Abstract
Red coral (Corallium rubrum, L. 1758) is an over-exploited Mediterranean gorgonian. The gonadal development cycle of this gorgonian is examined at the Costa Brava (NW Mediterranean) taking into account for the first time colony size, depth and spatial horizontal variability. This study compares the gonad development and fertility in two colony size classes (colonies <6-cm height, and >10-cm height, both at 40–45-m depth), and two populations at different depths (16–18-m depth, and 40–45-m depth, both consisting of <6-cm high colonies) in a 15-month period. The fertility of seven size classes (<2 cm to >12 cm high colonies, in 2 cm intervals) was examined in the deep population, where large colonies were present. Furthermore, reproductive output was compared in 6 populations (distributed along more than 70-km coastline) one month before spawning (June). Red coral was found to be dioecious and gonochoric with a sex ratio of 1:1, which differs from other NW Mediterranean populations. On the other hand, fertility of different size classes indicates that small colonies of 2-cm height already produce gonads, which is in line with previous studies. Female and male polyp fertility and sperm sac size increase significantly with colony size [sperm sac diameter: 476±144 μm (mean±SD) and 305±150 μm in the >10-cm and <6-cm height colonies, respectively), whereas no significant effect on oocyte diameter was found (oocyte diameter: 373.7±18.7 μm). Depth staggered spawning, that is, an earlier release of gonads in the shallow populations, was observed in summer 2003, coinciding with the highest temperature gradient between shallow and deep water during the study period. Colonies of <6-cm height were significantly less fertile than colonies >12 cm, thus the recommendation of this study is that a minimum height should be incorporated into fishing regulations. The six studied populations at the Costa Brava showed a comparable reproductive potential, which demonstrates little variability within the homogenous population structure and range of size classes (due to overharvesting) found at the Costa Brava. The study of reproductive output is an important tool for ecosystem management, and this work recommends basing specific exploitation laws for distinctive populations on colony size, which is found to have a larger effect on reproductive potential than mesoscale variability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reproductive cycle of a species and factors affecting the reproductive potential of a population are crucial for a better understanding of population dynamics (e.g., Dayton 2003). These kinds of studies are especially relevant for key- and exploited species. Gorgonian octocoral populations are characteristic components that contribute significantly to biomass and energy flow of many ecosystems (e.g., Brazeau and Lasker 1989; Coma and Gili 1998; Arntz et al. 1999), yet little is known about the spatial variability of their reproductive output.
The Mediterranean red coral (Corallium rubrum, L. 1758) is a long-lived species with slow growth, but an exception to the general K-selection pattern in gorgonians, as it has a low age at first reproduction (Vighi 1972; Grigg 1989; Santangelo et al. 2003a, 2003b). Its age structure is generally dominated by young, small colonies (Santangelo and Abbiati 2001; Santangelo et al. 2003a), and the species may be at risk of local extinction in some places. Recent data demonstrate that this is the effect of red coral being systematically harvested for use in the jewellery industry since the seventeeth century (Tescione 1973; Santangelo and Abbiati 2001; Tsounis 2005). Thus there is a need to understand better how colony size and environmental factors affect its reproductive potential, in order to develop optimal harvesting strategies and protective measures.
Colony size of modular organisms affects the allocation of energy into reproduction (Hall and Hughes 1996; Beiring and Lasker 2000) and the ability of an octocoral to feed (Kim and Lasker 1997). Knowing the relationship between reproductive output and colony size allows an improved harvesting strategy that does not harvest the stock down to colonies of lower fertility (Grigg 1976). We hypothesize that polyp fertility and gonad diameter increase with colony size in red coral as it does in other gorgonians (Coma et al. 1995b; Beiring and Lasker 2000).
Population dynamics of modular organisms are frequently influenced by the depth at which they live, as depth affects the feeding capabilities of corals, and consequently their potential energy reserves (see Harland et al. 1992). This has a direct effect on larval recruitment, as a depth-dependent settlement of new recruits has been demonstrated in scleractinian corals (Mundy and Babcock 2000). Also, spawning has been found to occur earlier in shallow water colonies than in deep ones (Vighi 1972; Santangelo et al. 2003b). As harvesting of red coral is highly depth-selective, favouring shallow water (Tsounis 2005), it is vital to elucidate the contribution of colonies at various depth zones to the recoverability potential of the population. As C. rubrum thrives in the aphotic zone, we hypothesized an increase of reproductive output with depth.
A recent study demonstrated considerable micro- and mesoscale variability in the red coral recruitment (Bramanti et al. in press), as larval settlement and mortality were different off the Costa Brava (NW Mediterranean Spanish coast) and Calafuria (NW Mediterranean Italian coast). Given the direct effect of reproductive output on larval viability in red coral populations (Bramanti et al. 2004), spatial variability of reproductive output may have implications, as studies restricted to a limited spatial scale may be insufficient to give a base for the management of the fishery of this benthic species.
In contrast to previous studies concentrated in a particular area, this study elucidates the spatial variability of reproductive output between different populations. This is because one of the main difficulties in managing the red coral banks in the Mediterranean is due to coral-fishermen insisting that, in selected areas, the coral growth and reproduction are different and require a discriminating exploitation law. One of the goals of this study therefore, is to demonstrate the spatial reproduction patterns of red coral populations in order to provide a base for the decision if a common protocol rule or law should be proposed.
Material and methods
Sex ratio and size at first reproduction
In order to examine the size at first reproduction, seven colony size classes were sampled, ranging from 0–2 cm to >12 cm colony height. Twenty branches from 20 different colonies were sampled per class on 11 July 2002 at 40–45-m depth. This was shortly before the release of the gonads, thus ensuring that the sampling did not underestimate the fertility of the colonies. In total, 586 colonies were sampled in June 2002 in order to test the sex ratio of the Medas Islands population.
In all cases red coral samples were taken using cutting pliers extracting a 2 cm long apical coral branch, placing them in a seawater filled PVC bottle that was kept in ice until fixation with 10% Formalin (usually within 30 min). The coral samples were processed by opening coral polyps of the branches on the central part with fine tipped forceps under a binocular dissecting microscope (50× magnification) and counting oocytes and sperm sacs, as well as measuring their diameter with a calibrated eyepiece micrometer. Five polyps per branch were subsampled at random.
Gonadal development cycle and reproduction timing
Gonad development and number were studied in two populations at the Medas Islands Marine Protected Area in monthly sampling intervals between April 2002 and August 2003 from 20 different colonies <6 cm in height (sampled at random in each population). The sampled colonies measured 4–6 cm in height, representing the most abundant size class in the Mediterranean (Fig. 1) (Santangelo et al. 2003a; Garrabou et al. 2001; Tsounis 2005). One population (following the definition of Lincoln et al. 1998) is situated at an overhang at 16–18-m depth, while the other grows on a wall between 40 and 45 m. The distance between the two sites is 300 m, both being affected by the same main current (Pascual et al. 1995) (Fig. 2). Although red coral larvae survive for several days (Weinberg 1979) and can be swept considerable distances by currents, recent data suggest that there is only a negligible gene flow between the two populations at the Medas Islands and the opposite coast of Montgri (del Gaudio et al. 2004). About 600 colonies were sampled that measured 4–6 cm in colony height (the maximum distance from the base of the coral to the branch tips).
Frequency distribution of colony size of C. rubrum colonies at the Costa Brava. Height is measured as the linear distance from the base of the stem to the farthest tips (from Tsounis 2005)
Differences between size classes
In order to examine the influence of colony size on gonad development, an additional 300 samples from colonies larger than the 4–6 cm were taken from the population at 40 m, where enough large colonies could be found. These colonies measured 10–12 cm in height and were taken in the same monthly intervals as the other samples. This allowed a comparison of gonadal development and reproductive output of the two size classes.
Spatial comparison
Reproduction features of six red coral populations from different sites along the Costa Brava (see Fig. 2 and Table 1) have been sampled by SCUBA between 20 and 24 June 2003. Twenty colonies <6 cm height were sampled from each population (at 18 and 45-m depth, respectively) in the same way as described earlier. All populations are characterised by a typical rocky shore sublitoral hardbottom community, the “coralligene” (e.g. Sará 1969; Gili and Ros 1984).
Data analysis
In order to test the hypothesis that the colony size classes varied in reproductive output, a one-factorial ANOVA was used to analyse differences in colony fertility and gonad size between red coral colony size classes. Similarly, ANOVA was used to test for differences in reproductive output between the two depth zones sampled, and likewise between the five populations. The sex ratio was tested by applying a chi-square test.
The information from the gonad development cycle, in order to compare gonad quantity and size, allowed the exclusion of immature gonads by using only data from April to July. Colony fertility was defined as the number of gonads in five polyps per colony, using 20 replicate colonies per month. Gonad size was defined as the average diameter of gonads encountered in five polyps per colony.
The data were not distributed normally and had different variances but met both of these criteria for parametric analysis after logarithmic transformation (Brown-Forsythe test and Levene test, P = 0.05; Shapiro Wilk test, P = 0.1), thus permitting the use of an ANOVA. An exception was the oocyte number data comparing the two size classes, which had to be transformed using a modified square root transformation (Zar 1996), in order to show comparable variances, and the oocyte diameter data comparing spatial differences had to be tested using a weighted, nonparametric (Welsh) ANOVA (JMP Synergy software), as data transformation did not result in comparable variances.
Results
General reproductive features
Red coral was found to be dioecious and gonochoric both at the colony and at the polyp level. The overall sex structure of the Medas Islands population did not significantly deviate from 1:1 (X 2 = 1.3; P>0.05). In total, 281 female and 305 male colonies were found. About 2.0±2.0 mature gonads per polyp were found.
Gonadal development cycle and reproduction timing
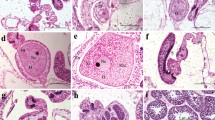
Gonad development in red coral followed an annual cycle with a synchronised release in summer. Oocyte development took more than 12 months, which manifested itself in the presence of two size classes of oocytes (Fig. 3). The ripe gonads measured 500–900 μm (Fig. 4) and were released in July/August, whereas the second, immature cohort consisted of oocytes of 50–400 μm that continued to grow until their release the following year (Fig. 5). Prior to the release in summer, there was a reabsorbing of oocytes, as oocyte numbers dropped between April and May (Fig. 6).
Sperm sac development followed an annual cycle with release of all sperm sacs in July (Fig. 7). Before release, they reached a maximum size of 900 μm. No sperm sacs were found in August 2002 (Fig. 8), and polyps started containing newly formed sperm sacs of 50–300-μm diameter in November (Fig. 9).
Differences between size classes
The sperm sac diameter was significantly larger in the large colonies (476±144 μm) (mean±SD) than in the small ones (305±130 μm) (ANOVA: F = 50.3; P ≤ 0.001). No significant differences in oocyte diameter were found between the two size classes (>10 cm: 418±178 μm; <6 cm: 388±144 μm). The average gonad number was higher in the large colonies (0.66 oocytes polyp−1 ± 0.024 and 2.42 sperm sacs × polyp−1 ± 0.166) than in the small ones (0.58 oocytes polyp−1 ± 0.024 and 1.74 sperm sacs × polyp−1 ± 0.18) (oocytes: ANOVA: F = 4.2; P < 0.05; sperm sacs: ANOVA: F = 7.6; P = 0.05).
The examination of six size classes revealed that all of them were fertile, including the class with colonies smaller than 2-cm height, however, colonies smaller than 2 cm contained less than 1 gonad polyp−1 (Fig. 10). Colonies of 2–4-cm height, which constituted the majority of population, showed 60% fertility and colonies of 4–6-cm height showed 100% fertile polyps (Fig. 11).
Differences due to depth
Gonads were significantly larger at 20-m depth (oocytes: 404.6±19.2 μm; sperm sacs: 426.5±17.1 μm) than at 40-m depth (oocytes: 342.8±18.1 μm; sperm sacs: 287.3±18 μm) (oocytes: ANOVA: F = 5.5; P = 0.05; sperm sacs: ANOVA: F = 31.6; P ≤ 0.001). No significant differences between the two depths were found in oocyte and sperm sac numbers. The gonad number data reveal that in 2002 the release was synchronized between the two depths, whereas in 2003 the release was depth staggered, with the shallow colonies releasing earlier than the deep ones (Figs. 12, 13).
Spatial comparison
The mean oocyte diameter differed significantly between populations (Table 2) (ANOVA: F: 2.6; P = 0.05), as diameters were larger at the shallow population at the Medas Islands than at Ses Ielles (eastern Cap de Creus, Fig. 2). However, no significant differences between the areas were found in oocyte and sperm sac numbers, and in sperm sac diameter (Figs. 14, 15).
Discussion
General reproductive features
All populations were found to be gonochoric at both the polyp and colony level, as previously found in other red coral and octocoral populations (Vighi 1972; Benayahu et al. 1989; Brazeau and Lasker 1990; Coma et al. 1995a; Santangelo et al. 2003b).
The sex ratio of C. rubrum of 1:1 at the Costa Brava differs from a recently studied population in Italy, where a bias towards females was found (Santangelo et al. 2003b). Other gorgonian species living in the same red coral habitat show the same sex ratio as C. rubrum (Paramuricea clavata, Coma et al. 1995a; Eunicella singularis, Weinberg and Weinberg 1979), and other tropical and Antarctic gorgonians show the same reproductive strategy (Kinzie 1974; Orejas et al. 2002). The difference in the sex ratio between the Italian and Spanish red coral populations may be explained by different population densities between the two sites (Santangelo et al. 1993; Tsounis 2005), which in turn may be related to different larval recruitment in both places (Bramanti et al. in press). It has been demonstrated that population density in gorgonians affects the optimal fertilization strategy and thus sex ratio (Brazeau and Lasker 1990). On the other hand, the number of mature oocytes per polyp (2.0±2.0) falls within the range of other studied populations (3–6, Vighi 1972; 1–2, Santangelo et al. 2003a, 2003b), and appears to be at the lower to middle spectrum of the range of octocorals (see Coma et al. 1995b).
The results of the present study show that size at first reproduction in the C. rubrum population of the studied area is remarkably small, corresponding to a young age, as previously observed by Santangelo et al. (2003b). Unlike most octocorals that are typically characterised as K-selected species with long lifetimes, low mortality and low reproductive output (Grigg 1989; Gotelli 1991; Santangelo et al. 1993; Coma et al. 1995b), red coral appears to have a small size/age at first reproduction (Table 3) that enables it to sustain its populations despite intensive harvesting (Grigg 1989). However, the polyp fertility increases considerably with colony size, as found in other octocorals (e.g., Hall and Hughes 1996; Beiring and Lasker 2000). Thus the results of this study show that polyp fertility is significantly lower in colonies smaller than 6-cm height, requiring a minimum harvestable size limit well above the current average size of Costa Brava populations of 5-cm height (Tsounis 2005).
Gonadal development cycle
The gonadal development cycle of red coral culminates in an annual summer spawning, a common phenomenon among octocorals in temperate seas (Weinberg and Weinberg 1979; Coma et al. 1995a; Santangelo et al. 2003b). Oocyte development in red coral takes longer than sperm development, which is a feature also shared with other octocorals (Benayahu and Loya 1984; Coma et al. 1995a) and scleractinians (Kojis and Quinn 1981; Szmant-Froelieh 1981). Oocyte development lasts longer than a year, so that immature oocytes are present in summer and are not released until they mature in the following year. Only the first cohort of ripe gonads is released. This has been observed earlier in a red coral population at the French coast (Vighi 1972), and also in the Mediterranean gorgonian P. clavata (Coma et al. 1995a). Production of sperm sacs begins in September and ends with their release in July, and is thus much shorter than gonad production. Oocyte numbers dropped between April and May due to a reabsorbing of gonad tissue. This little understood phenomenon has also been observed in the Caribbean gorgonian Plexaura “A” (Brazaeu and Lasker 1989).
The larval release follows the sudden drop in oocyte number and diameter, as stated in a recent study made in the Medas Islands (Bramanti et al. in press). In fact, recruitment seems to follow the same trends in terms of time, but not in number of larvae, in the Calafuria population (Italy) (Bramanti et al. 2003, 2004). In the same type of coralligenous habitat another octocoral (Eunicella singularis) also releases the larvae in July (Weinberg and Weinberg 1979). The stabilization of the thermocline has been suggested to be the main signal for larval expulsion in both octocorals (Weinberg 1979).
Differences between size classes
The results show a significant difference in reproductive output between the two size classes; large colonies contained significantly more oocytes and sperm sacs. Sperm sacs were also larger in the large colonies. The separate examination of colony fertility in the seven size classes revealed that even the small colonies of 2-cm height produce gonads, although in small numbers. Thus these data confirm the hypothesis that size has a positive effect on reproductive potential.
Small colonies produce fewer gonads as reproduction is of lesser priority during the young life phase. A greater resource allocation to growth at smaller size means a faster escape out of vulnerable size classes (Kojis and Quinn 1981; Szmant-Froelich 1985; Soong 1993; Ward 1995a; Hall and Hughes 1996; Beiring and Lasker 2000), improving their chances of survival, as small colonies suffer greater mortality (Connell 1973; Hughes and Jackson 1985; Jackson and Hughes 1985; Babcock 1991).
Colony size significantly affects the reproductive output (Ward 1995b), and has a direct relation to the number of released planulae (Tioho et al. 2001). The higher fertility of large colonies has a special implication in the case of red coras, as it is a harvested species with its population structure distorted towards young colonies (Santangelo and Abbiati 2001; Tsounis 2005). In the Mediterranean gorgonian P. clavata large colonies constitute 3% of the population, but contribute 40% of female gametes and 33% of male gametes (Coma et al. 1995b). In the tropical blue coral Heliopora coerulea, colonies larger than 6-cm radius constitute less than 22% of the colonies, but produce 80% of total annual gonad production (Babcock 1984).
However, not only is polyp fertility higher in large colonies, but the total number of reproductive modules increases exponentially with colony size of modular organisms, especially if they are highly branched (Stiller and Rivoire 1984; Sakai 1998; Santangelo et al. 2003b). Thus large colonies contribute disproportionately to gonad production, as in the case of Plexaura flexuosa, where large colonies (>70 cm in height) produced on average six times more gonads per polyp than small colonies, and 98% of the gonads were produced by half of the colonies (Beiring and Lasker 2000). These studies demonstrate the importance of large colonies for the survival of an octocoral population. In red coral this is of even higher relevance due to the size-selective harvesting.
Differences due to depth
The significantly larger gonad diameters in the shallow population are the result of depth-staggered spawning induced by seasonal differences in temperature stratification. Water temperature at 18 m usually rises above 22°C in summer, whereas at 40 m the water temperature did not rise above 16°C in summer 2003 (Meteo Service of Estartit). A similar situation has been recorded in previous years in the same area (Cebrián et al. 1996). This increase in temperature shortens the time necessary to complete gametogenesis (Vighi 1972; Grigg 1977), thus the gonads in shallow colonies reach their terminal size earlier than deep ones. The same has been observed in the Californian gorgonians Muricea californica and Muricea fructicosa (Grigg 1977) and also in a nearby red coral population in France (Vighi 1972). A recent study on an Italian population also found that shallow colonies released gonads earlier than deep ones, and did not detect any differences in the gonad diameter nor in the number of gonads per polyp between depths (Santangelo et al. 2003b). The authors noted the fact that the studied depth range was narrow, compared to the range of red coral (20–200 m), and the modest temperature interval of ΔT = 0.5°C (Santangelo et al. 2003b). In the same Italian population, however, recruitment was found to be higher in the shallow colonies (Bramanti et al. 2003). The present study did not observe an asynchronous spawning in the studied depths in 2002; merely in summer 2003, which coincided with the strongest temperature differential during the study (Tsounis personal observation). Thus we suggest that depth-staggered spawning in red coral occurs only during pronounced temperature differences. Finally, although gonad numbers were not significantly different between the two populations, and assuming reproductive output to be the same at both depths, it is possible that the variability within the population may mask a difference in reproductive output. Future research on wider depth ranges may help to confirm the hypothesis that gorgonian fecundity varies with depth due to phenotypic plasticity (West et al. 1993).
Spatial comparison
The data show a relatively comparable reproductive potential in all sampled populations, as gonad numbers and sperm sac diameter were not significantly different between the sampling stations. This may well be an indication that other aspects of red coral biology, such as growth rate, are also homogenous on a mesoscale level. Growth rate does vary significantly on larger geographic scale though (Santangelo et al. 1993; Bramanti et al. 2004; Marschal et al. 2004). Mesoscale variability in reproductive output is negligible within the restricted range of colony size classes found at the Costa Brava due to overharvesting (Tsounis 2005; Fig. 1), so this study concludes that reproductive variability is negligible within populations of one geographic region that are comparable in colony size and population density. However, as size influences reproductive potential (seen previously), natural or anthropogenically induced variability in size has a strong effect on reproductive output, as observed in other cnidarians (Hall and Hughes 1996), and should be used as a primary base for management guidelines in order to ensure a minimum reproductive potential.
Given the crucial importance of reproduction to the recoverability of the species, we recommend the monitoring of reproductive output as a tool for management plans (see Lloret and Planes 2003). Although the results of this study show no significant differences in reproductive output within the red coral populations along the Costa Brava (which may be attributed to the narrow interval of size classes studied), we would still like to stress the importance of variability of the biological features of a species to ecosystem management. Some biological parameters, like resistance of red coral populations against mass mortality, are subject to a remarkable meso- and small-scale selectivity (Garrabou et al. 2001).
Conclusions
The reproductive output of red coral increases with increasing colony size, but was not found to be affected by spatial variation or depth. However, the release of larvae can be depth-staggered when high temperature gradients occur in summer. The size at first reproduction is relatively low in red coral and has been suggested to be the reason for the resilience of its populations to harvesting. However, this study confirms more recent results that show a lower fertility in small colonies. In fact, the present populations at the Costa Brava consist to a large part of colonies that have not yet reached 100% fertility. The spatial mesoscale variability in reproductive output within the present populations with homogenous demography (similar population density and range of size classes) appeared to be negligible. Management considerations are therefore better based on size variability (which affects reproductive potential) than mesoscale variability of reproductive output.
Finally, studies of the reproductive potential of exploited stocks are highly recommended to improve management strategies, as the exploitation is operating at the limit of the recoverability of this species. Future research should focus on red coral recruitment, which is the ultimate measure of reproductive efficiency, and promises to be valuable in this respect.
References
Arntz WE, Gili JM, Reise K (1999) Unjustifiably ignored: reflections on the role of benthos in marine ecosystems. In: Gray JS et al (eds) Biogeochemical cycling and sediment ecology. Kluwer Academic, The Netherlands, pp 105–124
Babcock RC (1984) Reproduction and distribution of two species of Goniastrea (Scleractinia) from the Great Barrier Reef Province. Coral Reefs 2:187–195
Babcock RC (1991) Comparative demography of three species of scleractinian corals using age- and size-dependent classification. Ecology 61:255–244
Beiring EA, Lasker HR (2000) Egg production by colonies of a gorgonian coral. Mar Ecol Prog Ser 196:169–177
Benayahu Y, Loya Y (1984) Life history studies on the red sea soft coral Xenia macrospiculata Gohar, 1940. I. Annual dynamics of gonadal development. Biol Bull 166:32–43
Benayahu Y, Berner T, Achituv Y (1989) Development of planulae within a mesogleal coat in the soft coral Heteroxenia fuscescens. Mar Biol 100:201–210
Bramanti L (2003) Dinamica di populazione ed adattamenti demografici di una popolazione costiera di corallo rosso (Corallium rubrum L. 1758) con particolare riferimento al reclutamento. Ph.D. Thesis, Universitá degli studi di Pisa
Bramanti L, Magagnini G, Santangelo G (2003) Settlement and recruitment: the first stages in the life cycle of two epibenthic suspension feeders (Corallium rubrum and Anomia ephippium). Ital J Zool 70:175–178
Bramanti L, Magagnini G, de Maio L, Santangelo G (2004) Recruitment, early survival and growth of the Mediterranean red coral Corallium rubrum (L 1758), a four year study. J Exp Mar Biol Ecol 314:69–78
Bramanti L, Rossi S, Tsounis G, Gili JM, Santangelo G (in press). Recruitment and early survival of red coral on settlement plates: some clues for demography and restoration. Hydrobiologia
Brazeau DA, Lasker HR (1989) The reproductive cycle and spawning in a Caribbean gorgonian. Biol Bull 176:1–7
Brazeau DA, Lasker HR (1990) Sexual reproduction and external brooding by the Caribbean gorgonian Briareum asbestinum. Mar Biol 104:465–474
Cebrián J, Duarte CM, Pascual J (1996) Marine climate in the Costa Brava (northwestern Mediterranean) littoral. Publ Espec Inst Esp Ocenogr 22:9–21
Coma R, Gili JM (1998) Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol Evol 13:316–321
Coma R, Ribes M, Zabala M, Gili JM (1995a) Reproduction and cyce of gonadal development in the Mediterranean gorgonian Paramuricea clavata. Mar Ecol Prog Ser 117:173–183
Coma R, Zabala M, Gili JM (1995b) Sexual reproductive effort in the Mediterranean gorgonian Paramuricea clavata. Mar Ecol Prog Ser 117:185–192
Connell JH (1973) Population ecology of reef building corals. In: Jones OA, Endean R (eds) Biology and geology of coral reefs. Academic Press, New York, pp 205–245
Dayton PK (2003) The importance of the natural sciences to conservation. Am Nat 162:1–13
Del Gaudio D, Fortunato G, Borriello M, Gili JM, Buono P, Calcagno G, Salvatore F, Sacchetti L (2004) Genetic typing of Corallium rubrum. Marine Biotecnol 6:511–515
Garrabou J, Perez T, Sartoretto S, Harmelin JG (2001) Mass mortality event in red coral Corallium rubrum populations in the Provence region (France, NW Mediterranean). Mar Ecol Prog Ser 217:263–272
Gili JM, Ros J (1984) L’estatge circalitoral de les Illes Medes: el coralligen. In: Ros J, Olivella I, Gili JM, Els Sistemes Naturals de les Illes Medes. Institut d’Estudis Catalans, Barcelona, pp 677–705
Gotelli NG (1991) Demographic models for Leptogorgia virgulata, a shallow-water gorgonian. Ecology 13:297–337
Grigg RW (1974) Growth rings: annual periodicity in two gorgonian corals. Ecology 55:876–881
Grigg RW (1976) Fisheries management of precious and stony corals in Hawaii. UNIHI-SEAGRANT-TR-77–03
Grigg RW (1977) Population dynamics of two gorgonian corals. Ecology 58:278–290
Grigg RW (1989) Precious coral fisheries of the Pacific and Mediterranean. In: JF Caddy (ed) Marine invertebrate fisheries: their assessment and management. Wiley, New York, pp 636–645
Hall VR, Hughes TP (1996) Reproductive strategies of modular organisms: comparative studies of reef-building corals. Ecology 77:950–963
Harland AD, Davies PS, Fixter LM (1992) Lipid content of some Caribbean corals in relation to depth and light. Mar Biol 113:357–361
Hughes TP, Jackson JBC (1985) Population dynamics and life histories of foliaceous corals. Ecol Monogr 55:141–166
Kim K, Lasker HR (1997) Flow-mediated resource competition in the suspension-feeding gorgonian Plexaura homomalla (Esper.). J Exp Mar Biol Ecol 215:49–64
Kinzie RA (1974) Plexaura homomalla: the biology and ecology of a harvestable marine resource. In: Bayer FM, Weinheimer AJ (eds) Prostaglandins from Plexaura homomalla. University of Miamy Press, Coral Gables, pp 22–57
Kojis BL, Quinn NJ (1981) Aspects of sexual reproduction and larval development in the shallow water hermatypic coral, Goniastrea australiensis (Edwards and Haime 1857). Bull Mar Sci 31:558–573
Lincoln R, Boxhall G, Clark P (1998) A dictionary for ecology, evolution and systematics. Cambridge University Press
Lloret J, Planes S (2003) Condition, feeding and reproductive potential of white seabream Diplodus sargus as indicators of habitat quality and the effect of reserve protection in the northwestern Mediterranean. Mar Ecol Prog Ser 248:197–208
Marschal C, Garrabou J, Harmelin JG, Pichon M (2004) A new method for measuring growth and age in precious red coral Corallium rubrum (L.). Coral Reefs 23:423–432
Mundy C, Babcock R (2000) Are vertical distribution patterns of scleractinian corals maintained by pre- or post-settlement processes? A case study of three contrasting species. Mar Ecol Prog Ser 198:109–119
Orejas C, López-González P, Gili JM, Teixidó N, Gutt J, Arntz W (2002) Distribution and reproductive ecology of the Antarctic octocoral Ainigmaptilon antarcticum in the Weddell Sea. Mar Ecol Prog Ser 231:101–114
Pascual J, Lloret L, Salat J, Zabala M (1995) Projecte de determinació de la circulació de les aigües de la Reserva Marina de les Illes Medes. Informe técnic per la Direcció General de Pesca MarÌtima, Generalitat de Catalunya
Rinkevich B, Loya Y (1984) The reproduction of the red sea coral Stylophora pistillata. I. Gonads and planulae. Mar Ecol Prog Ser 1:133–144
Sakai K (1998) Delayed maturation in the colonial coral Gonasteria aspera (Scleractinia): whole-colony mortality, colony growth and polyp egg production. Res Popul Ecol 40:287–292
Santangelo G, Abbiati M (2001) Red coral: conservation and management of an over-exploited Mediterranean species. Aquat Conserv: Mar Freshwater Ecosyst 11:253–259
Santangelo G, Abiatti M, Caforio G (1993) Age structure and population dynamics in Corallium rubrum. In: Cicogna F, Cattaneo-Vietti R (eds) Red coral in the Mediterranean Sea: art, history and science. Ministerio delle Risorse Agricole, Almentari e Forestali, Roma, pp 131–157
Santangelo G, Maggi E, Bramanti L, Bongiorni L (2003a) Demography of the over-exploited Mediterranean red coral (Corallium rubrum L. 1758) Sci Mar 68:199–204
Santangelo G, Carletti E, Maggi E, Bramanti L (2003b) Reproduction and population sexual structure of the overexploited Mediterranean red coral Corallium rubrum. Mar Ecol Prog Ser 248:99–108
Sará M (1969) Research on coralligenous formation; problems and perspectives. Publ staz zool Napoli 37:124–134
Soong K (1993) Colony size as a species character in massive reef corals. Coral reefs 12:77–83
Stiller MA, Rivoire G (1984) Biologie et ècologie du corail rouge en Méditerranée française. FAO Fish Rep 306:89–93
Szmant-Froehlich AM (1981) Reproductive ecology of caribbean reef corals. Coral reefs 5:43–54
Szmant-Froehlich AM (1985) The effect of colony size on the reproductive ability of the Caribbean coral Monastrea annularis (Ellis and Solander). Proc fifth Int Coral Reef Congr 4:295–300
Tescione G (1973) The Italians and their coral fishing. Fausto Fiorino, Naples, Italy
Tioho H, Tokrshi M, Nojima S (2001) Experimental analysis of recruitment in a scleractinian coral at high latitude. Mar Ecol Prog Ser 213:79–86
Tsounis G (2005) Demography, reproductive biology and trophic ecology of red coral (Corallium rubrum L.) at the Costa Brava (NW Mediterranean): ecological data as a tool for management. Ph.D. Thesis, University of Bremen, Germany
Vighi M (1972) Étude sur la reproduction du Corallium rubrum (L.). Vie Milieu vol XXIII fase 1, sér A, pp 21–32
Ward S (1995a) Two patterns of energy allocation for growth, reproduction and lipid storage in the scleractinian coral Pocillopora damicornis. Coral Reefs 14:87–90
Ward S (1995b) The effect of damage on the growth, reproduction and storage of lipids in the scleractinian coral Pocillopora damicornis (Linnaeus). J Exp Mar Biol Ecol 187:193–206
Weinberg S (1979) The light dependent behaviour of planula larvae of Eunicella singularis and Corallium rubrum and its implication for octocorallian ecology. Bijdr Dierk 49:145–151
Weinberg S, Weinberg F (1979) The life cycle of a gorgonian: Eunicella singularis (Esper 1794). Bijdr Dierk 48:127–137
West JM, Harvell CD, Walls AM (1993) Morphological plasticity in a gorgonian coral (Briareum asbestinum) over a depth cline. Mar Ecol Prog Ser 94:61–69
Zar JH (1996) Biostatistical Analysis. Prentice-Hall
Acknowledgements
We gratefully acknowledge helpful comments by C. Orejas and G. Santangelo and three anonymous referees that greatly improved the final version of the manuscript. N. Fernández provided invaluable help during SCUBA diving fieldwork and sample processing. We also thank C. Linares, D. Diaz and G. Mas for help during sampling for size of first reproduction. Thanks to J. Metzner, P. Claver and T. Padron for sample processing. We also thank the collaboration of the family Mörker at Roses and the family Riera at Cadaqués. The professional divers of the Trepa helped S. Rossi in the sampling at Cap de Creus. Many thanks to J.M. Llenas for support at the Medas Islands. G. Tsounis was supported by a Ph.D. scholarship from the University of Bremen, Germany. This study was supported by a IFOP grant from the Generalitat de Catalunya (Spain).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Tsounis, G., Rossi, S., Aranguren, M. et al. Effects of spatial variability and colony size on the reproductive output and gonadal development cycle of the Mediterranean red coral (Corallium rubrum L.). Marine Biology 148, 513–527 (2006). https://doi.org/10.1007/s00227-005-0100-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0100-8