Abstract
Seventeen immature green turtles Chelonia mydas were tracked concurrently by automated ultrasonic receivers at a coral reef off North-Eastern Australia (September–December 2010, 16.4°S, 145.6°E). The majority (n = 11) were tracked for the entire 100-day study, the remainder for 23–85 days. Detection data aggregated at 30-min intervals produced median 6.5–35 daily locations for individual turtles. Home range areas (95 % utilisation distribution) were ≤1 km2, \( {\bar{\text{x}}} \) ± SD = 0.74 km2 ± 0.159. To the best of our knowledge, these are the first home range estimates for C. mydas foraging at offshore tropical reefs. The findings are important for conservation in revealing near-continuous presence of the same individuals within a small geographic area. Time between detections was very short (median <3 min) demonstrating passive ultrasonic technology can track multiple turtles in a foraging environment with higher temporal resolution than typically achieved by satellite tracking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine turtles have attracted expanding research effort over recent decades (Avise 2008; Godley et al. 2008), but important knowledge gaps remain, including a paucity of data regarding turtles in their geographically diverse foraging areas (Bjorndal 1999; Hamann et al. 2010). This gap may seem surprising for the globally threatened green turtle Chelonia mydas (Seminoff 2004), given that they spend the major part of their lives in neritic foraging grounds (Musick and Limpus 1997; Plotkin 2003) and suffer multiple anthropogenic impacts at some foraging sites (Lutcavage and Lutz 1997; Hazel and Gyuris 2006). However, the data deficit might in part be explained by technology constraints.
Satellite tracking has effectively revealed long distance migrations by turtles (Godley et al. 2008; Hart and Hyrenbach 2010; Hazen et al. 2012), but fine-scale tracking studies are hampered by the relatively low spatial resolution of satellite-derived locations. Furthermore, even with enhanced accuracy offered by recent marine variants of GPS technology, temporal resolution of satellite tracking remains a fundamental constraint (Hazel 2009). The problem is particularly acute for turtles in foraging areas because they spend the vast majority of time submerged (Hazel et al. 2009). During submergence, no satellite-derived locations can be obtained because radio signals necessary for satellite communication are blocked by seawater (Hill and Robinson 1962); therefore, researchers need to consider other methods for fine-scale tracking of submerged animals.
Ultrasonic acoustic signals transmit effectively over short distances underwater and allow active boat-based tracking with a directional hydrophone. This is a labour-intensive technique because the tracking boat follows one animal at a time, and work is further constrained by weather and sea conditions (e.g. Mendonca 1983; Zeller 1997; Holland et al. 1999; Seminoff and Jones 2006). Newer technology offers vastly expanded scope for passive tracking with omni-directional receivers capable of detecting many coded transmitters on a single ultrasonic frequency. These automated receivers can be deployed as fixed arrays in diverse situations (see review by Heupel et al. 2006). Prompt adoption and widespread studies attest to the utility of this new technology in fish research (e.g. among many others Heupel and Simpfendorfer 2005; Yeiser et al. 2008; Knip et al. 2011; O’Toole et al. 2011). In contrast, there has been sparse and limited usage for marine turtles where (i) ultrasonic signals were interpreted simply as turtle presence or absence near a particular receiver and the detection data were not used for calculating geographic position estimates (see Simpfendorfer et al. 2002); (ii) additional methods supplemented the ultrasonic data, e.g. Taquet et al. (2006) incorporated systematic diver observations, Okuyama et al. (2010) relied in part on depth recorders, Hart et al. (2012) relied primarily on satellite tracking. Thus potential utility of this technology as a stand-alone method for turtles has remained difficult to assess.
The present study had two objectives: (i) to estimate home ranges of green turtles foraging at an offshore coral reef by means of automated ultrasonic receivers and (ii) to report in detail on performance of the receiver array. Achievement of objective (i) would constitute the first step in alleviating a wide data gap of conservation importance. More than 2000 offshore coral reefs exist within Australia’s Great Barrier Reef (Hopley et al. 2007). Many of these reefs host substantial foraging aggregations of green turtles (Chaloupka and Limpus 2001) implying such coral reefs collectively represent significant habitat for the species. However, prior to the present study, no spatial ranges could be found for green turtles foraging at Australian offshore coral reefs, nor for this species in similar habitat elsewhere in the world. Objective (ii) was expected to assist other turtle researchers in assessing the potential utility of automated ultrasonic tracking for future studies.
This paper uses the term ‘home range’ in a broad sense to describe the geographic extent of turtle movements recorded during the study period. Quantitative measures (detailed below under Home range measures) were pragmatic choices based on recommendations in scientific literature. We acknowledge diverse alternatives and ongoing debate regarding the definition, calculation and reporting of home range measures (e.g. see Laver and Kelly 2008 and references therein). Such debate lies beyond present scope.
Methods
Field research
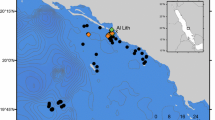
Low Isles (16.4°S, 145.6°E) is situated on the continental shelf of North-Eastern Australia and lies entirely within a Marine National Park Zone of the Great Barrier Reef Marine Park (GBRMPA 2004). Our study site comprised a small coral reef supporting a vegetated sand cay and a mangrove cay connected by a shallow reef flat that is partially exposed at extreme low tides (Fig. 1). Boat searches of the reef flat were conducted at mid- to high tide in water depths 0.5–2.5 m, and green turtles were hand-captured by an assistant jumping from the boat. Each study turtle was released within 1 h after being processed onboard the boat close to the capture site.
Study site at Low Isles (16.4°S, 145.6°E) off the north-east coast of Australia. Dashed line indicates approximate extent of the coral reef complex that supports a small sand cay to the west and a larger mangrove cay to the east. Light grey indicates sand, dark grey indicates vegetation, small squares indicate locations of receiver-stations. Circles with radius 200 m allow visual evaluation of receiver-station spacing in relation to signal detection range tests presented in Fig. 2
Ultrasonic transmitters (V16-4L, Vemco, Halifax, Nova Scotia, Canada) were embedded in streamlined epoxy fairing and attached to the posterior carapace (Supplementary material Fig. S1) with epoxy adhesive-putty (Knead-It Aqua, Selleys, Padstow NSW, Australia) taking care to avoid obstructing the transducer end. The transmitters produced a coded signal with a nominal delay of 90 s, meaning that the interval between successive transmissions varied randomly between 60 and 120 s. A variable transmission rate was necessary to reduce the risk of signals from multiple transmitters being emitted concurrently. Signals overlapping in time can cause a form of interference termed ‘signal collision’ that prevents successful decoding (Manufacturer’s advice, Vemco, Halifax, Nova Scotia, Canada.)
Underwater receivers (VR2W, Vemco, Halifax, Nova Scotia, Canada) were attached to concrete weights anchored to the substrate. Each receiver deployed in deep water was attached to a rope supported by a small sub-surface float, in order to maintain upright orientation. Receivers deployed at inter-tidal locations were supported in PVC tubes embedded in concrete weights and placed directly on the substrate (Supplementary material Fig. S1). The same locations, hereafter termed ‘receiver-stations’, were used throughout the study. The receivers operated automatically, constantly ‘listening’ for signals on the same ultrasonic frequency as the V16 transmitters and recording all signal detections in archival memory. Each receiver was brought to the surface at approximately 4-week intervals for cleaning and data download and promptly replaced.
Design of the receiver array was guided by range tests at the study site that showed a V16 transmitter within 50 m of a receiver produced near-perfect detection (99 %). More than 50 % of signals were detected up to 300 m, decreasing to 11 % detection at 300–400 m. (Fig. 2). Distances between adjacent receiver-stations varied (approx 200–350 m) to accommodate complex shorelines and to avoid placing equipment on coral or in boat transit lanes and high-use anchorage areas. Wave intensity prevented the placement of receivers along the southern and eastern edges of the reef (Fig. 1). Completion of a 15-receiver array defined the start of our 100-day study on 16 September 2010. An additional receiver was installed later (6 November 2010) at the most easterly position shown on the site map (Fig. 1). Data from this additional receiver-station were included in analyses of turtle activity but excluded from diagnostic metrics.
Detections were recorded by submerged acoustic receivers (Vemco VR2 W) at various distances from an ultrasonic transmitter (Vemco V16) moored at a fixed location. Detection efficiency is presented here as the percentage of emitted ultrasonic signals that were successfully detected during range testing at the study site
Data analysis: diagnostic data
Each VR2W receiver provided two types of data: (a) a list of transmitter signals successfully decoded, termed Detection data and (b) information relevant to efficient functioning of the receiver, termed Events data. We used Events data to calculate three diagnostic metrics: Code Detection Efficiency, Rejection Coefficient and Noise Quotient (Pincock 2008; Simpfendorfer et al. 2008; Vemco 2010) as defined in Table 1.
Data analysis: detection data
Successfully decoded signals were matched, via signal ID codes, to the turtle carrying the corresponding transmitter. For each turtle, we calculated tracking duration, number of days on which the turtle was detected, detections per day and elapsed time between successive detections. As an additional measure of receiver performance, total detections were standardised by the number of individual turtles detected on each day of the study.
We used detection data for each turtle to estimate locations of centres of activity during successive 30-min time steps (Δt = 30) following Simpfendorfer et al. (2002). Coordinates of these locations were calculated as the arithmetic means of latitude and longitude for all receiver-stations detecting the turtle during that time step, weighted by the number of detections at each station. The same calculations were repeated with other time step values (Δt = 5, 15 and 60 min) to assess the influence of this parameter. If this comparison showed greater utility of a different time step, the initial pragmatic choice of Δt = 30 could be changed accordingly. Minimum straight line distance was calculated between consecutive locations and termed ‘continuous distance’ when there were no intervening periods of non-detection, or ‘interrupted distance’ when locations were separated by variable periods of non-detection. Displacement rate (distance/time) was calculated from continuous distances only. Local sunrise and sunset times were used to define day and night.
Home range measures
Utilisation distributions were estimated by the fixed kernel method (recommended by many authors e.g. Worton 1989, 1995; Kernohan et al. 2001). To avoid confounding our comparisons, we applied consistent kernel parameters across all data sets (fixed kernel smoothing, user-defined bandwidth and grid resolution). Utilisation distribution (UD) contours were used to depict each turtle’s range of activity in two-dimensional space with the 99 % contour (UD 99 %) interpreted as maximum recorded activity range during the study period, UD 95 % as the area of routine use, equivalent to ‘home range’ sensu Burt (1943) and White and Garrott (1990), and UD 50 % as the core area of activity. UD contours were plotted on a map of the study area for visual evaluation. Bhattacharyya’s Affinity statistic (Bhattacharyya 1943; Fieberg and Kochanny 2005) was used to quantify the degree of similarity between UDs estimated from locations at four different time steps (Δt = 5, 15, 30, 60 min). The Utilization Distribution Overlap Index (Fieberg and Kochanny 2005) was used to measure the degree to which individual turtles shared space. Our usage followed recommendations by Fieberg and Kochanny (2005) where detailed information and tests of the above statistics are provided. Calculations were conducted in R (R Development Core Team 2010) with package adehabitat (Calenge 2006) used for UDs.
Results
Nineteen green turtles with curved carapace length (CCL) from 65.5 to 80.6 cm were equipped with tracking transmitters deployed in July 2010 (n = 7) and September 2010 (n = 12), of which 17 provided transmitter signals at the start of our 100-day study period (16 September 2010).
Tracking duration and detection frequency
Eleven of 17 available turtles were tracked throughout the 100-day study, five for more than half the period (51 to 85 d) and one for 23 d. Nine turtles recorded multiple detections each day without exception (detections d−1 \( {\bar{\text{x}}} \) ± SD = 209 ± 87.8). The other eight turtles were detected on 95–99 % of days within their tracking duration (detections d−1 \( {\bar{\text{x}}} \) ± SD = 88 ± 42.1). For all individuals, the time between successive detections was predominantly very short (median = 1.7 min, range <0.5 min to 8 days). Gaps in detection >24 h for 8 turtles collectively comprised 13 non-detection periods of 1–3 days, one of 6 days and one of 8 days. Table 2, Supplementary material Fig. S2.
Space use by study turtles
Detection data aggregated at 30-min time steps provided a per-turtle median of 1,599 locations, range 375 to 3,514 (Table 2). These locations indicated study turtles collectively used an area of 1.63 km2 (UD 99 %) over the 100-day study period, with routine use (UD 95 %) of 1.22 km2 and a core area (UD 50 %) of 0.29 km2 in the North-West sector of the study site (Fig. 3). Individual turtles had home range areas (routine use UD 95 %) \( {\bar{\text{x}}} \) ± SD = 0.74 km2 ± 0.159, range 0.47–1.04 km2 and core areas (UD 50 %) \( {\bar{\text{x}}} \) ± SD = 0.14 km2 ± 0.043, range 0.08–0.24 km2. Individual utilisation patterns were diverse, as shown quantitatively by the Utilization Distribution Overlap Index (UDOI) median 0.43, range 0–1.87. UDOI values were consistent with qualitative evaluation of the utilisation maps (Supplementary material Fig. S3). For example, turtles A7 and A19 used geographically similar areas in accord with UDOI = 1.87, whereas A3 and A8 UDOI = 0 used geographically distinct areas. In the latter case, A3 favoured the eastern side of the study site and A8 favoured the west, and they overlapped only within their UD 99 % (Supplementary material Fig. S3, Table S1). Day and night utilisation patterns for individual turtles were geographically similar, but night areas were smaller than day areas, UD 95 % day-night difference \( {\bar{\text{x}}} \) ± SD = 0.15 km2 ± 0.129, UD 50 % day-night difference \( {\bar{\text{x}}} \) ± SD = 0.08 ± 0.052.
Receiver performance
Receiver Event data showed the mean daily Code Detection Efficiency was 0.386 (median = 0.396, range 0–1) indicating that on average, less than 40 % of transmissions were successfully decoded over the 100-day study. However, the daily Rejection Coefficient was very low (mean = 0.017, median = 0.013, range 0–0.33), and Noise Quotient was generally moderate with occasional extreme values (first quartile = −252.0, mean = −112.7, median = −63.0, third quartile = 52.0, range = −2,449 to 1,793). Variation was irregular and sometimes high, but no consistent temporal trends emerged in any of the three diagnostic metrics (Fig. 4a–c). However, daily totals for signals detected (median = 1,992, range 1,048–4,712) showed an irregular but overall diminishing trend, evident in raw numbers and when totals were standardised by the number of turtles detected on each day Fig. 4d.
Performance data for the array of acoustic receiving stations (n = 15) over the 100-day study period. Three diagnostic metrics (defined in Table 1) indicated considerable variation but no consistent trends in a Code detection efficiency, b rejection coefficient and c noise quotient. Box plots show daily values for all receiver-stations pooled; horizontal bar indicates median; box length indicates inter-quartile range; whiskers extend to largest values within 1.5 × inter-quartile range; all more extreme data points shown as open circles. Detections standardised d shows total signals detected standardised by the number of individual turtles recorded on each day
Influence of time step value
Increasing the time step value (Δt = 5, 15, 30 and 60 min) for calculating locations from detections progressively increased the proportion of time steps with locations derived from one or more receiver-stations and reduced the proportion of time steps without detection. Increasing Δt increased the median distance between successive locations, as would be expected for consistency with longer elapsed time, but concurrently the inferred displacement rate decreased. Bhattacharyya’s Affinity statistic BA ≥ 0.99 for all pairs of comparisons indicated the utilisation distributions were very closely similar for the four Δt values although spatial areas of UD contours decreased slightly with longer time step values (Table 3, Fig. 3).
Discussion
Tracking duration and detection frequency
The majority of turtles (11 out of 17) remained in contact, via acoustic signal detection, for the full duration of the 100-day pilot study and only one individual ceased contact during the first half of the period. Study turtles were detected multiple times per day, either on every day (n = 9) or on 95–99 % of days (n = 8) indicating they maintained near-continuous presence at the small tropical reef complex at Low Isles.
Occasional non-detection intervals >24 h could have indicated brief departures from the study site or un-recorded movements within the site. We estimated that a return trip from Low Isles to another reef (the nearest 12 and 16 km distant) would involve at least 2 days of travel, based on mean swimming speeds <1 km h−1 recorded for green turtles of similar and larger sizes in fine-scale tracking studies (Seminoff and Jones 2006; Brooks et al. 2009; Hazel 2009). Therefore, within the two longest gaps in detection (6 and 8 days), a potential excursion to another reef might hold relatively low foraging benefit relative to the turtle’s energy expenditure for travel. Alternatively, detection gaps could have occurred if some turtles moved temporarily to southern or eastern parts of the Low Isles reef complex where their transmitter signals would have been out of range of our receivers. A more extensive receiver array would have been useful for reducing this ambiguity.
A premature end of detection for a minority of turtles might have been caused by active departure from the study area, or by turtle death (resulting in the carcase drifting away), transmitter failure, or transmitter detachment. Only the latter explanation has been confirmed. Two study turtles were re-encountered at the study site subsequent to the tracking period. They were in good to very good body condition (see Heithaus et al. (2007) for visual assessment criteria) but had lost their transmitters. Close inspection of the carapace suggested transmitter loss was due to scute-shedding rather than adhesive failure (J. H. pers. obs). Data are lacking regarding scute-shedding by wild C. mydas at any life stage, thus it remains unclear whether natural scute-shedding might represent an important limitation for adhesive attachment. For the present study, we relied entirely on adhesive attachment (i) to avoid drilling the carapace for mechanical fastenings and (ii) to facilitate eventual safe shedding of equipment because recapture for manual removal was not assured at our site. For tracking studies of extended duration, other researchers might consider supplementing adhesives with bolts or wires, subject to ethical considerations and regulatory conditions.
Space use by study turtles
All study turtles used home range areas ≤1 km2. These are to our knowledge the first home range estimates for green turtles foraging at an offshore coral reef and no prior data could be found for direct comparison. However, there is notable contrast between the small home ranges of turtles at Low Isles and larger ranges reported at various coastal sites: for example 3.5 km2 over 22–51 days (Mendonca 1983, values for ‘summer’ periods of study), 3.2 km2, over 4–26 days (Whiting and Miller 1998); 16.6 km2 over 34–96 days (Seminoff et al. 2002), 2.4 km2 over 55–66 days (Makowski et al. 2006) and 4.6 km2 over 4.5 days (Hazel 2009).
Unlike coastal sites, the Low Isles reef complex is entirely surrounded by open water with depths 15–30 m (Australian Hydrographic Service 2002). Such depths do not preclude foraging or resting by green turtles at some locations (e.g. Taquet et al. 2006). However, we suspect only scant forage exists in deeper waters around Low Isles because seagrass is restricted by low light penetration in turbid water (Abal and Dennison 1996). The latter is an enduring characteristic of our study region due to high rainfall, terrestrial runoff and re-suspension of sediments (e.g. see Hamilton 1994; Larcombe and Woolfe 1999). In addition, this part of the continental shelf features gentle gradients without underwater reefs (Australian Hydrographic Service 2002). Consequently the surrounding open water lacks coral and rock outcrops that turtles at Low Isles evidently favour for resting (J.H. pers. obs). Therefore, we suggest a spatial concentration of forage and shelter resources within the reef complex at Low Isles may explain the small home ranges of our study turtles.
Within the study area, there was sparse seagrass and macro-algae scattered across the reef flat while coral outcrops along the reef slopes offered shelter for resting turtles all around the perimeter of the site (J.H. pers. obs.). Similar sparse forage and abundant rest sites were present in the North-West area that received higher use by study turtles considered collectively (see UD 50 % in Fig. 3). However, that area might have been slightly favoured by turtles because it was partly sheltered from prevailing South-East winds and swell. Clearly, there was no strong preference for any particular area, given that individuals had different core areas. Indeed, their diverse home ranges (Supplementary material Fig. S2) suggested each study turtle found its own optimal pattern of resource use within the Low Isles reef complex.
The finding that study turtles used slightly larger areas by day than by night was consistent with the understanding that green turtles tend to move about more widely while foraging during the day and tend to rest during much of the night. Evidence of diurnal activity and nocturnal rest has also been inferred from the diving patterns of green turtles (Seminoff et al. 2001; Hazel et al. 2009). However, our data did not show a geographic distinction between day and night sites, as has been reported in some situations (Mendonca 1983; Taquet et al. 2006). General similarity between areas used by day and by night at Low Isles may reflect site characteristics, because wherever a turtle forages within this site, it can find multiple potential rest sites nearby. In general, Low Isles turtles can be observed to rest intermittently during the day (J. H. pers. obs.) and might occasionally forage at night (not feasible to observe). Thus there was no expectation that turtles would use clearly differentiated areas by day and night, nor was the tracking method designed to detect small differences in habitat use. Very precise locations cannot be obtained from ultrasonic detection data due to variations in detection efficiency (Fig. 2) and imperfect spatial coverage, notably around the perimeter of the array. Other methods should be considered for studies where geographic accuracy is the highest priority, for example standard GPS or Fastloc GPS.
Receiver performance
The low level of Code Detection Efficiency (<40 %) prompted the question, why was decoding unsuccessful for about 60 % of transmissions that had a correct Sync value? A potential explanation could lie in interference from a high rate of signal collisions, despite all transmitters by design emitting signals at variable intervals to minimise collision risks. However, this explanation lacked support for two reasons. Firstly, the manufacturer’s guidance indicates Rejection Coefficient would be high if collision rate was high (Vemco 2010). However, Rejection Coefficients in the present study were consistently low (Fig. 4c). Secondly, opportunities for signal collisions would be rare unless study turtles persistently aggregated in one area. The latter was contradicted by their diverse patterns of space use (Supplementary material Fig. S2).
More generally, low Code Detection Efficiency and intermittent extremes in Noise Quotient could be ascribed to multiple sources of signal disruption such as wind, waves, turbidity, signal echoes and underwater noise (Heupel et al. 2006). Our study site was characterised by wind and wave exposure (due to offshore location), turbidity (see above Space use by study turtles) and hard coral reef surfaces that could generate acoustic echoes. Sources of biological noise included fish and reef organisms while vessel traffic contributed anthropogenic noise. Insight into the relative importance of these factors might be gained by assessing variation in receiver performance in relation to time of day, wind strength, tidal height and vessel traffic. However, the intra-day performance of our receivers could not be evaluated because Events data provided only 24-h values. If feasible in future upgrades, an option to aggregate Events data at shorter intervals would represent a useful enhancement of the Vemco system.
The reef habitat of our study animals was not optimal for acoustic tracking, given multiple sources of signal disruption mentioned above, but natural sources of interference could not be controlled. Given that detection degrades with distance (Fig. 2), more frequent detection could be achieved by deploying a greater number of receivers with closer spacing provided site characteristics allow, for example space for placing receivers without damage to coral. However, costs and benefits of deploying more receivers or using different technology would be case-specific. Despite relatively low performance metrics, we were satisfied with the abundance of location data that our receiver array provided for multiple turtles concurrently. In terms of temporal resolution, our median time between detections (<3 min) compared very favourably with satellite tracking of marine turtles, in which many studies have received fewer than four locations per day, the latter further reduced by discarding low quality locations (e.g. Hays et al. 2001; Godley et al. 2003; Hart et al. 2012).
The diminishing trend in daily detections over time (Fig. 4d) defied explanation. It ran contrary to the trend in weather conditions that might have conferred an enhancement in acoustic transmission from progressively decreasing winds and wave action (JH pers. obs.; Australian Bureau of Meteorology data for Low Isles showed weekly average wind speed reduced by 10 km/h over the study period). Bio-fouling, which can degrade signal transmission (Heupel et al. 2006), was not observed during any underwater sightings of turtles equipped with transmitters. Bio-fouling of receivers was avoided by their regular servicing. The possibility of transmitter efficiency decreasing over time could not be ruled out. However, it seemed unlikely over the study duration, given the manufacturer’s estimate of 10-year transmitter life. This puzzling finding highlights the need for future research into temporal variation in the efficiency of ultrasonic signal detection, for example by long-duration deployment of test transmitters at fixed locations within an array.
Influence of time step value
Results of the time step comparison (Table 3) were consistent with intuitive expectations. A time step without detection occurred more often when analysis was based on short time steps, resulting in a greater proportion of ‘missing’ locations. Equally, longer time steps allowed greater numbers of detections to be recorded within the time step and greater opportunity for detection by multiple receiver-stations. The latter could be beneficial because locations derived from multiple receiver-stations could in principle offer better geographic resolution than those derived from single or few stations, albeit at the cost of reducing temporal resolution. The trade-off between geographic and temporal resolution was demonstrated by a diminishing displacement rate (Table 3) indicating detail of turtles’ movement trajectories had been lost with longer Δt values. Lost detail could also account for the slight decrease in spatial areas of UD contours derived from longer Δt values, although the effect was small for UD contours because they incorporated the very numerous locations accumulated over the study duration.
Short Δt produced a high proportion of time steps without detection. However, since each ‘missing’ location represented a very short time span, this was of little importance. Notably the actual time between detections (independent of Δt) was predominantly very short, median across all turtles <3 min (Table 2). At the same time, the potential for a long Δt value to enhance geographic resolution applied to only a small proportion of locations, for example with Δt = 60 min, only 24 % of locations were derived from 3 or more receiver-stations. In summary, there was no clear benefit to be gained by using longer or shorter time steps in the present study, and therefore, we retained the initial choice of Δt = 30 min. The trade-off between adopting shorter or longer Δt values may be resolved differently in other studies, taking into account different research objectives, site characteristics, array design and typical movement rates for the study species. Importantly, the selected Δt value needs to be reported so that subsequent comparisons between studies can take into account the differential influences on quantitative measures derived from locations inferred for diverse Δt values.
Implications for turtle conservation management
Green turtles in Queensland waters are understood to maintain long-term associations with particular foraging areas, based on recaptures of marked individuals (Limpus et al. 1992; Limpus and Chaloupka 1997). However, repeat encounters with marked animals were infrequent and widely separated in time, meaning that the continuity of presence or absence of marked individuals during intervening periods (typically years) could not be assessed by mark-recapture methods. In contrast, our data reveal that multiple individuals maintained continuous or near-continuous occupation of a small geographic area during the 100-day study period.
The combination of long-term fidelity to foraging sites (shown by mark–recapture studies) and continuity of occupation (shown by the present study) indicates that individual turtles could suffer long-term exposure to any anthropogenic risks at a particular site. Thus, the need for site-specific mitigation of risk is heightened. At the same time, public support for mitigation measures could be enhanced if people in the local area know turtles are not merely transient visitors and appreciate that ‘their’ resident turtles will benefit from long-term protection.
References
Abal EG, Dennison WC (1996) Seagrass depth range and water quality in Southern Moreton Bay, Queensland, Australia. Mar Freshw Res 47:763–771
Australian Hydrographic Service (2002) Chart AUS 831 Australia East Coast—Queensland—Low Islets to Cape Flattery
Avise JC (2008) Conservation genetics of marine turtles—10 years later. In: Fulbright TE, Hewitt DG (eds) Wildlife science: linking ecological theory and management applications. CRC Press, Boca Raton, Florida, pp 295–315
Bhattacharyya A (1943) On a measure of divergence between two statistical populations defined by their probability distributions. Bull Calcutta Math Soc 35:99–109
Bjorndal KA (1999) Priorities for research in foraging habitats. In: Eckert KL, Bjorndal KA, Abreu-Grobois FA, Donnelly M (eds) Research and management techniques for the conservation of sea turtles, vol Publication No. 4. IUCN/SSC Marine Turtle Specialist Group
Brooks LB, Harvey JT, Nichols WJ (2009) Tidal movements of East Pacific green turtle Chelonia mydas at a foraging area in Baja California Sur, Mexico. Mar Ecol Prog Ser 386:263–274
Burt WH (1943) Territoriality and home range concepts as applied to mammals. J Mammal 24:346–352
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Chaloupka M, Limpus C (2001) Trends in the abundance of sea turtles resident in southern Great Barrier Reef waters. Biol Conserv 102(3):235–249. doi:10.1016/s0006-3207(01)00106-9
Fieberg J, Kochanny CO (2005) Quantifying home-range overlap: the importance of the utilization distribution. J Wildl Manag 69(4):1346–1359
GBRMPA (2004) Great barrier reef marine park zoning plan 2003. Australian Government, Townsville
Godley BJ, Lima EHSM, Akesson S, Broderick AC, Glen F, Godfrey MH, Luschi P, Hays GC (2003) Movement patterns of green turtles in Brazilian coastal waters described by satellite tracking and flipper tagging. Mar Ecol Prog Ser 253:279–288
Godley BJ, Blumenthal JM, Broderick AC, Coyne MS, Godfrey MH, Hawkes LA, Witt MJ (2008) Satellite tracking of sea turtles: where have we been and where do we go next? Endanger Species Res 4(1–2):3–22. doi:10.3354/esr00060
Hamann M, Godfrey MH, Seminoff JA, Arthur K, Barata PCR, Bjorndal KA, Bolten AB, Broderick AC, Campbell LM, Carreras C, Casale P, Chaloupka M, Chan SKF, Coyne MS, Crowder LB, Diez CE, Dutton PH, Epperly SP, FitzSimmons NN, Formia A, Girondot M, Hays GC, Cheng IJ, Kaska Y, Lewison R, Mortimer JA, Nichols WJ, Reina RD, Shanker K, Spotila JR, Tomas J, Wallace BP, Work TM, Zbinden J, Godley BJ (2010) Global research priorities for sea turtles: informing management and conservation in the 21st century. Endanger Species Res 11(3):245–269. doi:10.3354/esr00279
Hamilton LJ (1994) Turbidity in the Northern Great Barrier Reef lagoon in the wet season, March 1989. Aust J Mar Freshw Res 45:585–615
Hart KM, Hyrenbach KD (2010) Satellite telemetry of marine megavertebrates: the coming of age of an experimental science. Endanger Species Res 10(1–3):9–20. doi:10.3354/esr00238
Hart KM, Sartain AR, Fujisaki I, Pratt HL Jr, Morley D, Feeley MW (2012) Home range, habitat use, and migrations of hawksbill turtles tracked from Dry Tortugas National Park, Florida, USA. Mar Ecol Prog Ser 457:193–207
Hays GC, Akesson S, Godley BJ, Luschi P, Santidrian P (2001) The implications of location accuracy for the interpretation of satellite-tracking data. Anim Behav 61(Part 5):1035–1040
Hazel J (2009) Evaluation of fast-acquisition GPS in stationary tests and fine-scale tracking of green turtles. J Exp Mar Biol Ecol 374:58–68
Hazel J, Gyuris E (2006) Vessel-related mortality of sea turtles in Queensland, Australia. Wildl Res 33:149–154
Hazel J, Lawler IR, Hamann M (2009) Diving at the shallow end: green turtle behaviour in near-shore foraging habitat. J Exp Mar Biol Ecol 371(1):84–92
Hazen EL, Maxwell SM, Bailey H, Bograd SJ, Hamann M, Gaspar P, Godley BJ, Shillinger GL (2012) Ontogeny in marine tagging and tracking science: technologies and data gaps. Mar Ecol Prog Ser 457:221–240
Heithaus MR, Frid A, Wirsing AJ, Dill L, Fourqurean JW, Burkholder D, Thomson J, Bejder L (2007) State-dependent risk-taking by green turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. J Anim Ecol 76:837–844
Heupel MR, Simpfendorfer CA (2005) Quantitative analysis of aggregation behavior in juvenile blacktip sharks. Mar Biol (Berl) 147(5):1239–1249. doi:10.1007/s00227-005-0004-7
Heupel MR, Semmens JM, Hobday AJ (2006) Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Mar Freshw Res 57(1):1–13. doi:10.1071/mf05091
Hill MN, Robinson AR (1962) Physical oceanography. Harvard University Press, Cambridge
Holland KN, Wetherbee BM, Lowe CG, Meyer CG (1999) Movements of tiger sharks (Galeocerdo cuvier) in coastal Hawaiian waters. Mar Biol (Berl) 134(4):665–673. doi:10.1007/s002270050582
Hopley D, Smithers SG, Parnell KE (2007) The geomorphology of the Great Barrier Reef: development, diversity, and change. Cambridge University Press, Cambridge
Kernohan BJ, Gitzen RA, Millspaugh JJ (2001) Analysis of animal space use and movements. In: Millspaugh JJ, Marzluff JM (eds) Radio tracking and animal populations. Academic Press, San Diego
Knip DM, Heupel MR, Simpfendorfer CA, Tobin AJ, Moloney J (2011) Ontogenetic shifts in movement and habitat use of juvenile pigeye sharks Carcharhinus amboinensis in a tropical nearshore region. Mar Ecol Prog Ser 425:233–246. doi:10.3354/meps09006
Larcombe P, Woolfe KJ (1999) Increased sediment supply to the Great Barrier Reef will not increase sediment accumulation at most coral reefs. Coral Reefs 18:163–169
Laver PN, Kelly MJ (2008) A critical review of home range studies. J Wildl Manag 72(1):290–298
Limpus C, Chaloupka M (1997) Nonparametric regression modelling of green sea turtle growth rates (southern Great Barrier Reef). Mar Ecol Prog Ser 149(1–3):23–34. doi:10.3354/meps149023
Limpus CJ, Miller JD, Parmenter CJ, Reimer D, McLachlan N, Webb R (1992) Migration of green (Chelonia mydas) and loggerhead (Caretta caretta) turtles to and from eastern Australian rookeries. Wildl Res 19:347–358
Lutcavage ME, Lutz PL (1997) Diving physiology. In: Lutz PL, Musick JA (eds) The biology of sea turtles, vol 1. CRC Press, Boca Raton, FLA, pp 387–409
Makowski C, Seminoff JA, Salmon M (2006) Home range and habitat use of juvenile Atlantic green turtles (Chelonia mydas L.) on shallow reef habitats in Palm Beach, Florida, USA. Marine Biol 148:1167–1179
Mendonca MT (1983) Movements and feeding ecology of immature green turtles (Chelonia mydas) in a Florida lagoon. Copeia 4:1013–1023
Musick JA, Limpus CJ (1997) Habitat utilization and migration in juvenile sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles, vol 1. CRC Press, Boca Raton, pp 137–163
Okuyama J, Shimizu T, Abe O, Yoseda K, Arai N (2010) Wild versus head-started hawksbill turtles Eretmochelys imbricata: post-release behavior and feeding adaptions. Endanger Species Res 10(1–3):181–190. doi:10.3354/esr00250
O’Toole AC, Danylchuk AJ, Goldberg TL, Suski CD, Philipp DP, Brooks E, Cooke SJ (2011) Spatial ecology and residency patterns of adult great barracuda (Sphyraena barracuda) in coastal waters of The Bahamas. Mar Biol (Berl) 158(10):2227–2237. doi:10.1007/s00227-011-1728-1
Pincock DG (2008) False detections: what they are and how to remove them from detection data. Vemco Division, Amirix Systems Inc., Halifax, Nova Scotia
Plotkin P (2003) Adult migrations and habitat use. In: Lutz PL, Musick JA, Wyneken J (eds) Biology of sea turtles, vol II. CRC Press, Boca Raton, pp 225–241
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Seminoff JA (2004) Chelonia mydas. In: IUCN 2012. IUCN red list of threatened species version 2012.1. www.iucnredlist.org. Accessed 04 September 2012
Seminoff JA, Jones TT (2006) Diel movements and activity ranges of green turtles (Chelonia mydas) at a temperate foraging area in the Gulf of California, Mexico. Herpetol Conserv Biol 1(2):81–86
Seminoff JA, Resendiz A, Smith TW, Yarnell L (2001) Diving patterns of green turtles (Chelonia mydas agassizii) in the Gulf of California. In: Coyne MS, Clark RD (eds) Proceedings of the twenty-first annual symposium on sea turtle biology and conservation. NOAA, Philadelphia
Seminoff JA, Resendiz A, Nichols WJ (2002) Home range of green turtles Chelonia mydas at a coastal foraging area in the Gulf of California, Mexico. Mar Ecol Prog Ser 242:253–265
Simpfendorfer CA, Heupel MR, Hueter RE (2002) Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can J Fish Aquat Sci 59(1):23–32
Simpfendorfer CA, Heupel MR, Collins AB (2008) Variation in the performance of acoustic receivers and its implication for positioning algorithms in a riverine setting. Can J Fish Aquat Sci 65(3):482–492. doi:10.1139/f07-180
Taquet C, Taquet M, Dempster T, Soria M, Ciccione S, Roos D, Dagorn L (2006) Foraging of the green sea turtle Chelonia mydas on seagrass beds at Mayotte Island (Indian Ocean), determined by acoustic transmitters. Mar Ecol Prog Ser 306:295–302. doi:10.3354/meps306295
Vemco (2010) Frequently asked questions. Amirix Systems, Inc. www.vemco.com. Accessed 21 March 2012
White GC, Garrott RA (1990) Analysis of wildlife radio-tracking data. Academic Press, San Diego
Whiting SD, Miller JD (1998) Short term foraging ranges of adult green turtles (Chelonia mydas). J Herpetol 32(3):330–337
Worton BJ (1989) Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70(1):164–168
Worton BJ (1995) Using monte carlo simulation to evaluate kernel-based home range estimators. J Wildl Manag 59(4):794–800
Yeiser BG, Heupel MR, Simpfendorfer CA (2008) Occurrence, home range and movement patterns of juvenile bull (Carcharhinus leucas) and lemon (Negaprion brevirostris) sharks within a Florida estuary. Mar Freshw Res 59(6):489–501
Zeller DC (1997) Home range and activity patterns of the coral trout Plectropomus leopardus (Serranidae). Mar Ecol Prog Ser 154:65–77
Acknowledgments
Equipment and logistical support were provided by private donors, James Cook University, Queensland Parks and Wildlife and Quicksilver Connections. Grateful thanks to Ian Bell, Col Limpus, Colin Simpfendorfer and Michelle Heupel for expert advice, to Sam Dibella and volunteers for fieldwork assistance, to Clement Calenge and colleagues for adehabitatHR and to peer reviewers for helpful suggestions. Field research was conducted under the authority of James Cook University Ethics Approval A1474 and scientific research permits G10/33206.1, G10/33897.1 and WISP06563509.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. D. R. Houghton.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hazel, J., Hamann, M. & Lawler, I.R. Home range of immature green turtles tracked at an offshore tropical reef using automated passive acoustic technology. Mar Biol 160, 617–627 (2013). https://doi.org/10.1007/s00227-012-2117-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2117-0