virginica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To predict impacts of climate change on individual species, it is vital to understand how the link between temperature and physiology changes on a seasonal and latitudinal basis. In species with large geographic ranges, there are often morphological and physiological differences among populations that can lead to differential responses to elevated temperatures (Dittman et al. 1998). Variability among populations over a large geographic range can thus complicate attempts to predict species-specific metabolic responses to climate change (Jansen et al. 2007; Cherkasov et al. 2010). Morphological changes in response to temperature over a latitudinal range are especially complex in estuarine habitats, where changes in water temperature on a latitudinal and seasonal basis are accompanied by variation in salinity, food availability, and other environmental factors (Brown and Hartwick 1988; Irie 2005; Schöne et al. 2006; Waldbusser et al. 2011). Calcifying organisms display a particularly complex reaction to environmental conditions since calcification rates change based on factors such as temperature, salinity, pH, nutrients, and calcium carbonate (calcite and aragonite) saturation states (Morse and Mackenzie 1990; Dissard et al. 2010). For example, in Mytilus edulis, a temperature increase from 4 to 20 °C more than doubles calcification rates (Malone and Dodd 1967). It is therefore necessary to integrate experimental data with large-scale geographic patterns in order to assess the impact of latitudinal temperature gradients on metabolism and morphology and to predict the influence of climate change (Jansen et al. 2007).
In marine ectotherms, such as oysters, metabolic rate is positively correlated with temperature (e.g., Newell et al. 1977; Shumway and Koehn 1982; Haure et al. 1998; Jihong et al. 2004; Mao et al. 2006; Pernet et al. 2008). Temperature varies with latitude and season, so oysters and other species with broad distributions have intraspecific variations in metabolic rates on a spatial and temporal basis. Previous studies relating temperature to metabolism have measured responses to changes in temperature at one site (Shumway and Koehn 1982; Boucher and Boucher-Rodoni 1988; Mao et al. 2006; Martin et al. 2006) or measured in situ metabolic rates along a geographic temperature gradient (Dittman et al. 1998; Jansen et al. 2007). Some species, such as the bivalves M. edulis and Macoma balthica, reduce their metabolism in summer due to temperatures above their breakpoint temperature (maximum respiration rate), while other mollusks, such as Crassostrea gigas and Crepidula fornicata, display their highest metabolic rates during the summer (Boucher and Boucher-Rodoni 1988; Mao et al. 2006; Martin et al. 2006).
For calcifying organisms, shell growth and calcification patterns are also affected by seawater carbonate chemistry, which is dependent on temperature and salinity (Morse and Mackenzie 1990; Ferguson et al. 2008; Waldbusser et al. 2011). Shell growth varies with temperature seasonally (Kanazawa and Sato 2008; Waldbusser et al. 2011), latitudinally (Irie 2005), and on a climatological scale (Schöne et al. 2006; Miyaji et al. 2010). Temperature can impact the defensive abilities of calcifying organisms such as mollusks by altering shell strength or shell thickness, affecting vulnerability to crushing (crabs), or drilling predators (gastropods) (Nicol 1967; Palmer 1992; Trussell and Etter 2001). Large-scale patterns of shell thickness can also be affected by biological factors such as predator density, which can induce shell-thickening in intertidal snails including Nucella lapillus, Littorina obtusata, and L. littorea (Trussell and Etter 2001; Trussell et al. 2003), clams including Mercenaria mercenaria (Nakaoka 2000), and oysters including Crassostrea virginica and C. ariakensis (Newell et al. 2007; Lord and Whitlatch 2012).
The eastern oyster, C. virginica, increases calcification rates and shell growth rates at higher temperatures (Dame 1972; Waldbusser et al. 2011). The metabolism of C. virginica from Long Island Sound, USA is also strongly temperature dependent (Shumway and Koehn 1982). Studies of metabolic rates of marine invertebrates typically use oxygen consumption (VO2) as a proxy (Jansen et al. 2007). Species such as C. virginica that have temperature-dependent metabolism and shell growth will be affected by predicted 2–4 °C increases in global ocean temperature by 2100 (Pachauri and Reisinger 2007).
Changes in growth and metabolism of eastern oyster populations may have large ecological and economic impacts, since oysters are a commercially important species throughout their range and are ecosystem engineers (Coen et al. 2007). Oysters not only filter estuarine water and play a large role in benthic–pelagic coupling, but oyster reefs provide feeding habitat and refuge from predation for several benthic and pelagic species (e.g., Grabowski et al. 2005; Coen et al. 2007; Stunz et al. 2010). In order to predict future climate-induced changes in oyster populations and in oyster distribution, it is necessary to understand the way that oyster shell characteristics and metabolism respond to increased temperatures along a latitudinal gradient.
The goals of this study were to determine the effect of temperature on C. virginica metabolism and shell growth by testing the responses of oysters to experimentally elevated temperatures in the laboratory, and in the field at sites spanning a wide latitudinal range. We tested metabolic responses and measured shell thickness on both local and latitudinal scales because experimentally determined thermal effects on shell growth and metabolism are often generalized for an entire species, despite large variability among populations and along a latitudinal gradient (Dame 1972; Singh and Zouros 1978; Shumway and Koehn 1982; Dittman et al. 1998; Jansen et al. 2007; Waldbusser et al. 2011). We tested three main hypotheses in this study: (1) oysters from the northeastern USA would show increased growth rates when grown in warmer laboratory conditions since they live far from the southern range limit of the species, (2) shell thickness would increase from north to south because warmer temperatures result in lower solubility of calcium carbonate, which facilitates shell deposition and (3) southern C. virginica populations would be more stressed by elevated temperatures during the summer than northern populations since they are closer to the upper temperature limit for the species, i.e., ~36 °C (Galtsoff 1964). We also quantified the density of a major oyster predator, the oyster drill Urosalpinx cinerea, in order to test whether predator density affects shell thickness over a large geographic range. We conducted field surveys and experiments at 24 sites over a large latitudinal gradient (26–47°N) along the east coast of North America, enabling us to assess the role of temperature in describing and predicting large-scale patterns in C. virginica metabolism and morphology.
Materials and methods
Laboratory experiments
All laboratory experiments were conducted in the J. S. Rankin Jr. Seawater Research Laboratory at the University of Connecticut, Avery Point. Oysters were provided by Fisher’s Island Oyster Farm, where they were grown in brackish Ocean Pond and then in an open ocean environment on Fisher’s Island, New York. They were tagged with Hallprint® shellfish tags super-glued to the left valve (cupped side of the shell). Oysters were kept in 20- and 25-L flow-through seawater tanks, each connected to a heat exchanger that controlled the temperature of the flowing seawater. Each tank was fed by a separate seawater hose that coiled through a heat exchanger, so all water sources were independent and supplied seawater at approximately 1 L min−1. Aqua Logic® digital temperature controllers with temperature probes in the experimental tanks turned the heaters on and off in order to regulate the temperature of the heat exchangers and produce the desired temperature outflow in each experimental tank. Temperature controllers were precise to within 1 °C and were used to create four temperature treatments of five tanks each: control (ambient seawater temperature), +2 °C above ambient, +4 °C above ambient, and +6 °C above ambient. Temperatures in the tanks were further monitored by HOBO® data loggers which recorded water temperature every hour for the 3 month duration of the experiment. Ambient seawater temperatures increased from 8 °C at the start of the experiment to 14 °C by the end and elevated temperature treatments went up accordingly.
Each of the five tanks in the control treatment contained 10 small (1 cm long) and 10 large (6 cm long) oysters (total 50 per size class), with no significant differences in size among treatments at the start of the experiment. Each of the elevated temperature treatment tanks contained 5 large and 5 small oysters (5 tanks per treatment = 25 per size class per treatment). Twice as many oysters were used in the control treatment to account for the possibility of food limitation; under food-limited conditions, control oysters should display thicker shells than in other treatments (Brown and Hartwick 1988). Oysters were obtained in December 2011,̄ stored in flowing ambient seawater temperature tanks for 1 month, and then transferred to the experimental tanks for 3 months from January to April 2012. Prior to the start, and at the end of the experiment, all oysters were weighed in air (wet weight) and weighed in water (immersed weight) as in Palmer (1982) in order to estimate the shell weight of live oysters. The immersed weight correlates linearly with shell weight (Suppl. Fig. 1). A regression was then determined by dissecting and weighing the shells of 20 non-experimental oysters, and used to convert immersed weight to shell weight (Palmer 1982). All oysters were also photographed before and after the experiment, and the area of the left valve was measured with the image analysis software ImageJ (NIH). These data were used to determine growth rates in terms of shell weight, shell thickness, shell area, and tissue weight for C. virginica at different temperatures over the 3-month experimental period. In order to control for variability in the initial size of the oysters, all growth rates were calculated as new growth divided by initial size in the unit being measured. Shell growth differences were quantified with ANOVA tests between treatments, with Tukey’s tests for pairwise comparisons.
Latitudinal patterns in shell thickness
Oyster shells were collected from natural populations in 24 different intertidal locations along the east coast of North America, from the northernmost end of the range of C. virginica (New Brunswick, Canada) to south Florida (Fig. 1; Table 1). To assess changes in shell morphology with latitude, shell thickness was determined to be the most useful measure of morphological differences, since growth rates could not be determined over short sampling intervals and shell lengths and weights tend to be highly variable (Palmer and Carriker 1979). Shell thickness was inferred from shell weights (from dissected oysters) divided by surface area of the left valve (measured via photograph analysis). This was compared with actual maximum shell thickness, determined by measuring shell thickness of longitudinal cross sections with digital calipers for 171 oysters (~10 from each site sampled in March) to the nearest 0.1 mm. There was a strong correlation between inferred thickness (g cm−2) and measured shell thickness (mm) indicating that this inferred thickness estimates actual shell thickness (Regression ANOVA F (1,169) = 323.37, R 2 = 0.66, p < 0.001) (Suppl. Fig. 2). While shell thickness was not measured directly, the same method was used for all 24 sites and for laboratory experiments and was an accurate measure of the amount of shell material deposited by oysters per square centimeter of left valve area.
Differences in inferred shell thickness between sites were analyzed by comparing residuals from a common regression in order to account for size-based differences in shell weights. A regression between left valve shell area (from photographs and image analysis) and shell weight was created from a pooled dataset including all oysters from all 24 sites. Residuals were then calculated as the difference between each actual oyster shell weight and the regression-predicted shell weight for that oyster. This method reduced variability in inferred thickness comparisons and eliminated concerns that between-site variability could be dependent on the size of C. virginica sampled at each site.
At each site, 17 C. virginica were collected haphazardly along a 25-m transect, weighed on an electronic balance accurate to 0.01 g, and photographed for measurement of shell area. Oysters were then dissected, with wet tissue and wet shell weights measured separately on an electronic balance in order to determine the relative amounts of shell and tissue. Some oysters at each site were excluded from analysis if they had broken or damaged shells that would influence measurements of area or weight, but at least 12 oysters were dissected and measured at all sites.
Several other abiotic and biotic measurements were made at each site to determine possible correlations with oyster shell morphology. Water temperature and dissolved oxygen levels were measured with a YSI® Ecosense DO200A dissolved oxygen and temperature meter, and salinity was measured with a refractometer. Average annual seawater temperature estimates for each region were obtained from the NOAA National Oceanographic Data Center, which provides monthly temperature data for the USA coast. Surveys for oyster drills (U. cinerea and Eupleura caudata) were conducted in order to assess correlations between predator density and shell growth patterns. At each site, one 15-m transect was run parallel to the shoreline through the center of the oyster bed in the area from which oysters were collected. Oyster drills within 0.5 m on either side of this transect (1-m wide swath) were counted; loose oysters and rocks were turned over to search for drills, but surveys were otherwise non-destructive.
Latitudinal patterns in respiration
Respiration experiments were conducted at 11 sites in March 2012 and 13 sites in August 2012, (Table 1; Fig. 1). At each site, 30 C. virginica were collected and placed (left valve downward) in haphazard order into closed 355-mL glass jars with in- and out-flowing air tubing. These closed chamber respirometers were placed in coolers filled with seawater; 15 chambers in a cooler at ambient seawater temperature, and 15 chambers in a cooler at seawater temperature elevated 3 °C above ambient. Chambers were aerated for 45 min, so oysters could acclimate before the experiment, after which the air tubes were capped, sealing the chamber. Using closed chambers to measure bivalve respiration is common and effective (Buxton et al. 1981; Shumway and Koehn 1982; Vedpathak et al. 2011), and laboratory experiments confirmed a strong correlation between field respirometer and Strathkelvin respirometer results (regression ANOVA, F (1,28) = 578.9, p < 0.0001, R 2 = 0.954) (Suppl. Fig. 3).
Experiments were conducted for 2 h during the winter and 30 min during the summer due to dramatically increased respiration rates during warmer months. At the end of the experimental period, the lids of the respirometer chambers were removed and dissolved oxygen levels in each chamber measured with a YSI® Ecosense DO200A dissolved oxygen (DO) meter in mL L−1. Each treatment had two control chambers that went through the same aeration and experimental process but did not contain oysters, so oxygen consumption (VO2) for each oyster was measured as control DO level minus experiment DO level in Ml L−1. Each oyster was weighed on a digital balance, and tissue weight was estimated from regressions between wet weight and tissue weight that were created from dissected oysters at each site. To compare respiration between oysters of different sizes, VO2 for each oyster was corrected to mean dry tissue weight (0.88 g) using the allometric equation VO2 = mass0.8 (from Bougrier et al. 1995) as in Lannig et al. (2006). Therefore, all respiration measurements are in units of mL O2 L−1 h−1 0.88 g−1 tissue. Statistical analyses were conducted using SAS® and Minitab®.
Results
Shell growth and metabolic patterns of C. virginica varied with temperature in both laboratory and field experiments. Strong latitudinal gradients were found in shell thickness, while respiration rate varied with both latitude and season.
Laboratory experiments
Differences within treatments were highly nonsignificant (p > 0.4), so data points (small and large oysters) within each treatment were pooled (Underwood 1997). Shell weight of large oysters was significantly greater in +4 °C treatments than in control or +2 °C treatments (Tukey’s test, p < 0.05). Shell growth in the +6 °C treatment was significantly higher than in all other treatments. (Tukey’s test, p < 0.05: ANOVA for difference between treatments, F (3,111) = 21.07, p < 0.001) (Fig. 2a). Small oysters showed a similar pattern of shell weight growth, with significantly more shell production at 4 and 6 °C above ambient temperature (ANOVA, F (3,116) = 6.90, p < 0.001, Tukey’s test for treatment comparisons, p < 0.05) (Fig. 2c). Inferred shell thickness showed a comparable pattern for both large (ANOVA, F (3,107) = 8.63, p < 0.001) and small (ANOVA, F (3,115) = 5.23, p < 0.01) oysters, with individuals in +4 and +6 °C treatments producing significantly thicker shell than those in ambient and +2 °C treatments for both size classes (Tukey’s tests, p < 0.05) (Fig. 2b, d). Control oysters did not produce thicker shells than other treatments despite having twice the number of oysters per tank, suggesting that food limitation was not the mechanism driving observed shell growth patterns.
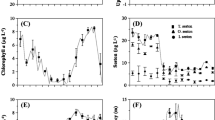
Sampling locations for Crassostrea virginica studies in March and August 2012. Open diamonds represent sites sampled only in March; open circles, in August; filled diamonds both March and August. See Table 1. Latitude on right margin
Laboratory C. virginica growth results (control, +2, +4, and +6 °C treatments). Large oyster growth measured in terms of wet shell weight (a) and inferred shell thickness (b). Small juvenile oysters showed similar shell weight (c) and thickness (d) patterns (±SE bars). Letters indicate significant differences between groups
Both large and small oysters showed significant but non-directional differences in tissue growth between treatments (ANOVA, small F (3,117) = 10.4, p < 0.001, larger F (3,110) = 9.21, p < 0.001). Mean tissue weights for small oysters in each temperature elevation treatment were as follows: control, 0.13; +2 °C, 0.06; +4 °C, −0.05; +6 °C, −0.02, and for large oysters were as follows: control, 0.01; +2 °C, 0.17; +4 °C, 0.02; +6 °C, −0.11. Differences in large and small oyster lateral shell growth (shell area) were not significantly different between cooler (control, 2 °C) and warmer (+4, 6 °C) temperature treatments (small cool X ± SE = 0.39 ± 0.45 cm2; small warm 0.38 ± 0.27 cm2; larger cool 1.36 ± 0.48 cm2; larger warm 1.18 ± 0.51 cm2).
Latitudinal patterns in shell thickness
Inferred shell thickness increased significantly with increasing average annual temperature (regression ANOVA, F (1,22) = 22.9, R 2 = 0.51, p < 0.001) (Fig. 3; Table 2). The difference between minimum and maximum shell thickness values was 64.7 %, with higher shell thickness at warmer sites to the south. Because latitude displayed strong collinearity with site temperature, it was not included as a second variable in a multiple regression. Site salinity did not add significantly (ANOVA, variables added-in-order test, F = 0.97, p > 0.3) to the regression model that included temperature and was not considered to be a major driver of shell thickness patterns. There was also no correlation (p > 0.3) between shell thickness and oyster drill (U. cinerea + E. caudata) density at each site. While the effect of temperature cannot be separated from that of latitude, temperature is likely to be a major driver underlying oyster shell growth patterns.
Latitudinal patterns in metabolism
Respiration rates were strongly correlated with temperature and not with any other environmental factor measured. Respiration rates showed an exponential increase with water temperature for both March and August experiments (regression ANOVA on log-transformed data, F (1,22) = 59.4, R 2 = 0.73, p < 0.001) (Fig. 4; Table 2). There was an approximately fivefold increase in respiration rates from March to August at all latitudes and temperatures, indicating a strong seasonal difference in metabolism superimposed on latitudinal differences (Fig. 4). Dissolved oxygen (DO) levels were at 100 % prior to all experiments due to air supply during 45-min acclimate period, and even in the warmest treatment (heated, MacArthur Beach, Florida) dropped to an average of 73 %. Few chambers at any site had DO levels drop below 60 %, the minimum level used in previous studies (Mao et al. 2006).
Metabolism of C. virginica at all sites during both March (n = 11) and August (n = 13) experiments at ambient seawater temperatures. Respiration rate is exponentially related to site temperature (Table 2)
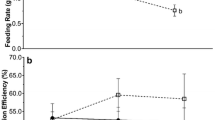
While oysters displayed higher respiration rates at sites with higher temperatures and at experimentally elevated temperatures within sites (Figs. 4, 5), responses to elevated temperatures were lower than expected based on the exponential temperature–respiration curve established in control treatments (Fig. 5). The elevated temperature treatments tested acclimation to local water temperatures by exposing oysters to temperatures 3 °C above their local level. Respiration rates in the elevated treatment were lower than predicted by the control function at all latitudes and during both summer and winter as shown by the significant difference in control and elevated regressions with temperature (general linear model with log-transformed data, group difference F (1,44) = 4.61, p = 0.037) (Fig. 5; Table 2).
Discussion
Several temperature-related patterns emerged from both laboratory-controlled and latitudinal thermal gradient studies, highlighting the role of temperature as a driver of large-scale patterns in oyster growth and metabolism. Our hypothesis that oysters from Long Island Sound would display increased growth rates at experimentally elevated temperatures was supported by growth data from a three-month experiment exposing C. virginica to flowing seawater temperature 2, 4, and 6 °C above ambient temperatures. This is not novel in itself and has been shown by other researchers (e.g., Dame 1972), but the added shell thickness component in this experiment is valuable. The observed increase in shell thickness and weight growth rates at +4 and +6 °C was likely a result of the lower solubility of calcium carbonate, specifically calcite, at higher temperatures which facilitates shell deposition (Burton and Walter 1987; Morse and Mackenzie 1990; Gazeau et al. 2010). This pattern has been observed in several species of calcifying organisms including oysters, cowries, and barnacles (Schöne et al. 2006; Kawai 2009; Waldbusser et al. 2011). Higher shell growth rates at warmer temperatures could also be due to increased metabolic rates, which could accelerate the processes involved in shell deposition, but this scenario would be accompanied by higher somatic tissue growth, which was not observed. Shell and tissue growth rates could have been limited by food availability in the tanks due to the high filtration rates of oysters, but this effect would be the same for all of the treatments and would not explain the patterns found.
Oysters can filter large volumes of water (5 L h−1 g−1 dry mass) (Newell 1988), so it is possible that C. virginica in flowing seawater tanks were food-limited, especially in the control treatment that had twice the number of oysters. While oysters often produce thicker shells at low food concentration, clearance rates estimated by Riisgard (1988) at 28 °C suggest that the highest total clearance rate in any treatment should be 20 L h−1 and water was supplied at 60 L h−1 (Brown and Hartwick 1988). Our shell growth experiments were conducted in winter at temperatures of 8–20 °C, depending on treatment, which would cause even lower feeding rates. In addition, there were significant differences in shell deposition between +2, +4, and +6 °C treatments, which each had the same number of oysters, making it unlikely that the number of control oysters influenced results. Increased shell growth at warmer temperatures indicates that shell deposition rates are higher under these conditions, irrespective of the allocation to shell thickness or lateral growth. These results show a strong effect of temperature changes on shell growth patterns for oysters from a single population and suggest that temperature could be a mechanism behind latitudinal patterns in shell morphology.
Previous studies on calcifying species (Nicol 1967; Pytkowicz 1969; Graus 1974; Trussell and Etter 2001; Waldbusser et al. 2011) support our finding that oyster shell thickness is greater at lower latitudes, with a 65 % increase from north to south. Inferred shell thickness (correlated with actual thickness) showed a strong negative correlation with latitude and a positive correlation with temperature (Table 2; Fig. 3). The 65 % increase in shell thickness from north to south shows a large latitudinal gradient that is likely due in part to temperature-induced increases in shell deposition rate. Oysters do increase calcification rates (Waldbusser et al. 2011) and shell growth rates (Fig. 2) at experimentally elevated temperatures, supporting the hypothesis that these latitudinal patterns are temperature related. Increased shell thickness at warmer latitudes has also been reported for littorine snails in the Northwest Atlantic and cowries in the Northwest Pacific (Trussell and Etter 2001; Irie 2005). These studies hypothesize that the impact of water temperature on shell deposition is driving latitudinal thickness patterns, an idea that is supported by laboratory results for C. virginica (Fig. 2) (Waldbusser et al. 2011).
Shell deposition rates are inferred from shell thickness patterns but cannot be definitively established without growth data at all sites, which would require either long-term experiments or aging of specimens—both beyond the scope of this study. Latitudinal shell thickness patterns corresponded well with laboratory temperature experiments, but collinearity between latitude and temperature makes it difficult to establish causation on a large geographic scale. Increasing predation intensity toward the equator has been proposed as a mechanism behind latitudinal patterns in shell morphology for some mollusks and cannot be ruled out for C. virginica (Vermeij and Veil 1978). Eastern oysters can increase shell thickness as an inducible defense in response to crab (Rhithropanopeus harrisii and Callinectes sapidus) and oyster drill (U. cinerea) predators (Newell et al. 2007; Johnson and Smee 2012; Lord and Whitlatch 2012). Whereas surveys in the present study ruled out U. cinerea and E. caudata density as a factor impacting large-scale shell thickness patterns, predators such as crabs or other species of oyster drills (e.g., Stramonita spp.) tend to increase in abundance to the south and could impact C. virginica morphology.
The final hypothesis that populations of southern C. virginica would be more stressed at experimentally elevated temperatures during the summer than northern oysters was not supported by experimental respiration data, as oysters showed similar thermal responses at warmer and cooler sites during summer and winter (Fig. 5). The oxygen consumption measurements in the present study were brief and provide snapshots of respiration responses to thermal stress at two different times of year across latitudes. As such, there may be long-term differences in acclimation capacity between seasons that were not captured by these experiments. Respiration rates (proxy for metabolism) were strongly and exponentially related to temperature during both March and August sampling seasons, as expected based on previous work on thermal responses of oysters (Shumway and Koehn 1982) (Fig. 4). As a result, respiration rates were up to fivefold higher during summer than winter, indicating large seasonal swings in metabolic rate of C. virginica. Since oysters play a large and active role in estuaries in terms of filtration and benthic–pelagic coupling, high seasonal variability in metabolic rate indicates seasonal shifts in the ecological influence of oyster reefs (Coen et al. 2007). Seasonal acclimatization plays a role as well, as evidenced by lower than expected respiration rates at elevated temperatures at all sites (Fig. 5).
While respiration rates in elevated temperature treatments were generally higher than the ambient temperature controls, they fell consistently below the regression between control temperature and respiration rate. This means that the temperature-specific respiration rates were higher for control than elevated treatments at all latitudes, suggesting relatively finely tuned acclimatization in C. virginica. Seasonal acclimatization, or the adjustment to natural seasonal changes in temperature, may allow oysters to gradually adjust their metabolic rate based on seasonally shifting water temperatures (Gatten et al. 1988). This would explain the higher temperature-specific respiration rates in control treatments, as oysters were acclimatized to that specific temperature and could not fully acclimate in elevated temperature treatments (Fig. 5). The fact that oysters from all latitudes responded similarly to temperatures elevated 3 °C above their local seasonal maximum suggests a high level of local adaptation or acclimatization. The large degree of seasonal and latitudinal acclimatization displayed by C. virginica suggests that studies conducted at one location or point in time (e.g., Dame 1972; Buxton et al. 1981; Shumway and Koehn 1982; Hummel et al. 2000; Mao et al. 2006) should take a cautious approach when attempting to draw broad conclusions about the metabolic response of a species.
Adaptation or even acclimation was not directly measured during this study since it took place over a short period of time, but a few conclusions can be drawn about the latitudinal metabolic patterns. The latitudinal compensation hypothesis described by Levinton (1983) suggests that populations could evolutionarily shift their temperature–metabolism curve to optimize performance under local environmental conditions. This does not appear to be the case for C. virginica, as oxygen consumption at all sites fell along a similar exponential curve related to site temperature (Fig. 4). In addition, populations over a latitudinal gradient responded similarly to experimental temperature increases (Fig. 5), suggesting a low degree of adaptation to local temperatures. Metabolic rates increased concomitantly with site temperature as expected from previous studies (Shumway and Koehn 1982), so there also does not appear to be any type of counter-gradient variation in metabolic rate (Levins 1968). The overall pattern of oxygen consumption with latitude appeared to be well-constrained by site temperature alone, which explained over 60 % of the variability in temperature between sites. However, there are several factors that can influence oyster feeding and metabolism (reviewed by Cranford et al. 2011), so temperature is likely to be just one of the several factors (e.g., food availability) that influence respiration rate.
In conclusion, we demonstrated that temperature can exhibit strong control on oyster shell growth and deposition in a controlled setting. Ocean temperature may also be a major driver of latitudinal patterns in shell thickness, as we observed a strong correlation between temperature and inferred shell thickness at 24 sites over 21 degrees of latitude on the east coast of North America. Latitudinal differences in metabolic rates were highly correlated with site temperature, but oysters showed a significant level of seasonal acclimatization to local water temperatures. Growth experiments and shell thickness surveys suggest that warmer temperatures resulting from climate change will likely cause increases in both oyster shell growth and shell thickness across latitudes. The strong positive correlation between seawater temperature and shell thickness may be caused by decreased solubility of calcium carbonate in warmer water, facilitating shell deposition with global warming. We illustrate that oyster metabolic rates, acclimatization, and shell deposition vary widely with latitude and season and that differences in temperature may be one of the major mechanisms underlying this variation.
References
Boucher G, Boucher-Rodoni R (1988) In situ measurement of respiratory metabolism and nitrogen fluxes at the interface of oyster beds. Mar Ecol Prog Ser 44:229–238
Bougrier S, Geairon P, Deslous-Paoli J, Bacher C, Jonquieres G (1995) Allometric relationships and effects of temperature on clearance and oxygen consumption rates of Crassostrea gigas (Thunberg). Aquaculture 134:143–154
Brown J, Hartwick E (1988) Influences of temperature, salinity and available food upon suspended culture of the Pacific oyster, Crassostrea gigas: I. Absolute and allometric growth. Aquaculture 70:231–251
Burton EA, Walter LM (1987) Relative precipitation rates of aragonite and Mg calcite from seawater: temperature or carbonate ion control? Geology 15:111–114
Buxton C, Newell R, Field J (1981) Response-surface analysis of the combined effects of exposure and acclimation temperatures on filtration, oxygen consumption and scope for growth in the oyster Ostrea edulis. Mar Ecol Prog Ser 6:73–82
Cherkasov A, Taylor C, Sokolova IM (2010) Seasonal variation in mitochondrial responses to cadmium and temperature in eastern oysters Crassostrea virginica (Gmelin) from different latitudes. Aquat Toxicol (Amsterdam, Netherlands) 97:68–78
Coen L, Brumbaugh R, Bushek D, Grizzle R, Luckenbach M, Posey M, Powers S, Tolley S (2007) Ecosystem services related to oyster restoration. Mar Ecol Prog Ser 341:303–307
Cranford P, Ward JE, Shumway S (2011) Bivalve filter feeding: variability and limits of the aquaculture biofilter. In: Shumway S (ed) Shellfish aquaculture and the environment. Wiley, Hoboken, pp 81–124
Dame R (1972) The ecological energies of growth, respiration and assimilation in the intertidal American oyster Crassostrea virginica. Mar Biol 17:243–250
Dissard D, Nehrke G, Reichart GJ, Bijma J (2010) The impact of salinity on the Mg/Ca and Sr/Ca ratio in the benthic foraminifera Ammonia tepida: results from culture experiments. Geochim Cosmochim Acta 74:928–940
Dittman D, Ford S, Haskin H (1998) Growth patterns in oysters, Crassostrea virginica, from different estuaries. Mar Biol 132:461–469
Ferguson JE, Henderson GM, Kucera M, Rickaby REM (2008) Systematic change of foraminiferal Mg/Ca ratios across a strong salinity gradient. Earth Planet Sci Lett 265:153–166
Galtsoff PS (1964) The American oyster, Crassostrea virginica (Gmelin). Fishery Bull Fish Wildl Serv USA 64:1–480
Gatten R Jr, Echternacht A, Wilson M (1988) Acclimatization versus acclimation of activity metabolism in a lizard. Physiol Zool 61:322–329
Gazeau F, Gattuso J, Dawber C (2010) Effect of ocean acidification on the early life stages of the blue mussel Mytilus edulis. Biogeosciences 7:2927–2947
Grabowski J, Hughes A, Kimbro D, Dolan M (2005) How habitat setting influences restored oyster reef communities. Ecology 86:1926–1935
Graus R (1974) Latitudinal trends in the shell characteristics of marine gastropods. Lethaia 7:303–314
Haure J, Penisson C, Bougrier S, Baud J (1998) Influence of temperature on clearance and oxygen consumption rates of the flat oyster Ostrea edulis: determination of allometric coefficients. Aquaculture 169:211–224
Hummel H, Bogaards R, Bachelet G, Caron F, Sola J, Amiard-Triquet C (2000) The respiratory performance and survival of the bivalve Macoma balthica (L.) at the southern limit of its distribution area: a translocation experiment. J Exp Mar Biol Ecol 251:85–102
Irie T (2005) Geographical variation of shell morphology in Cypraea annulus (Gastropoda: Cypraeidae). J Moll Stud 72:31–38
Jansen JM, Pronker AE, Kube S, Sokolowski A, Sola JC, Marquiegui MA, Schiedek D, Wendelaar Bonga S, Wolowicz M, Hummel H (2007) Geographic and seasonal patterns and limits on the adaptive response to temperature of European Mytilus spp. and Macoma balthica populations. Oecologia 154:23–34
Jihong Z, Fang J, Hawkins A, Pascoe P (2004) The effect of temperature on clearance rate and oxygen consumption of scallops, Chlamys farreri. J Shell Res 23:715–721
Johnson KD, Smee DL (2012) Size matters for risk assessment and resource allocation in bivalves. Mar Ecol Prog Ser 462:103–110
Kanazawa T, Sato S (2008) Environmental and physiological controls on shell microgrowth pattern of Ruditapes philippinarum (Bivalvia: Veneridae) from Japan. J Moll Stud 74:89–95
Kawai K (2009) Shell growth, reproduction and mortality of Ovula ovum in southern Kyushu, Japan. J Moll Stud 75:35–40
Lannig G, Flores JF, Sokolova IM (2006) Temperature-dependent stress response in oysters, Crassostrea virginica: pollution reduces temperature tolerance in oysters. Aquat Toxicol 79:278–287
Levins R (1968) Thermal acclimation and heat resistance in Drosophila species. Am Nat 103:483–499
Levinton J (1983) The latitudinal compensation hypothesis: growth data and a model of latitudinal growth differentiation based upon energy budgets. I. Interspecific comparison of Ophryotrocha (Polychaeta: Dorvilleidae). Biol Bull 165:686–698
Lord JP, Whitlatch RB (2012) Inducible defenses in the eastern oyster Crassostrea virginica Gmelin in response to the presence of the predatory oyster drill Urosalpinx cinerea Say in Long Island Sound. Mar Biol 159:1177–1182
Malone P, Dodd J (1967) Temperature and salinity effects on calcification rate in Mytilus edulis and its paleoecological implications. Limnol Oceanogr 12:432–436
Mao Y, Zhou Y, Yang H, Wang R (2006) Seasonal variation in metabolism of cultured Pacific oyster, Crassostrea gigas, in Sanggou Bay, China. Aquaculture 253:322–333
Martin S, Thouzeau G, Chauvaud L, Jean F, Guérin L, Clavier J, Thouzeau G, Jean F, Guerin L (2006) Respiration, calcification, and excretion of the invasive slipper limpet, Crepidula fornicata L.: implications for carbon, carbonate, and nitrogen fluxes in affected areas. Limnol Oceanogr 51:1996–2207
Miyaji T, Tanabe K, Matsushima Y, Sato S, Yokoyama Y, Matsuzaki H (2010) Response of daily and annual shell growth patterns of the intertidal bivalve Phacosoma japonicum to Holocene coastal climate change in Japan. Palaeogeogr Palaeoclimatol Palaeoecol 286:107–120
Morse JW, Mackenzie FT (1990) Geochemistry of sedimentary carbonates. Elsevier, New York
Nakaoka M (2000) Nonlethal effects of predators on prey populations: predator-mediated change in bivalve growth. Ecology 81:1031–1045
Newell RIE (1988) Ecological changes in Chesapeake Bay: are they the result of overharvesting the American oyster, Crassostrea virginica? Understanding the estuary. Adv Chesap Bay Res 129:536–546
Newell RIE, Johson L, Kofoed L (1977) Adjustment of the components of energy balance in response to temperature change in Ostrea edulis. Oecologia 30:97–110
Newell RIE, Kennedy VS, Shaw KS (2007) Comparative vulnerability to predators, and induced defense responses, of eastern oysters Crassostrea virginica and non-native Crassostrea ariakensis oysters in Chesapeake Bay. Mar Biol 152:449–460
Nicol D (1967) Some characteristics of cold-water marine pelecypods. J Paleontol 41:1330–1340
Pachauri RK, Reisinger A (2007) Climate change 2007. Synthesis report. Contribution of working groups I, II, and III to the fourth assessment report. Intergovernmental panel on climate change, Geneva, Switzerland
Palmer A (1982) Growth in marine gastropods. A non-destructive technique for independently measuring shell and body weight. Malacologia 23:63–73
Palmer A (1992) Calcification in marine molluscs: how costly is it? Proc Nat Acad Sci 89:1379–1382
Palmer RE, Carriker MR (1979) Effects of cultural conditions on morphology of the shell of the oyster Crassostrea virginica. Proc Nat Shellfish Assoc 69:58–72
Pernet F, Tremblay R, Redjah I, Sévigny JM, Gionet C (2008) Physiological and biochemical traits correlate with differences in growth rate and temperature adaptation among groups of the eastern oyster Crassostrea virginica. J Exp Biol 211:969–977
Pytkowicz R (1969) Chemical solution of calcium carbonate in sea water. Am Zool 9:673–679
Riisgard HU (1988) Efficiency of particle retention and filtration rate in 6 species of Northeastern American bivalves. Mar Ecol Prog Ser 45:217–223
Schöne B, Rodland D, Fiebig J (2006) Reliability of multitaxon, multiproxy reconstructions of environmental conditions from accretionary biogenic skeletons. J Geol 114:267–285
Shumway S, Koehn R (1982) Oxygen consumption in the American oyster Crassostrea virginica. Mar Ecol Prog Ser 9:59–68
Singh S, Zouros E (1978) Genetic variation associated with growth rate in the American oyster (Crassostrea virginica). Evolution 32:342–353
Stunz G, Minello T, Rozas L (2010) Relative value of oyster reef as habitat for estuarine nekton in Galveston Bay, Texas. Mar Ecol Prog Ser 406:147–159
Trussell GC, Etter RJ (2001) Integrating genetic and environmental forces that shape the evolution of geographic variation in a marine snail. Genetica 112–113:321–337
Trussell GC, Ewanchuk P, Bertness M (2003) Trait-mediated effects in rocky intertidal food chains: predator risk cues alter prey feeding rates. Ecology 84:629–640
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Vedpathak A, Muley R, Tangade D (2011) Variations in the rate of oxygen consumption, ammonia excretion and O: N ratio of freshwater bivalve, Indonaia caeruleus (Prashad, 1918) in relation to body size during summer. Bioscan 6:315–318
Vermeij G, Veil J (1978) A latitudinal pattern in bivalve shell gaping. Malacologia 17:57–61
Waldbusser GG, Voigt EP, Bergschneider H, Green MA, Newell RIE (2011) Biocalcification in the eastern oyster (Crassostrea virginica) in relation to long-term trends in Chesapeake Bay pH. Est Coast 34:221–231
Acknowledgments
Jeremy Calini was instrumental in August 2012 sampling at all sites. Additional thanks to Dr. Evan Ward and Dr. Dane Frank for providing valuable input into the analysis of the data and preparation of the manuscript. Marine laboratories that provided lodging and facilities included Virginia Institute of Marine Science, VIMS Eastern Shore Lab, UNC-Institute of Marine Sciences, Duke Marine Lab, Skidaway Institute of Oceanography, USC Belle Baruch Field Station, and Whitney Marine Lab. Collecting permits were granted by the Florida Fish and Wildlife Conservation Commission (SAL 12-1368-SR and SAL 12-1401-SR), Florida DER Division of Recreation and Parks (Permit # 07171210), MacArthur Beach State Park (Permit # 5-12-26), Georgia DNR, South Carolina DNR and North Carolina Estuarine Research Reserve (Permit # 3-2012). Funding provided by University of Connecticut predoctoral fellowship and Connecticut Sea Grant College Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Grassle.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lord, J., Whitlatch, R. Latitudinal patterns of shell thickness and metabolism in the eastern oyster Crassostrea virginica along the east coast of North America. Mar Biol 161, 1487–1497 (2014). https://doi.org/10.1007/s00227-014-2434-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2434-6