Abstract
Alcyonium acaule (Cnidaria, Octocorallia) is a common, hard-bottom soft coral in the northwestern Mediterranean Sea. This study describes sexual reproduction and the gamete development cycle of this soft coral. A population at 15–18 m depth in the Marine Protected Area of the Medes Islands (42º02′55″ N, 3º13′30″ E) was sampled from July 1994–August 1995. A. acaule is gonochoristic and a surface brooder, spawning once a year in early summer. The mean diameter of ripe spermatic sacs was 400 ± 91 (SD) μm, and the mean diameter of mature oocytes was 473 ± 37 (SD) μm. There were 30 spermatic sacs polyp−1 in males and 14 oocytes polyp−1 in females. Different phases of gametogenesis in female and male colonies were examined separately with respect to seasonal changes in bottom temperature and solar irradiance. The data suggest that the relatively constant temperatures in January–April are probably not related to oocyte maturation, but that rising temperatures in May could affect sperm maturation. Rapidly increasing solar irradiance in March may be the trigger for vitellogenesis and oocyte maturation, although the mechanism for this in anthozoans is not understood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reproduction is a key feature of an organism’s life strategy and plays a major role in the structure, dynamics and distribution of benthic populations. Benthic coastal communities are particularly vulnerable to diverse threats (global warming, eutrophication, pollution, bottom trawling, etc.), especially in a semi-enclosed basin like the Mediterranean Sea, where changes may be faster and more pronounced (Garrabou et al. 2009). One of the best tools for marine conservation is a better knowledge of the reproductive cycles and the potential gonadal output of marine species. Life history traits are also related to dispersal potential (Kinlan et al. 2005) and predict whether or not a species may colonize new surfaces. Among benthic organisms, Anthozoa (phylum Cnidaria) have an important role as habitat-forming species in circalittoral hard and soft bottoms (Gili and Coma 1998). Studies linking environmental and biological cycles are crucial in defining which factors influence reproductive output and timing, and in understanding how different disturbances may affect fitness and how populations may recover (Kinlan et al. 2005).
The numerous studies describing reproductive biology of anthozoans mainly deal with hexacorals and octocorals of tropical reefs, and several authors have compiled comprehensive reviews (e.g. Harrison and Wallace 1990; Richmond and Hunter 1990; Kahng et al. 2011). Annual reproductive patterns are typical for the majority of anthozoans, but the timing and the degree of synchronization of spawning events vary geographically, ranging from the highly coordinated mass phenomena described for the Great Barrier Reef (Babcock et al. 1986) to the continuous or random breeding throughout the year reported for a few species in shallow and deep waters (Dahan and Benayahu 1997; Ben-David-Zaslow et al. 1999; Cordes et al. 2001, all soft corals; Waller and Tyler 2005, scleractinians).
Photoperiod and solar radiation (Pearse and Eernisse 1982; Babcock et al. 1994), lunar and tidal cycles (Babcock et al. 1986), food availability (Hartnoll 1975; Coma et al. 1995) and local seasonal environmental phenomena (e.g. seasonal rainfall [Mendes and Woodley 2002] or typhoons [Fan et al. 2005]) have been suggested as temporal cues or environmental pressures regulating the breeding time, as an alternative to or in combination with temperature cycles.
In the warm temperate Mediterranean Sea, marked seasonal patterns of sea water temperature and primary production (Estrada 1996) are ultimately driven by the photoperiod and irradiance cycles characteristic of intermediate latitudes. The reproductive cycles of Mediterranean gorgonians (Octocorallia, Alcyonacea) studied to date were correlated with changes in temperature and food availability (e.g. Coma et al. 1995; Tsounis et al. 2006; Gori et al. 2007; Ribes et al. 2007; Rossi and Gili 2009), but the potential role of irradiance and photoperiod has so far been neglected.
Much attention has been paid to spawning events, overlooking other phases of the reproductive cycle (gamete proliferation and maturation) in which different endogenous and exogenous factors may act in different ways (Giese and Pearse 1974; Harrison and Wallace 1990; Olive 1995). Spawning is the culmination of gametogenesis, a process that has evolved to generate mature gametes at the appropriate time. Vitellogenesis in these cnidarians requires an especially large energy investment, and coordination of its seasonal onset with environmental cycles might be as important as synchronous spawning in ensuring reproductive success.
Alcyonium acaule is a soft coral representative of the widespread pre-coralligene and coralligene communities (Gili and Ros 1985) in the NW Mediterranean. It occurs over a broad depth range (10–90 m, Gori pers. com.), dwelling on rocky bottoms. This octocoral is long-lived reaching lifespans of >20 years (Teixidó pers. com.), with a slow growth rate and relying on sexual reproduction as probably the only means of population maintenance (Garrabou 1999). The genus Alcyonium is among the invertebrate taxa with the most diverse sexual reproduction patterns, including species that vary both in mode of reproduction (broadcast spawners, internal brooders and surface brooders) and sexual expression (gonochoristic, hermaphroditic and a putative parthenogenetic species) (McFadden et al. 2001). To our knowledge, there are only two studies on sexual reproduction of a Mediterranean species of the genus Alcyonium. Lacaze-Duthiers (1900) identified the internal brooding behaviour of Alcyonium coralloides (as Sympodium coralloides) and the release of larvae in spring (May to July); Lo Bianco (1909) carried out a preliminary study of the reproductive cycle of A. acaule using samples taken over a very limited period of time.
This study describes for the first time the general features of sexual reproduction and the annual development of the gametes of A. acaule. The reproductive pattern of this species was compared with other Mediterranean octocorals and other species of Alcyonacea from other habitats. The relationship between the reproductive cycle and seasonal changes in temperature and solar radiation was analysed, in an attempt to identify the main factors triggering the onset of gametogenesis, gamete maturation and spawning.
Materials and methods
Study area and sampling
The study population of A. acaule was located in the Marine Protected Area of the Medes Islands (NW Mediterranean Sea), in Tascons Grossos (42º02′55″ N, 3º13′30″ E). Sampling was conducted at 15–18 m depth by SCUBA diving. This species dwells on rocky bottom at 10–50 m depth in the study area, coexisting with other benthic suspension feeders and coralline algae (Gili and Ros 1985). To minimize the impact on the studied population, monthly samples of only 10–12 colonies were collected from July 1994–August 1995. The samples were obtained by cutting off the tips of the finger-like branches of massive digitate colonies having more than three fingers (colonies can be up to 21 cm width and have up to 45 fingers, but mature colonies have 10–12 fingers on average, Gori personal observation). The samples were immediately fixed in 10 % buffered formalin in sea water.
Gamete development cycle
Samples from each month (25 polyps for each sex) were dissected under a binocular microscope, in order to quantify and measure the sexual products. Sex determination was initially carried out by observing colour, texture and appearance of reproductive structures, following the work of previous authors (Coma et al. 1995; Tsounis et al. 2006; Ribes et al. 2007; Rossi and Gili 2009). The oocytes and spermatic sacs were counted in five haphazardly selected polyps of each colony and their diameters measured with an eyepiece micrometer (precision ± 10 μm). Size–frequency distributions of the spermatic sac and oocyte diameters for each month were analysed for male and female colonies, as well as monthly changes in the average diameter and fecundity (number of spermatic sacs or oocytes polyp−1).
Histological study
Histological sections were examined to identify gametogenic events and the correspondence between gamete size and developmental stage. Fragments from three male and three female colonies were decalcified in 10 % formic acid for 10 min, rinsed in running sea water for 15 min to remove excess acid from the tissues, dehydrated in an ethanol series (70, 80, 96, 100, 100 %, xylene) and embedded in paraffin. Histological sections 6–8 μm thick were stained with Ramón y Cajal’s Triple Stain (Gabe 1968), and the diameters of reproductive structures were measured.
To compare the diameter of oocytes measured in histological sections and on hydrated material observed under the stereomicroscope, we calculated the shrinkage suffered by the oocytes due to dehydration. The diameters of five oocytes from each size class were measured before and after the same dehydration process (ethanol series then xylene) and the per cent reduction calculated. Oocyte diameter is one of the main gametogenic characters used to compare different octocoral species.
Environmental parameters
Sea water temperature, photoperiod and irradiance data sets were assembled from different sources in order to test the possible coupling of gamete development dynamics with the seasonal changes of environmental parameters. Sea water temperature records were registered by the Meteorological Station of l’Estartit (see Pascual and Flos 1984; Rossi and Gili 2005) every 4–5 days at different depths (5, 20, 50 and 80 m) ~300 m away from the sampling point (1994–1995). Monthly mean day length at the latitude of the study area was calculated from sunrise and sunset times (available at the NASA website http://aom.giss.nasa.gov/srlocat.html).
Monthly mean values of daily solar horizontal irradiation recorded at the nearest station (Narbonne, France) were downloaded from the database of the Joint Research Centre of the European Commission: (http://re.jrc.ec.europa.eu/pvgis/apps/radmonth.php?lang=es&map=europe).
Horizontal irradiation is defined as the amount of solar radiation energy received by 1 m2 in a horizontal plane in one day (Page 1986) and is expressed in W h m −2d −1.
Statistics
A Spearman rank correlation (Zar 1999) was used to examine the relationship between oocyte diameters and environmental variables (solar irradiation, monthly changes of solar irradiation, temperature, monthly changes of temperature), as well as between spermatic sac diameters and the same variables. The rates of monthly change in irradiation and temperature were calculated as the ratio between the average value for a given month and the average value for the previous month. Statistics were performed using STATISTICA v6.
Results
Environmental parameters
Monthly mean temperature at 20-m depth in the study area ranged from 12 °C (January 1995) to 22 °C (August 1995), displaying the annual pattern characteristic of coastal waters in the Mediterranean Sea (Rossi and Gili 2005). The two-year temperature cycle at different depths (Fig. 1) shows how the seasonal conditions alternate between a stratified water column in summer and an isothermal water column in winter, with transitions during the formation of the thermocline in late spring and its breakdown in autumn storms. Warming and the consequent stratification started in May 1994 and 1995 (Fig. 1), with a lag of 2 months relative to the increase in solar irradiation. During the summer, wide variations in temperature (up to 5 °C) were found at 20 m depth, due to the vertical oscillations of the thermocline, probably driven by currents and winds in the study area (Rossi and Gili 2005).
At latitude 43º N, horizontal daily irradiation gradually increases in winter and spring (the lowest monthly mean value is 1,456 kWh m−2 d−1 in December) to reach a maximum in July (monthly mean value of 6,523 kWh m−2 d−1) and then decreases in the following seasons. Variation in the photoperiod (at this latitude, day length is 15 h 22 min at the summer solstice and 9 h at the winter solstice) and variation of sun altitude on the horizon mainly account for the seasonal changes in daily solar irradiation. The fastest increase in irradiation occurs in March (monthly mean was 160 % of that in February, Fig. 2).
General features of sexual reproduction
Alcyonium acaule was found to be gonochoric, and no hermaphrodite polyps were found among the 140 colonies examined. A. acaule is a surface brooder, and the mature oocytes released are retained for a few days by the mucus secreted on the surface of female colonies, where fertilization probably occurs. The reproductive cycle followed an annual pattern with breeding in summer (Figs. 2, 3).
The number of reproductive structures per polyp was highly variable among colonies in both females and males (Fig. 4), and only 5 % of the colonies examined were sterile. The average female fecundity prior to the release in July 1995 was 14.3 (±8.3 SD) oocytes polyp−1 (Fig. 4a), and in a few cases, up to 34 oocytes were counted in a single polyp. In male colonies, fecundity was higher, and the mean value in the same month was 30.4 (±9.5 SD) spermatic sacs polyp−1 (Fig. 4b), while up to 87 were counted in a single polyp in June 1995.
a Number of oocytes per polyp (monthly mean ± SD; N = 4–6 colonies each month; 5 polyps per colony observed) of A. acaule period (dark line). b Number of sperm sacs per polyp (monthly mean ± SD; N = 4–6 colonies each month; 5 polyps per colony observed) of A. acaule (dark line). Monthly means of gonad diameter are also shown (grey lines)
Oocytes had the greatest mean diameter in June and July 1995, a mean diameter of 472 μm (±42 SD) and 473 μm (±37 SD), respectively (Fig. 2), while the largest oocyte measured was 540 μm. Spermatic sacs before spawning had a mean diameter of 333 μm ± 127 SD in July 1994 and 400 μm ± 91 SD in July 1995 (Fig. 3). The maximum diameter was 580 μm.
Gamete development cycle and reproductive timing
The annual development cycle of male and female reproductive products culminated in summer. Size–frequency distributions of oocytes and spermatic sac diameters are shown in Figs. 5 and 6.
In both sexes, the formation of new gametes occurred over 10 mo as indicated by the presence of small gametes (0–100 μm diameter) from July to April. Gametogenesis seems to start prior to the release of ripe gametes and to continue during the maturation of the previous cohorts. The coexistence of two cohorts of oocytes and sperm sacs was observed in colonies sampled in July 1994. Average oocyte and spermatic sac diameter increased exponentially from September 1994 to July 1995 (Figs. 3, 4). Both female and male gametogenesis took 12–13 mo.
In female colonies, oocytes grew slowly from September (68 ± 23 μm mean diameter) to January (242 ± 75 μm), reaching 50 % of the mean diameter of mature oocytes (Fig. 2). After 3 mo of apparent rest, the female gametes doubled their mean diameter in a few months from March (238 ± 102 μm) to June (472 ± 42 μm). During ripening of the oocytes, standard deviations of monthly mean diameter gradually decreased (Fig. 2). In June and July, oocytes of uniform size (>400 μm) were present, while size classes <400 μm diameter almost disappeared (Fig. 5).
Spermatic sac diameter increased slowly from September 1994 (mean diameter 54 ± 19 μm) to April 1995 (mean diameter 171 ± 53 μm corresponding to 43 % of the pre-spawning diameter, see Fig. 3). By May, in a single month, the spermatic sacs were found to have doubled in diameter and during the following 2 mo reached the final size (400 ± 91 μm in July 1995), displaying a short and highly synchronized maturation.
The degree of reduction in the diameter of the oocytes caused by ethanol dehydration in the histological preparation was 7.7 % on average. However, the percentage of shrinkage decreased from about 10.6 % in oocytes of ~100 μm to 8 % for oocytes of ~400 μm. This difference between hydrated and dehydrated oocytes can be used for comparative purposes. Although these differences are negligible, this shrinkage should be taken into account when comparing the data on maximum diameters of mature oocytes among different studies.
The histological results are shown in Fig. 1 ESM. The pre-vitellogenic oocytes reach a maximum diameter of ~70 μm (Fig. 1D ESM). Larger oocytes start to increase the number of lipid droplets in the ooplasm (Fig. 1E ESM) until the ooplasma is filled with these lipid reserves (Fig. 1A ESM). Special attention was given to the 200–300 μm size class, where the increment in oocyte diameter ceases for 3 months (Jan–Mar). These oocytes are clearly vitellogenic (Fig. 1B ESM). Spermatic sacs had a thinner mesogloeal envelope than oocytes and numerous heads of developing spermatic sacs (Figs. 1F, G ESM).
The release of ripe reproductive products occurred in July as, by August, the polyps contained only small ones (<200 μm) (Figs. 5, 6). In September, 100- to 200-μm-diameter oocytes and spermatic sacs disappeared, probably reabsorbed.
There was a significant positive correlation between oocyte diameter and monthly changes in solar irradiation (R s = 0.599; P < 0.05) as well as between oocyte diameter increase and temperature (R s = 0.613; P < 0.05). Spermatic sac diameter was also significantly correlated with monthly changes in solar radiation (R s = 0.625; P < 0.05) and temperature (R s = 0.556; P < 0.05).
Discussion
Alcyonium acaule is a gonochoric species, a strategy described in most Alcyonacea (Hwang and Song 2007) and other Octocorallia (Ribes et al. 2007; Kahng et al. 2011). This differs from the simultaneous hermaphroditism, so common among the Hexacorallia (Harrison and Wallace 1990; Richmond and Hunter 1990; Kahng et al. 2011). However, most studies assessing gonochorism, including the present one, could not exclude sex changes during the life of these long-lived organisms (Fadlallah 1983; Loya and Sakai 2008; Kahng et al. 2011). Pluri-annual studies on tagged colonies would be necessary to exclude this possibility (but see Benayahu and Loya 1983).
Within the genus Alcyonium, internal brooding is the dominant mode of reproduction (McFadden et al. 2001), while broadcast spawning seems to be the rule among tropical reef-dwelling alcyoniid genera (Benayahu et al. 1990; Kahng et al. 2011). External brooding is uncommon in alcyonacean species (only 4 species of 64, Hwang and Song 2007), while it is more frequent among gorgonians (5 of 20 species, reviewed by Ribes et al. 2007). Surface brooding may be a successful adaptation for passive suspension feeders. For example, the Mediterranean gorgonian Paramuricea clavata is a surface brooder (Coma et al. 1995) and may achieve a 70 % fertilization rate (Linares et al. 2008). Lasker (2006) found high fertilization success (>90 %) in the Caribbean surface-brooding gorgonian Pseudopterogorgia elisabethae, demonstrating that surface brooding is an efficient mechanism for “harvesting” sperm released upstream of female colonies by extending the period over which eggs are likely to encounter sperm. Colony density and spawning synchronization are key determinants of fertilization rates (Lasker et al. 1996), especially in low-density populations such as A. acaule in the study area (5 colonies m−2, Gili and Ballesteros 1991). Also, external surface brooding enhances larval survival compared to planktonic development (Kahng et al. 2011), but can be seriously reduced by SCUBA diving disturbance (Tsounis et al. 2012).
Since in A. acaule the polyp cavity can be deeper and wider in bigger colonies, slight differences in colony size and shape may have an effect on gamete volume polyp−1 and may contribute to the variability in mean polyp fecundity among colonies. Reaching an optimal final size seems to be critical for the lecithotrophic oocytes of A. acaule, in contrast to the male spermatic sac, where greater size only implies a higher number of mature sperm. Pre-spawning spermatic sacs varied greatly in size as previously described for Clavularia hamra (Benayahu 1989), while in female colonies, the final oocyte size had low variability in June and July. The uniformity in final egg size in A. acaule contrasts with the variation in egg size in individual broods in other lecithotrophic species (see Kahng et al. 2011 for the octocoral range in oocyte diameter) and described as a strategy for producing offspring with variable dispersal potential (Marshall and Bolton 2007). An optimal oocyte final size may represent an effective strategy to ensure production of high-quality oocytes, thus minimizing energy waste and increasing the probability of fertilization and reproductive success (Ramirez-Llodra 2002; Excoffon et al. 2011).
Alcyonium acaule had an annual cycle of gamete development and release, which seems to be common among the alcyonaceans reviewed in this study with few exceptions (see Table 1 ESM). In order to detect seasonal timing cues for the synchronization of reproductive cycles, the different phases of the gamete development cycle were considered separately. The onset of gametogenesis, ripening (i.e. vitellogenesis in oocytes) and spawning may respond to different seasonal cues and have different exogenous and endogenous triggers. As Giese (1959) pointed out, description of breeding cycles from observations on spawning alone is not adequate for establishing the factors controlling breeding (Olive 1995).
The onset of gametogenesis (proliferation of germ cells and their differentiation into gametes) in A. acaule is almost continuous, as indicated by the presence of small gonads over 10 mo of the year. No clear pattern emerges among alcyonaceans, as the formation of new gametes may be continuous or last only a few (1–2) to several (7–8) mo (see Table 1 ESM), generally starting after or during the release of the previous generation. The production of new germ cells may not require high energy investment and may be insensitive to selective pressures (e.g. food availability), requiring synchronization with environmental seasonal cycles. Therefore, this process could be controlled by inherited, endogenous rather than external environmental factors. On the other hand, the ripening of gametes, especially of oocytes, is an energy-intensive process and, therefore, extremely sensitive to selective pressures (Ramirez-Llodra 2002). Vitellogenesis is especially demanding, as the production and the storage of yolk requires a large energy investment apart from important changes in metabolism. Therefore, these processes must be tightly linked to seasonal food availability (Coma et al. 1998).
In A. acaule oocytes grew exponentially in diameter. Data sets available for Mediterranean species at different sites, depth ranges and years (for references see Table 1) allowed us to identify a pattern that seems to be constant independent of these variables. For the three gorgonians Eunicella singularis, P. clavata and Corallium rubrum as well as for A. acaule in this study, oocyte growth rate increases notably at the end of winter (February–March) indicating an increase in the rate of vitellogenesis. Their diameter increases from ~60 to 100 % of their maximum size by June, independent of year, depth or site of study, while the moment of spawning can vary in relation to these factors. Such a coherent pattern is likely to respond to a reliable cue that synchronizes metabolic effort with food availability.
Traditionally, sea water temperature cycles have been associated with reproductive timing, but environmental signals other than temperature could be involved in pushing the energy-demanding process of vitellogenesis. In the Mediterranean Sea, an average temperature of 12 °C (±1 °C with little interannual variation, Rossi and Gili 2005) is maintained throughout the water column in the winter (January and February), with a negligible increase in March and April of ~0.5 °C. Therefore, we may exclude temperature as an influence on oocyte maturation in A. acaule, although it may be important in sperm maturation starting in May. The final maturation took only 3 months, and its onset was delayed relative to oocyte maturation in females, but culminated in synchronization between the sexes.
Considerable changes in solar irradiation occur around the equinoxes at intermediate latitudes as photoperiod and solar altitude increase, and the maximum rate of change occurs at the spring equinox (March in the northern hemisphere). These changes may provide a reliable cue to reset the biological clock and trigger the physiological changes related to vitellogenesis (Mc Clintock and Watts 1990; Rees and Olive 1999). We found a positive correlation between oocyte diameter increase in A. acaule and monthly changes in solar irradiation that supports this hypothesis. The effects of changing photoperiod and solar irradiation on gametogenic cycles of anthozoans have been largely overlooked, with the exception of occasional studies such as Mechawar and Anctil (1997) who described seasonal cycles of melatonin levels in the sea pansy Renilla köllikeri, with an annual increase coinciding with the first stages of sexual maturation. Melatonin is an important photoperiodic and seasonal messenger in vertebrates, but its role and regulatory mechanisms in primitive metazoans are still unclear. The depth range of A. acaule is 10–90 m (Rossi and Gori pers. obs.), where blue light still penetrates in warm temperate seas. Therefore, it is possible that physiological changes associated with vitellogenesis in A. acaule are induced by seasonal variation in irradiation. To assess the effect of light on coral reproductive behaviour, the crucial issue is the presence of photoreceptors in these basal invertebrates, a relatively unexplored area of research.
The formation and oscillations of the seasonal thermocline during summer result in wide (a few degrees) and frequent (daily temporal scale) variations in surface water temperature down to 100 m in the study area. Changes in temperature, therefore, may constitute a confusing signal, while reaching a minimum threshold temperature (i.e. A. acaule spawns at 18 °C in June in Mediterranean) may be a clearer signal for spawning in temperate seas with a wide thermal range (12–23 °C). However, experimental or longer field studies are needed to corroborate this hypothesis.
Knowing the environmental features relevant to the reproductive cycles of eco-engineering species (sensu Jones et al. 1994) is essential for future conservation planning. The potential role of solar irradiation as a trigger in oocyte maturation in A. acaule is a new concept that should be taken into account with others like temperature and food availability in future management models.
References
Babcock RC, Bull GD, Harrison PL, Heyward AJ, Oliver JK, Wallace CC, Willis BL (1986) Synchronous spawning of 105 scleractinian coral species on the Great Barrier Reef. Mar Biol 90:379–394
Babcock RC, Willis BL, Simpson CJ (1994) Mass spawning of corals on a high latitude coral reef. Coral Reefs 13:161–169
Benayahu Y (1989) Reproductive cycle and developmental processes during embryogenesis of Clavularia hamra (Cnidaria, Octocorallia). Acta Zool (Stockh) 70:29–36
Benayahu Y (1991) Reproduction and developmental pathways of Xeniidae (Octocorallia: Alcyonacea). Hydrobiologia 216(217):125–130
Benayahu Y, Loya Y (1983) Surface brooding in the red sea soft coral Parerythropodium fulvum fulvum (Forskaal, 1775). Biol Bull 165:353–369
Benayahu Y, Loya Y (1984) Life history studies on the red sea soft coral Xenia macrospiculata (Gohar,1940). I. Annual dynamics of gonadal development. Biol Bull 166:32–43
Benayahu Y, Loya Y (1986) Sexual reproduction of a soft coral: synchronous and brief annual spawning of Sarcophyton glaucum (Quoy and Gaimard, 1833). Biol Bull 170:32–34
Benayahu Y, Weil D, Kleinman M (1990) Radiation of broadcasting and brooding patterns in coral reef alcyonaceans. Adv Invertebr Reprod 5:323–328
Ben-David-Zaslow Y, Henning G, Hofmann DK, Benayahu Y (1999) Reproduction in the red sea soft coral heteroxenia fuscescens: seasonality and long-term record (1991–1997). Mar Biol 133:553–559
Choi EJ, Song JI (2007) Reproductive biology of the temperate soft coral Dendronephthya suensoni (Alcyonacea: Nephtheidae). Integrat Biosci 11:215–225
Coma R, Ribes M, Zabala M, Gili JM (1995) Reproduction and cycle of gonadal development in the Mediterranean gorgonian Paramuricea clavata. Mar Ecol Prog Ser 117:173–183
Coma R, Ribes M, Gili JM, Zabala M (1998) An energetic approach to the study of life-history of two modular colonial benthic invertebrates. Mar Ecol Prog Ser 162:89–103
Cordes EE, Nybakken JW, VanDykhuizen G (2001) Reproduction and growth of Anthomastus ritteri (Octocorallia: Alcyonacea) from Monterey Bay California, USA. Mar Biol 138:491–501
Dahan M, Benayahu Y (1997) Reproduction of Dendronephthya hemprichi (Cnidaria: Octocorallia): year-round spawning in an azooxanthellate soft coral. Mar Biol 129:573–579
Estrada M (1996) Primary production in the northwestern Mediterranean. Sci Mar 60(2):55–64
Excoffon AC, Navella ML, Acuña FH, Garese A (2011) Oocyte production, fecundity, and size at the onset of reproduction of Tripalea clavaria (Cnidaria: Octocorallia: Anthothelidae) in the Southern Atlantic. Zool Studies 50:434–442
Fadlallah YH (1983) Sexual reproduction, development and larval biology in scleractinian corals. Coral Reefs 2:129–150
Fan TY, Chou YH, Dai CF (2005) Sexual reproduction of the Alcyonacean Coral Lobophytum pauciflorum in Southern Taiwan. Bull Mar Sci 76:143–154
Farrant PA (1986) Gonad development and the planulae of the temperate Australian soft coral Capnella gaboensis. Mar Biol 92:381–392
Gabe M (1968) Technique histologique. Massou et Cie, Paris
Garrabou J (1999) Life-history traits of Alcyonium acaule and Parazoanthus axinellae, (Cnidaria, Anthozoa), with emphasis on growth. Mar Ecol Prog Ser 178:193–204
Garrabou J, Coma R, Bensoussan N et al (2009) Mass mortality in Northwestern Mediterranean rocky benthic communities: effects of the 2003 heat wave. Glob Change Biol 14:1090–1103
Giese AC (1959) Comparative physiology: annual reproductive cycles of marine invertebrates. Annu Rev Physiol 21:547–576
Giese AC, Pearse JS (1974) Introduction: general principles. In: Giese AC, Pearse JS (eds) Reproduction of marine invertebrates. Acoelomate and pseudocoelomate metazoans, vol 1. Academic, New York, pp 1–49
Gili JM, Ballesteros E (1991) Structure of cnidarian populations in Mediterranean sublittoral benthic communities as a result of adaptation to different environmental conditions. In: Ros JD, Prat N (eds) Homage to Ramón Margalef; or why there is such pleasure in studying nature. Oecol Aquat 10: 243–254
Gili JM, Coma R (1998) Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol Evol 13:316–321
Gili JM, Ros JD (1985) Study and cartography of the benthic communities of the Medes Islands (NE Spain). Mar Ecol 6:219–238
Gori A, Linares C, Rossi S, Gili JM (2007) Spatial variability in reproductive cycle of the gorgonians Paramuricea clavata and Eunicella singularis (Anthozoa, Octocorallia) in the Western Mediterranean Sea. Mar Biol 151:1571–1584
Harrison PL, Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z (ed) Coral reefs. Elsevier Science Publishers, Amsterdam, pp 133–207
Hartnoll RG (1975) The annual cycle of Alcyonium digitatum. Est Coast Shelf Sci 3:71–78
Hartnoll RG (1977) Reproductive strategy in two British species of Alcyonium. In: Keegan BF, Ceidigh PO, Boaden PJS (eds) Biology of benthic organisms. Pergamon Press, Oxford, pp 321–328
Hwang SJ, Song JI (2007) Reproductive biology and larval development of the temperate soft coral Dendronephthya gigantea (Alcyonacea: Nephtheidae). Mar Biol 152:273–284
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Kahng SE, Benayahu Y, Lasker HR (2011) Sexual reproduction in octocorals. Mar Ecol Prog Ser 443:265–283
Kinlan BP, Gaines SD, Lester SE (2005) Propagule dispersal and the scales of marine community process. Diversity Distrib 11:139–148
Kruger A, Schleyer MH, Benayahu Y (1998) Reproduction in Anthelia glauca (Octocorallia: Xeniidae). I. Gametogenesis and larval brooding. Mar Biol 131:423–432
Lacaze-Duthiers H (1900) Coralliaires du Golfe du Lion. Alcyonaires. Arch Zool Expér Gén 3:352–462
Lasker HR (2006) High fertilization success in a surface-brooding Caribbean gorgonian. Biol Bull 210:10–17
Lasker HR, Brazeau DA, Calderon J, Coffroth MA, Coma R, Kim K (1996) In situ rates of fertilization among broadcast spawning gorgonian corals. Biol Bull 190:45–55
Linares C, Coma R, Mariani S, Díaz D, Hereu B, Zabala M (2008) Early life history of the Mediterranean gorgonian Paramuricea clavata: implication for population dynamics. Invertebr Biol 127:1–11
Lo Bianco L (1909) Notizie biologiche riguardanti specialmente il periodo di maturitá sessuale degli animali del golfo di Napoli. Mitt Zool Ston Neaple 19:513–761
Loya Y, Sakai K (2008) Bidirectional sex change in mushrooms stony corals. Proc R Soc B 275:2335–2343
Marshall DJ, Bolton TF (2007) Effects of egg size on the development time of non-feeding larvae. Biol Bull 212:6–11
Mc Clintock JB, Watts SA (1990) The effects of photoperiod on gametogenesis in the tropical sea urchin Eucidaris tribuloides (Lamarck) (Echinodermata: Echinoidea). J Exp Mar Biol Ecol 139:175–184
McFadden CS, Donahue R, Hadland BK, Weston R (2001) A molecular phylogenetic analysis of reproductive trait evolution in the soft coral genus Alcyonium. Evolution 55:54–67
Mechawar N, Anctil M (1997) Melatonin in a primitive metazoan: seasonal changes of levels and immunohistochemical visualization in neurons. J Comp Neurol 387:243–254
Mendes JM, Woodley JD (2002) Timing of reproduction in Montastraea annularis: relationship to environmental variables. Mar Ecol Prog Ser 227:241–251
Olive PJW (1995) Annual breeding cycles in marine invertebrates and environmental temperature: probing the proximate and ultimate causes of reproductive synchrony. J Thermal Biol 20:79–90
Page JK (1986) Prediction of solar radiation on inclined surfaces. In: Page JK (ed) Solar energy R&D in the European community, series F: solar radiation data, vol 3. D. Reidel Publishing Company, Dordrecht
Pascual J, Flos J (1984) Meteorología i oceanografía. In: Ros J, Olivella I, Gili JM (eds) Els Sistemes naturals de les Illes Medes. Institut d’Estudis Catalans, Barcelona, pp 75–114
Pearse JS, Eernisse DJ (1982) Photoperiodic regulation of gametogenesis and gonadal growth in the sea star Pisaster ochraceus. Mar Biol 67:121–125
Ramirez-Llodra E (2002) Fecundity and life-history strategies in marine invertebrate. Adv Mar Biol 43:87–170
Rees SW, Olive PJW (1999) Photoperiodic changes influence the incorporation of vitellin yolk protein by oocytes of the semelparous polychaete Nereis (Neanthes) virens. Comp Biochem Physiol A 123:213–220
Ribes M, Coma R, Rossi S, Micheli M (2007) The cycle of gonadal development of Eunicella singularis (Cnidaria: Octocorallia): trends on sexual reproduction in Mediterranean gorgonians. Invertebr Biol 126:307–317
Richmond RH, Hunter CL (1990) Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific, and the Red Sea. Mar Ecol Prog Ser 60:185–203
Rossi S, Gili JM (2005) Temporal variation and composition of near-bottom seston features in a Mediterranean coastal area. Est Coast Shelf Sci 65:385–395
Rossi S, Gili JM (2009) Reproductive features and gonadal development cycle of the soft bottom-gravel gorgonian Leptogorgia sarmentosa (Esper 1791) in the NW Mediterranean Sea. Inv Repro Develop 53:175–190
Santangelo G, Carletti E, Maggi E, Bramanti L (2003) Reproduction and population sexual structure of the overexploited Mediterranean red coral Corallium rubrum. Mar Ecol Prog Ser 248:99–108
Schleyer MH, Kruger A, Benayahu Y (2004) Reproduction and the unusual condition of hermaphroditism in Sarcophyton glaucum (Octocorallia, Alcyoniidae) in KwaZulu-Natal, South Africa. Hydrobiologia 530/531:399–409
Tsounis G, Rossi S, Araguren M, Gili JM, Arntz WE (2006) Effects of spatial variability and colony size on the reproductive output and gonadal development cycle of the Mediterranean red coral (Corallium rubrum L.). Mar Biol 143:513–527
Tsounis G, Martínez L, Bramanti L, Viladrich N, Martínez A, Gili JM, Rossi S (2012) Effects of human impact on the reproductive effort and allocation of energy reserves in the Mediterranean octocoral Paramuricea clavata. Mar Ecol Prog Ser 449:161–172
Waller RG, Tyler PA (2005) The reproductive biology of two deep-water, reef-building scleractinians from the NE Atlantic Ocean. Coral Reefs 24:514–522
Yamazato K, Sato M, Yamashiro H (1981) Reproductive biology of an alcyonacean coral, Lobophytum crassum Marenzeller. Proc 4th Int Coral Reef Symp 2:671–678
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle River
Acknowledgements
This work has been financed by the project D.G.I.C.Y.T. 1995-1998 PB94-0014-C02-0 and BENTOLARV CTM2009-10007. IF was funded by a FPU grant of Spain’s Ministry of Education and Science. SR was funded by a Ramón y Cajal contract (RYC-2007-01327). The authors are especially grateful to Josep Pascual for environmental data collection. We also thank C. Linares for the preliminary treatment of data. C. Orejas and G. Tsounis and three anonymous referees improved previous versions of this manuscript with their useful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. P. Grassle.
Electronic supplementary material
Below is the link to the electronic supplementary material.
227_2012_2126_MOESM1_ESM.pdf
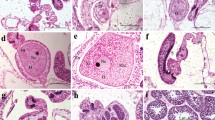
Fig. 1 Electronic Supplementary Material (ESM) Alcyonium acaule, histological sections. Female colony: A, transverse section through gastrovascular cavity showing a pre-vitellogenic oocyte (pvo) and four vitellogenic oocytes (vo), mesenteries (mes) and mesogloea matrix (meg); B, vitellogenic oocytes (vo); C, early stage of pre-vitellogenic oocytes, note nucleolus, nucleus and reduced basophilic ooplasm; D and E, oocytes starting vitellogenesis. Male colony: F, group of spermatic sacs; G, spermatic sac (PDF 23288 kb)
Rights and permissions
About this article
Cite this article
Fiorillo, I., Rossi, S., Alva, V. et al. Seasonal cycle of sexual reproduction of the Mediterranean soft coral Alcyonium acaule (Anthozoa, Octocorallia). Mar Biol 160, 719–728 (2013). https://doi.org/10.1007/s00227-012-2126-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2126-z