Abstract
Great barracuda (Sphyraena barracuda) were implanted with acoustic telemetry transmitters (n = 42) and monitored within a stationary acoustic receiver array (n = 53 receivers) in The Bahamas to examine residency, seasonal movements, and habitat use. Barracuda were monitored for up to 980 days and remained within the array area ~33% (median value) of the time. Most tagged barracuda were transient and would often disappear from the array for months at a time, particularly in the summer where they were usually last detected on receivers located in deeper shelf habitats, and then return at other times in the year. Habitat use across the footprint of the array differed, with most detections occurring in coastal areas and comparatively fewer in deeper mosaic or shelf habitats. Linear home range estimates revealed that some barracuda moved >12 km within a single day and are capable of migrating >100 km to other islands in the Bahamian Archipelago. Our results provide some of the first telemetry data for this apex marine predatory fish and the first reliable information on the residency and localized seasonal movements of adult great barracuda in the coastal waters of the Western Atlantic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Great barracuda (Sphyraena barracuda) are a predatory marine fish found in subtropical and tropical regions worldwide (Williams 1959; de Sylva 1963; Blaber 1982) and may hold an important position as an apex predator in nearshore systems (de Sylva 1963; Friedlander and de Martini 2002). As a species that is easily accessible from shore, barracuda are often targeted by recreational anglers (Dunaway 2008) and subsistence fishers for consumption, thus illustrating the economic and cultural importance of this species (de Sylva 1963; Villareal et al. 2007). Despite their potential ecological importance, abundance, and stature as a prized sportfish and food fish, there is a relative dearth of knowledge of great barracuda biology and natural history. In particular, barracuda movement patterns are poorly understood.
To date, only a limited number of studies have documented barracuda movement and behaviour at coarse scales, using mark-recapture tagging studies (Springer and McErlean 1961; Villareal et al. 2007), underwater visual observations (Paterson 1998), and natural body markings to monitor individuals (Wilson et al. 2006). Barracuda have often been observed to inhabit a range of habitat types, including nearshore reefs, tidal flats, and deeper pelagic environments (de Sylva 1963; Paterson 1998; Wilson et al. 2006). Their presence in these areas may be a function of life history stage or environmental conditions, while seasonal migrations are presumed to be associated with spawning activity or variation in water temperatures (de Sylva 1963; Paterson 1998; Faunce and Serafy 2008). A mark-recapture study has revealed that larger individuals move greater distances and are more likely to inhabit offshore reefs (Springer and McErlean 1961), while in contrast, Wilson et al. (2006), using unique natural color markings to identify individual fish, found that larger barracuda were re-sighted near their original location during a 10 month study near South Caicos, Turks and Caicos Islands, suggesting a degree of site fidelity. Interestingly, great barracuda have also been documented to migrate over 1,000 km across the Gulf of Mexico (Villareal et al. 2007), suggesting that they are capable of large-scale migrations across great distances. However, mark-recapture studies are known to be biased against the detection of movement and have limited spatial and temporal resolution (Klimley et al. 1998). Consequently, local residency, home range, and habitat use are poorly understood and warrant further investigation using additional and more robust techniques.
Large acoustic telemetry receiver arrays are increasingly being used to study the spatial ecology of marine fish species and are providing unprecedented advances in knowledge with respect to fish behaviour and critical habitat use (Heupel et al. 2006a, b; Yeiser et al. 2008; Meyer et al. 2007a, b). With increasing anthropogenic influence, large predatory fish such as great barracuda, may become more susceptible to disturbances in coastal regions, and basic biological information could inform future conservation initiatives.
The objective of the present study was to examine residency, seasonal movements, and habitat use of great barracuda in the coastal waters of The Bahamas. A large-scale acoustic telemetry array was deployed across a range of habitat types and fish were monitored for up to 33 months. To our knowledge, this is the first study to use acoustic telemetry to document movement and habitat use for great barracuda.
Materials and methods
Study site
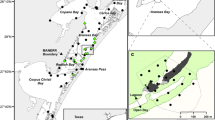
The study site was located near the Cape Eleuthera Institute off the coast of Powell Point, Eleuthera, The Bahamas (24°54′N; 76°20′W, Fig. 1). Situated at the junction of the shallow Grand Bahamas Bank and the deeper waters of the Exuma Sound, the marine environment adjacent to Powell Point contains a diversity of habitats including mangrove tidal creek systems, seagrass beds, patch reefs, and deeper, continental shelf environments. An acoustic telemetry array, designed to monitor the movement and activity of several marine fish species, was deployed along the coast of Powell Point, initially consisting of 27 receivers in 2007 (VR2 and VR2W; Vemco/Amirix systems, Shad Bay, Canada). The array was expanded in 2008 and 2009, and some receiver stations were re-located to optimize data collection. The greatest number of receivers deployed at one time was 53, with a total of 105 receiver stations used over the entire study period. The receiver array was arranged in three “curtains” extending from Powell Point, with additional receivers placed as a net formation between the curtains, a row along the edge of the continental shelf, and stations within tidal creeks and coastal flats (Fig. 1). The receivers were attached to a piece of iron bar that was fixed to a cement block and placed on the ocean floor. Range tests, performed according to manufacturer specifications upon initial receiver deployment using fixed tags in varied environments and depths as detailed in Murchie et al. (in press), indicated average detection ranges of approximately 500–600 m radius in deep water (i.e. continental shelf habitat) under optimal conditions, generally 200–250 m in shallower, dynamic coastal areas, and as small as 30 m radius in the shallow waters of tidal creeks. Inherent with all such arrays, we expected that detection efficiency was reduced during storms. For the most part, receiver detection zones did not overlap. Fish were monitored for 980 days from February 20, 2007, when the first barracuda was tagged, until the final receiver download on October 27, 2009, when the study ended due to the need to redeploy equipment for a different study.
Habitat type was divided into three categories: coastal, mosaic, and shelf. Coastal habitat (within approximately 1 km from shore) was generally less than 5 m deep, with typical characteristics including tidal creeks, mud flats, some seagrass, and small patch reefs. These habitats were often exposed to some wave and tidal action. Anthropogenic structures such as marinas or dredged cuts (regardless of water depth) were also included as coastal habitat due to the close proximity of these locations to other available coastal habitat. Mosaic habitat was generally less than 10 m deep, usually within 2 km from shore and characterized as having a mosaic of patch reefs, seagrass beds, and areas of sandy bottom. Shelf habitats were located along the continental shelf of the Exuma Sound, at depths greater than 10 m (the greatest depth of a receiver station was placed over the edge of the shelf at 42 m). They were characterised by some patch reefs and exposure to currents, and some of these shelf locations were located relatively close to shore (<1 km). Depth information was determined at the time of receiver deployment.
Between February 2007 and December 2008, acoustic telemetry transmitters (2007, n = 8; 2008, n = 34) were surgically implanted into 42 adult great barracuda. All 42 fish were tagged during the months of December, February, and April. Barracuda were captured by trolling with heavy-action recreational fishing gear (14 kg [30 lb] test fishing line, n = 38, see O’Toole et al. 2010 for details) and artificial lures, with spinning gear in shallow intertidal areas (n = 2), or in mangrove creeks with a seine net (n = 2). Once landed, the fish were transferred to a 100 l cooler of seawater that contained a sufficient concentration of the anaesthetic 3-aminobenzoic acid ethyl ester (MS222; approximately 100 mg l−1) to induce stage 4 anaesthesia (Summerfelt and Smith 1990) in 3–4 min. Anaesthetized fish were held in a supine position with gills submerged while a 2–3 cm incision was cut with a scalpel along the central mid-line halfway between the pelvic and anal fins, and disinfected transmitters were inserted into the body cavity. The incision was closed using 2–3 simple interrupted sutures (PDS II 3/0 absorbable sutures, Ethicon Ltd, NJ; Cooke et al. 2003). Halfway through the surgery, fresh seawater was added to the cooler to dilute the anaesthetic concentration and to begin the recovery process. All surgeries were performed by the same trained surgeon, and all handling and surgical procedures conformed to the guidelines of the Canadian Council for Animal Care administered through Carleton University, Ottawa, ON. All fish were allowed to recover in ambient seawater for approximately 45–60 min post-surgery before being released back into the study area. Some fish were released at the point of capture (n = 22), however, on some occasions barracuda were released from the marina at Powell Point (n = 20). The marina offered an area that was sheltered from poor weather and water conditions conducive for surgery procedures as well as low predator burden area for release post surgery and was less than 4 km from all capture locations.
Acoustic transmitters used during this study included V13 (average delay, 80 or 90 s), V16 (average delay, 60, 57.5, 80, or 90 s), and V9AP models (average delay, 45 or 90 s), each coded with a unique pulse series and operated at 69.0 kHz (Vemco/Amirix systems, Shad Bay, NS). The transmitters had an expected battery life ranging from 530 to 3,645 days, and all battery units were projected to last the duration of the study period (as a function of tagging date) except for the V9AP transmitters, which had a battery life expectancy of 65–160 days.
Data analysis
Residency
To quantify the number of barracuda present within the study area at any one time, a point was plotted on a graph for each day that an individual fish was detected anywhere in the array across the entire study period (Fig. 2a). Data were sorted by month and the proportion of fish present relative to the total number of tagged fish at large on a monthly basis over the entire study period (February 2007–October 2009, Fig. 2b) were plotted to determine seasonal trends in residency. Based on the distribution of seasonal data and coastal water temperature data (summer, mean = 28.6 ± 0.02°C; winter, mean = 24.9 ± 0.02°C), data were subsequently divided into two seasons (summer, April–September and winter, October–March). Since not all individuals were detected for an entire year, only data from the first 365 days post-tagging for individuals that were detected for at least 1 year (n = 10) were used to compare the difference between median number of days detected for winter and summer using a Wilcoxon Sign-Rank test (Zar 1999). Long-term telemetry data sets spanning multiple years are rare, thus, an additional analysis was completed to compare the median number of days detected in winter and summer for barracuda that were detected within the array for more than 1 year (extending from tagging date until final date of detection in the array) also using a Wilcoxon Sign-Rank test (Zar 1999).
Residency plots depicting a presence-absence data for each tagged S. barracuda detected within the acoustic telemetry array over the entire study period. Each data point represents a day during which a barracuda was detected at any location within the array, open triangles represent the tagging date for each fish, grey triangles represent the predicted date of transmitter battery depletion or final download date. b Proportion of tagged S. barracuda present within the array to the number of available tagged barracuda on a monthly basis over the duration of the study period. Seasonal changes are represented by ‘S’ (summer, April–September) and ‘W’ (winter, October–March)
A chi-square test was used to determine the probability that the number of tagged fish at large (with an expectation of 100% residency) differed from the number of fish observed for each month within the array during the study period. In addition, we assessed the interval at which each fish was absent from the array and noted the receiver at which the fish was last detected as well as the receiver where the fish was detected upon returning to the study site. The number of detections within the array from April to September was divided by the total number of detections in both seasons to determine the percentage of time each individual barracuda spent in the array during the summer. Fish that were tagged with transmitters that did not have sufficient battery life to last through both seasons (i.e., V9AP model) and fish that were not detected in the array during the entire study period were removed from the analysis. Individuals that were detected in the summer were characterised by capture location and total length.
A residency index (IR) was calculated for each fish by dividing the total number of days a fish was detected within the array by the number of days in the array (i.e. number of days between the date of release until the last day detected). An IR value of 0 indicates no residency and a value of 1 indicates continuous residency within the array (Abecasis and Erzini 2008; March et al. 2010). A correlation analysis was conducted to evaluate the relationship between fish size (TL) and residency variables (total number of days detected, number of days at large, and IR).
Habitat use
The position of many of the receivers within the array was periodically adjusted during the study period in an effort to refine spatial coverage and to accommodate a number of related studies of other fish species. As such, a correction factor was applied so that the total number of detections at each station was divided by total number of days the receiver was deployed at that location since receivers were not deployed for the same length of time they were not equally likely to detect fish throughout the duration of the study period (Murchie et al. 2010).
Minimum linear distance (MLD) and median distance travelled (MDT) were used to estimate barracuda home range. MLD was calculated by determining the straight-line distance through water between the two farthest receivers by which each fish was detected (Chapman et al. 2005), and MDT was calculated by finding the median distance between all receivers at which each fish was detected and the receiver where a fish was most frequently detected (Murchie et al. 2010). A correlation analysis was used to determine if there was a relationship between fish size and MLD, MDT, and the mean daily number of receivers at which each fish was detected.
A repeated measures analysis of variance (ANOVA) was used to determine if the number of detections per days deployed varied across different habitat types (i.e., coastal, mosaic, shelf) to further examine habitat use. A repeated measures analysis of variance (ANOVA) was also used to determine if the number of detections per days deployed differed across diel periods. Diel periods were defined as day (06:00–17:59) and night (18:00–05:59) according to average sunrise and sunset times for the local area. All statistical results were assessed at α = 0.05.
Results
Residency
Great barracuda (n = 42) ranging in total length from 60 to 125 cm (mean = 88.5 ± 2.4 cm) were monitored from February 20, 2007 to October 27, 2009 (Table 1). Only nine fish were detected within the array for less than 24 h, while some fish were detected for more than 400 days during the course of the study period (Table 1). The nine fish that were detected in the array for less than 24 h were excluded from further analysis (including two individuals, #9,531 and 9,532 that were never detected post-release, even within the first 24 h). The total number of detections per individual ranged from 0 to 74,174 (mean = 5,300 ± 2,395, median = 717 detections). One fish was harvested by a local subsistence fisher in June 2008 and the transmitter was returned to us but not re-deployed. Individual fish showed variable degrees of residency over time, with some fish being routinely detected throughout the monitoring period while others were more transient (Fig. 2a). Barracuda presence within the array fluctuated over time. Throughout the study period the number of fish detected was less than the total number tagged (χ2 = 232, df = 32, P < 0.001), 18–75% of the tagged fish were detected on a monthly basis. Results also showed a seasonal difference in number of days detected for fish that were present in the array for 365 days, with barracuda spending significantly more time within the array during the winter than in the summer (Z = 25.5, P = 0.006). When a similar analysis was completed for barracuda that were detected in the array for more than 1 year, a seasonal difference in median number of days detected was not apparent (Z = −10.5, P = 0.32).
Approximately 35% (n = 14) of all tagged fish left the footprint of the array for periods of more than 2 weeks during the summer months before returning. Of the individuals that left the array, 71% (n = 10) were last detected at a shelf habitat receiver and were then also detected again by a shelf receiver upon return to the study area, suggesting movement to deeper, oceanic habitat during summer. In fact, one individual (#2380) left the array at a western receiver located along the shelf in March 2008, was detected by a neighbouring telemetry array located near Stuart’s Cove, New Providence (a distance > 100 km) in June and July 2008, before returning back to our array at Cape Eleuthera in September 2008. The remaining 29% (n = 4) of barracuda appeared to primarily move in and out of the eastern portion of the array (i.e., receiver #39, 15, 17; Fig. 1). Eleven fish had a range of 15–59% of total detections during the summer. Of these 11 fish, four were the largest barracuda captured during the study (TL ranged 106–125 cm) and only one fish was initially captured from shelf habitat, while all other individuals were captured in mosaic or coastal habitat.
IR values ranged from 0 to 0.91 and the median IR value was 0.33 (mean = 0.40 ± 0.05). Eleven barracuda were detected in the array for less than 10 days and were not included in the residency index analysis because these fish were detected for a short period of time and thus, were given an artificially inflated residency index value. Total length of tagged barracuda was not correlated with total number of days detected (r2 = 0.22, n = 33, P = 0.21), number of days at large (r2 = 0.17, n = 33, P = 0.33), or IR (r2 = 0.17, n = 29, P = 0.39).
Habitat use and movements
The three receivers with the greatest number of detections per days deployed were receiver #33 (coastal), #49 (mosaic), and #28 (coastal; Fig. 1). Adult barracuda were detected more often in coastal and mosaic habitats than in shelf habitats, although some receivers in other mosaic and shelf habitats (i.e., receiver #74, mosaic; #98, shelf) detected more individual fish, but less frequently (Fig. 3). Some barracuda were detected by up to 21 receiver stations in a single day, and the mean number of receivers detecting barracuda per day ranged from 1.0 ± 0.0 to 5.0 ± 0.2 receivers (Table 2). Barracuda exhibited MDT values that ranged from 449 to 7,824 m (median = 1,926 m; mean = 2,299 ± 287 m) and MLD values ranging 898–15,950 m (median = 5,729 m; mean = 6,999 ± 825 m), not including fish that were detected for less than 24 h or by less than one receiver (n = 29, Table 2). Fish size was not significantly correlated with the number of receivers at which fish were detected (r2 = 0.19, n = 33, P = 0.30) or MLD (r2 = 0.27, n = 33, P = 0.13); however, MDT was weakly correlated with fish size (r2 = 0.37, n = 33, P = 0.03). Barracuda did not exhibit differences in the number of detections per days deployed across habitat types (F = 1.46, df = 2, P = 0.24) or between diel periods (F = 0.04, df = 1, P = 0.84).
Discussion
Residency
Median residency index values (IR) showed that great barracuda spent approximately 33% of the monitoring period within the receiver array. Although a previous study (Wilson et al. 2006) using individual-specific body color markings recorded with digital photographs reported that larger barracuda (TL > 70 cm) exhibited a high degree of site fidelity over extended time periods (up to 20 months) compared to smaller individuals, residency time within the array was not related to fish size in our study, although some of the barracuda that exhibited higher residence in the summer months were larger fish. While a dearth of knowledge exists on great barracuda reproductive ecology, de Sylva (1963) postulated that barracuda off the coast of southern Florida and Bimini, The Bahamas, spawn in offshore areas during the summer months (May–August), based on information from gonadal somatic indices and post-larval specimens collected in plankton tows. A more recent study conducted in the Florida Keys by Kadison et al. (2010) suggests a summer spawning period extending from March to September also based on gonadal somatic index values and timing of young-of-the-year barracuda presence in nearshore areas. A notable decrease in the presence of barracuda within our array occurred during the summer months (April–September) even among highly resident individuals. These movements could be related to spawning activities, seasonal changes in coastal water temperature, or ontogenetic factors. A large percentage of the barracuda that left the array during the summer (71%) were detected in shelf habitat before and after long-term absences from the study area, indicating movement to offshore environments, a pattern consistent with postulated spawning activity.
Individual great barracuda showed varied degrees of residency within the telemetry array and over the study period, the proportion of tagged fish present within the array fluctuated. It is to be expected that those periods with the highest proportion of tagged fish present in the array corresponded to when fish were being tagged (Fig. 2), indicating that some barracuda may have been transiting through the area when captured and may have moved outside of the study area shortly after release, suggesting that some tagged individuals were not locally resident prior to the commencement of the study. If barracuda were preyed upon or died in some other way, it would be likely that tags would have ended up on the ocean bottom. Some of the fish (21%; n = 9) were detected for less than 24 h post-release, and two of these fish did not register a single detection. The lack of detections for these fish may be associated with immediate movement outside of the detection range of the array, although lack of detection may also have been a result of transmitter malfunction. Despite allowing barracuda to recover for up to an hour post-surgery in an attempt to reduce potential for post-release predation, we cannot exclude the possibility that fish exhibited some level of behavioural impairment after release. In a study of barracuda catch-and-release angling that evaluated the immediate mortality of fish at time of capture in the same area, several barracuda were attacked and killed while being reeled in, which suggests a reasonable predator burden in the area where fish were tagged (O’Toole et al. 2010). Within the vicinity of where the barracuda were tagged, several shark species are common, including lemon sharks (Negaprion brevirostris, Murchie et al. 2010), bull sharks (Carcharhinus leucas), and Caribbean reef sharks (Carcharhinus perezi; O’Toole, pers obs). Post-release mortality of fish tagged in predator-rich environments is a significant concern because predation after tagging has been observed in a number of marine fish (Kerstetter et al. 2004; Danylchuk et al. 2007; Murchie et al. 2010). However, a number of sharks also carried acoustic tags and we saw no evidence of those tagged sharks mirroring the movements of tagged barracuda which would indicate post-release predation. It is unfortunately quite difficult to determine if non-detections were the result of mortality, transient behaviour, or a combination of the two. Given that one fish was detected over 100 km away from the tagging site, there is clearly potential for evaluating barracuda movements using other technology that could better document transient behaviour and large-scale movements.
Habitat use and movements
Although great barracuda were detected more often in coastal and mosaic habitats than on the shelf, the three receivers with the most detections per days deployed (i.e., receiver #33, 49, 28; Fig. 3) were located less than 1 km apart and frequently detected only a small number of individuals (predominately, #1441, 2609, 9524, and 9526). Some of the other receivers in other mosaic and shelf habitats (i.e., receiver #74, 98) detected more individual fish, but less frequently (Fig. 3; see Table A in the online appendix). Nonetheless, for those barracuda that were resident in the array for extended periods, most individuals used all three habitat types. It is evident that some barracuda maintain a territory and although these longer term resident individuals sometimes left the study area, they would typically return to a certain area (i.e., #2606, 9524, 2609). For example, barracuda #2606 was often detected in the vicinity of the tidal creeks located along the eastern side of the array even after prolonged absences or movements across the study area. In addition, continental shelf habitat in this region may be considered a transitory area for barracuda, an idea that has also been proposed by Paterson (1998).
Individual barracuda showed variation in home range with some fish utilizing more of the available area within the array than others. In fact, some individuals sometimes transited across the entire expanse of the array within a single day (for example, #2606 moved at least 12 km from receiver #15, around Powell Point to receiver #78, Fig. 1). The metrics used to estimate home range (MLD and MDT) may not fully encompass the full range of movement for the individuals in our study since our residency results suggest that many of the barracuda left the study area. Although fish size was not associated with the number of receivers at which a fish was detected or with MLD, it is noteworthy that there was a weak relationship between fish size and MDT. Great barracuda are capable of transiting great distances, particularly larger individuals (>60 cm, TL) that were shown to move up to 193 km over a 90 days period (Springer and McErlean 1961). Conversely, Wilson et al. (2006) found that larger barracuda (>73 cm) demonstrated negligible movement (<100 m) from original sighting locations, albeit long time periods often separated the sightings of many of these individuals, potentially suggesting migrations out of the area. Intraspecific variation (Albula vulpes, Humston et al. 2005) and seasonal changes in habitat use and residency are common in other marine teleost species, even among highly resident individuals (e.g., Caranx ignobilis, Meyer et al. 2007a; Aprion virescens, Meyer et al. 2007b; Pseudocaranx dentex, Afonso et al. 2009).
Due to the transient nature of many of the tagged barracuda and open nature of the array design, we were unable to monitor individuals once they left the footprint of the array. Additionally, in an effort to cover a broader overall geographic area, the detection ranges of the receiver stations often did not overlap, somewhat limiting our ability to assess continuous movement even when the barracuda were present within the confines of the array. Non-detections are a common issue with arrays of this design (Meyer et al. 2007a, b; Afonso et al. 2009). However, one tagged barracuda was detected on a telemetry array operated off the coast of New Providence suggesting this species is capable of large scale movements. Thus, greater behavioural resolution would be greatly improved by using pop-up satellite tag technology (Arnold and Dewar 2001) to monitor barracuda spatial ecology at continuous temporal scales and across larger geographic ranges.
Conclusions
The great barracuda is an economically, culturally, and ecologically important predator that plays a key role in the ecology of coastal marine environments. Developing conservation strategies for this species, however, suffers greatly from the lack of basic biological information about its life history, reproductive ecology, and behaviour. Indeed, if great barracuda predominately inhabit coastal areas, they may be subject to anthropogenic impacts such as habitat degradation, pollution, or overfishing. Our data suggest that some fish are resident for long periods of time, however, there also seems to be a segment of the population that is transient and mobile. The extent of those movements remains unknown. Successful conservation, therefore, depends upon increased research efforts to fill in key information gaps and future studies should consider the use of pop-up satellite tags to track barracuda at larger spatial scales than what was assessed in the current study.
References
Abecasis D, Erzini K (2008) Site fidelity and movements of gilthead sea bream (Sparus aurata) in a coastal lagoon (Ria Formosa, Portugal). Estuar Coast Shelf Sci 79:758–763
Afonso P, Fontes J, Holland KN, Santos RS (2009) Multi-scale patterns of habitat use in a highly mobile reef fish, the white trevally Pseudocaranx dentex, and their implications for marine reserve design. Mar Ecol Prog Ser 381:273–286
Arnold G, Dewar H (2001) Electronic tags in marine fisheries research: a 30-year perspective. In: Sibert J, Nielsen J (eds) Methods and technologies in fish biology and fisheries, vol 1. Academic Press, Dordrecht, pp 7–64
Blaber SJM (1982) The ecology of Sphyraena barracuda (Osteichthyes: Perciformes) in the Kosi system with notes on the Sphyraenidae of other Natal estuaries. S Afr J Zool 17:171–176
Chapman DD, Pikitch EK, Babcock E, Shivji MS (2005) Marine reserve design and evaluation using automated acoustic telemetry: a case-study involving coral reef-associated sharks in the Mesoamerican Caribbean. Mar Technol Soc J 39:42–55
Cooke SJ, Graeb BDS, Suski CD, Ostrand KG (2003) Effects of suture material on incision healing, growth and survival of juvenile largemouth bass implanted with miniature radio transmitters: case study of a novice and experienced fish surgeon. J Fish Biol 62:1366–1380
Danylchuk AJ, Danylchuk SE, Cooke SJ, Goldberg TL, Koppelman JB, Philipp DP (2007) Post-release mortality of bonefish, Albula vulpes, exposed to different handling practices during catch-and-release angling in Eleuthera, The Bahamas. Fish Manag Ecol 14:149–154
de Sylva DP (1963) Systematics and life history of the great barracuda, Sphyraena barracuda. University of Miami Press, Coral Gables
Dunaway V (2008) Sportfish of Florida. Wickstrom, Miami
Faunce CH, Serafy JE (2008) Selective use of mangrove shorelines by snappers, grunts, and great barracuda. Mar Ecol Prog Ser 356:153–162
Friedlander AM, de Martini EE (2002) Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators. Mar Ecol Prog Ser 230:253–264
Heupel MR, Semmens JM, Hobday AJ (2006a) Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Mar Freshwater Res 57:1–13
Heupel MR, Simpfendorfer CA, Collins AB, Tyminski JP (2006b) Residence and movement patterns of bonnethead sharks, Sphyrna tiburo, in a large Florida estuary. Environ Biol Fish 76:47–67
Humston R, Ault JS, Larkin MF, Luo J (2005) Movement of bonefish (Albula vulpes) in Biscayne Bay determined using ultrasonic transmitters and a passive acoustic receiver array. Mar Ecol Prog Ser 291:237–248
Kadison E, D’Alessandro EK, Davis GO, Hood PB (2010) Age, growth, and reproductive patterns of the great barracuda, Sphyraena barracuda, from the Florida Keys. Bul Mar Sci 86:773–784
Kerstetter DW, Polovina JJ, Graves JE (2004) Evidence of shark predation and scavenging on fishes equipped with pop-up satellite archival tags. Fish Bull 102:750–756
Klimley AP, Voegeli F, Beavers SC, Le Boeuf BJ (1998) Automated listening stations for tagged marine fishes. Mar Technol Soc J 2:94–101
March D, Palmer M, Alós J, Grau A, Cardona F (2010) Short-term residence, home range size and diel patterns of the painted comber Serranus scriba in a temperate marine reserve. Mar Ecol Prog Ser 400:195–206
Meyer CG, Holland KN, Papastamatiou YP (2007a) Seasonal and diel movements of giant trevally Caranx ignobilis at remote Hawaiian atolls: implications for the design of marine protected areas. Mar Ecol Prog Ser 333:13–25
Meyer CG, Papastamatiou YP, Holland KN (2007b) Seasonal, diel, and tidal movements of green jobfish (Aprion virescens, Lutjanidae) at remote Hawaiian atolls: implications for marine protected area design. Mar Biol 151:2133–2143
Murchie KJ, Schwager E, Cooke SJ, Danylchuk AJ, Danylchuk SE, Goldberg TL, Suski CD, Philipp DP (2010) Spatial ecology of juvenile lemon sharks (Negaprion brevirostris) in tidal creeks and coastal waters of Eleuthera, The Bahamas. Environ Biol Fishes 89:95–104
Murchie KJ, Danylchuk AJ, Cooke SJ, O’Toole AC, Shultz A, Haak C, Brooks E, Suski CD (in press) Considerations for tagging and tracking fish in tropical coastal habitats: lessons from bonefish, barracuda, and sharks tagged with acoustic transmitters. In: Amer Fish Soc Special Pub—Handbook of Fish Telemetry, Bethesda
O’Toole AC, Danylchuk AJ, Suski CD, Cooke SJ (2010) Consequences of catch-and-release angling on the physiological status, injury, and immediate mortality of great barracuda (Sphyraena barracuda) in the The Bahamas. ICES J M Sci 67:1667–1675
Paterson SE (1998) Group occurrence of great barracuda (Sphyraena barracuda) in the Turks and Caicos Islands. Bul Mar Sci 63:633–638
Springer VG, McErlean AJ (1961) Tagging of great barracuda, Sphyraena barracuda (Walbaum). Trans Am Fish Soc 90:497–500
Summerfelt RC, Smith LS (1990) Anaesthesia, surgery, and related techniques. In: Schreck CB, Molyle PB (eds) Methods for fish biology. American Fisheries Society, Bethesda, pp 213–272
Villareal TA, Hanson S, Qualia S, Jester ELE, Granade HR, Dickey RW (2007) Petroleum production platforms as sites for the expansion of ciguatera in the northwestern Gulf of Mexico. Harmful Algae 6:253–259
Williams F (1959) The barracuda (genus Sphyraena) in British East African waters. Ann Mag Nat Hist 2:92–128
Wilson SK, Wilson DT, Lamont C, Evans M (2006) Identifying individual great barracuda Sphyraena barracuda using natural body marks. J Fish Biol 69:928–932
Yeiser BG, Heupel MR, Simpfendorfer CA (2008) Occurrence, home range and movement patterns of juvenile bull (Carcharhinus leucas) and lemon (Negaprion brevirostris) sharks within a Florida estuary. Mar Freshw Res 59:489–501
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall, Englewood Cliffs
Acknowledgments
The authors would like to acknowledge the staff and volunteers at the Cape Eleuthera Institute and The Island School. Thank you to A. Shultz, C. Haak, K. Murchie, K. Hanson, C. Pullen, K. Pullen, and J. Cooke for field and laboratory assistance and to Aleksandra Maljkovic for sharing movement information. The Bahamas Department of Marine Resources provided scientific collection permits and animal care approval was obtained from the Canadian Council on Animal Care through Carleton University. The receiver array was partially supported by grants from Bonefish and Tarpon Trust, the Charles A. and Anne Morrow Lindbergh Foundation, and the Baldwin Foundation. Additional financial support was provided by Ontario Ministry of Research and Innovation (through an Early Researcher Award to S.J.C.), the Canada Foundation for Innovation, the Canada Research Chairs Program, and Carleton University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Righton.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
O’Toole, A.C., Danylchuk, A.J., Goldberg, T.L. et al. Spatial ecology and residency patterns of adult great barracuda (Sphyraena barracuda) in coastal waters of The Bahamas. Mar Biol 158, 2227–2237 (2011). https://doi.org/10.1007/s00227-011-1728-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1728-1