Abstract
In the Japanese pygmy squid Idiosepius paradoxus, females often pick up the spermatangium using their mouth (buccal mass) after copulation. To examine whether the female I. paradoxus directly transfers sperm into the seminal receptacle via this picking behaviour, or removes the spermatangium, we conducted detailed observations of picking behaviour in both virgin and copulated females and compared the sperm storage conditions in the seminal receptacle between females with and without spermatangia picking after copulation in virgin females. In all observations, elongation of the buccal mass occurred within 5 min after copulation. However, sperm volume in the seminal receptacle was not related to spermatangia picking. Observations using slow-motion video revealed that females removed the spermatangia by blowing or eating after picking. These results suggest that picking behaviour is used for sperm removal but not for sperm transfer. Moreover, the frequency of buccal mass elongation was higher in copulated females than in virgin females, consistent with the sequential mate choice theory whereby virgin females secure sperm for fertilisation, while previously copulated females are more selective about their mate. Female I. paradoxus may choose its mate cryptically through postcopulatory picking behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the reproduction of coastal decapod cephalopods such as loliginid squids and cuttlefish, the basic consequence of events during the copulation is as follows: a male provides a female with its spermatophore using the hectocotylus, which is a specialised arm for holding the spermatophore (Hanlon and Messenger 1996). The spermatangium is ejaculated from the spermatophore by a spermatophoric reaction and is attached to the female body by the cement body of the spermatangium (Drew 1919; Marian 2012; Takahama et al. 1991). In the cuttlefish, males pass the spermatangium to females in a head-to-head position, and the spermatangium is deposited on the female buccal membrane near the seminal receptacle (Hanlon et al. 1999; Naud et al. 2004; Wada et al. 2010, 2006). In loliginid squids, spermatangia may be deposited at two positions by alternative mating behaviours (Hanlon et al. 2002; Iwata et al. 2005; Iwata and Sakurai 2007; Buresch et al. 2009). A male deposits a spermatangium inside her mantle cavity by male-parallel position (Iwata et al. 2011). Alternatively, a female can have a seminal receptacle in the buccal membrane (Hanlon and Messenger 1996). When the male copulates in a head-to-head position, a spermatangium is deposited on the buccal membrane near the seminal receptacle, as in cuttlefish. Many squids, including the loliginids, have seminal receptacles on the buccal membrane (e.g. Todarodes pacificus and T. sagittatus) (Ikeda et al. 1993; Nigmatullin et al. 2002). Spermatozoa from a spermatangium deposited on the buccal membrane are transferred to the female’s seminal receptacle and then stored until spawning. However, the method by which the sperm are transferred from the spermatangium into the seminal receptacle has not been determined. The opening duct of the spermatangium faces the outside and does not connect to the opening of the seminal receptacle (Drew 1919; Takahama et al. 1991). Therefore, even if the spermatangium were deposited on the seminal receptacle, sperm would not transfer directly.
Two possible methods of sperm transfer exist in decapod cephalopods. One is that sperm released from the spermatangia can swim in seawater and reach the seminal receptacle. The tip of the spermatangium has an opening duct, and sperm are released into seawater from the opening duct in Loligo pealei (Drew 1919) and T. pacificus (Takahama et al. 1991). The other method is that sperm is transferred passively by a female. The female may use her arm or mouth to transfer sperm directly at the seminal receptacle or may move the seminal receptacle itself. Previous studies on Caribbean reef squids (Sepioteuthis sepioidea) suggested that the female may transfer sperm to the seminal receptacle directly using her arms (Moynihan and Arcadio 1982; Hanlon and Forsythe in Hanlon and Messenger 1996). However, to date, neither method has actually been demonstrated, and few studies have investigated the possibility of the latter idea in particular.
The female Japanese pygmy squid Idiosepius paradoxus has a single seminal receptacle which is located in the ventral portion of the buccal membrane surrounding the mouth (buccal mass) (Sato et al. 2010). The male attaches spermatangia at the base of the female’s arm. Recent studies have reported that the female frequently picks up the spermatangium using her extendable buccal mass after copulation (Kasugai 2000; Sato et al. 2010). This behaviour may be used to transfer sperm from the spermatangia into the seminal receptacle. In this study, to examine whether the female I. paradoxus directly transfers sperm into the seminal receptacle, the sperm storage conditions in the seminal receptacle were compared between females with and without spermatangia picking after copulation in virgin females.
Although the picking behaviour may work as a sperm transfer method, this behaviour might alternatively be used to remove sperm. Cryptic female choice (CFC) is the process by which a female chooses the sperm used for fertilisation, thus biasing offspring parentage towards a preferred phenotype (Thornhill 1983; Eberhard 1996). Female I. paradoxus may selectively store sperm in the seminal receptacle by this picking behaviour. To investigate the possibility of CFC by I. paradoxus, we conducted detailed observations of the picking behaviour. In particular, we focused on differences between the behaviour of virgin females and copulated females. Halliday (1983) hypothesised that, in polyandrous species, the first male that a female encounters ensures the fertilisation of its eggs, and the female then maximises the quality of her progeny by subsequently mating with higher quality males. Many studies have shown that the criteria for choosing a mate are stricter in copulated females than in virgin females (e.g. Bakker and Milinski 1991; Gabor and Halliday 1997; Pitcher et al. 2003; Fedina and Lewis 2007; Izzo and Gray 2011). If the picking behaviour of I. paradoxus is related to CFC, this behaviour would be more frequently observed in copulated than in virgin females. We compared the reproductive behaviour of virgin females with that of copulated females.
To examine whether the female I. paradoxus directly transfers sperm into the seminal receptacle via this picking behaviour, or removes the spermatangium, in this study, we conducted detailed observations of picking behaviour in both virgin and copulated females and compared the sperm storage conditions in the seminal receptacle between females with and without spermatangia picking after copulation in virgin females.
Materials and methods
Study species
The Japanese pygmy squid (I. paradoxus) occurs around Japan, South Korea, southern Russia and central China (Lu and Dunning 1998; Nesis et al. 2002). This species has two life-history cycles per year in central Honshu (Kasugai and Segawa 2005; Sato et al. 2008). In one, the squids hatch in the spring (March–May) and spawn in the summer (June–September), and in the other, hatching occurs in the summer, with spawning in the spring. Most I. paradoxus individuals live for 5 months (Sato et al. 2008) and die after the spawning season (Natsukari 1970). Females spawn several times, with an interval of 2 days. All Idiosepius species have a unique ability to adhere to substrata, such as seagrass, using an adhesive organ on the dorsal mantle (Sasaki 1923; Moynihan 1983; Nabhitabhata 1998). I. paradoxus can elongate its buccal mass (Kasugai 2001). When it forages crustaceans, I. paradoxus inserts the buccal mass into the exoskeleton of the captured crustacean and eats the flesh. Males and females mate in a head-to-head position (Kasugai 2000; Nabhitabhata and Suwanamala 2008). In I. paradoxus, a male darts towards a female, grasps the female, and attaches his spermatangium to the base of the female’s arms, not directly to the seminal receptacle (Kasugai 2000; Sato et al. 2010). Squids do not form consort pairs, and females copulate with multiple males in aquariums, suggesting that I. paradoxus has a promiscuous mating system (Kasugai 2000; Sato et al. 2010).
Collection and rearing conditions
The collection site of squids was near small stocks of the seagrass Zostera marina in the nearshore waters of the Chita Peninsula, central Honshu, Japan (34°43′N, 136°58′E). The squids were collected with a small drag net (1 × 2 m, mesh size: 1.5 mm) on 12 December 2008 and on 15 January 2009 (Season 1), when many females were expected to be immature and virgin, and on 12 and 29 April 2009 (Season 2), when females should have had mating experience and kept sperm in their seminal receptacle. Live specimens in well-aerated seawater were transported via a parcel delivery service to the Usujiri Fisheries Station, Field Science Centre for Northern Biosphere, Hokkaido University, Japan (41°56′N, 140°56′E). Mortality was <1 % when the specimens arrived at the station. At the fisheries station, the specimens were maintained in four aquariums (60 × 45 × 45 cm) with a closed circulation system. Before being introduced into the aquarium, all squids were separated by sex. Their sex can be readily confirmed by morphological observations of hectocotylus. The squid density was 40 per aquarium. Twelve half-cut (longitudinally) plastic pipes (3 × 20 cm) were placed on the sand bottom of each aquarium to provide substrates onto which the squids could adhere. Lighting provided a 12/12 h light/dark photoperiod, and the water temperature was maintained at 22 °C. Squids were fed with live amphipods (Ampithoe sp.) twice daily and were kept in good body condition. All immature squids matured approximately 2 weeks after transportation. Maturation was determined by confirming the presence of white testes in males and ripe eggs and large white nidamental glands in females, as observed through their transparent bodies. Only mature squids were used for the experiments.
Experiment 1: observation of copulatory and postcopulatory behaviours
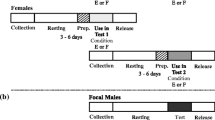
Prior to the experiments, we checked the virginity of females. Copulated females were confirmed by observing sperm in the seminal receptacle through the buccal membrane under anaesthesia with 1 % ethanol (Sato et al. 2010). However, we could not confirm the numbers of males with which the copulated female had mated using this method. All checked females were placed in a plastic bottle (1 L) to allow for recovery from the anaesthesia. All checked squids recovered successfully. Then, one female and one male were introduced into an experimental aquarium (30 × 40 × 20 cm). A plastic plate (1 × 15 cm) was placed on the sand bottom in each area as an adhering substrate for squids. Water temperature in the aquarium was the same as in the stock aquariums. Since the squids were nervous and needed several hours to become accustomed to the aquarium conditions, we split the aquarium into two areas with a partition and assigned each sex to an area (30 × 20 × 20 cm) for over 3 h before the experiment began. All trials were conducted during 1000–1900 hours.
At the start of the experiment, the partition was removed and then copulations were observed. Males copulated with females immediately after the partition was removed. After observing one copulation, we removed the male from the aquarium, and postcopulatory behaviours of the female were observed by eye for 30 min in Season 1 and by video recording for 1 h in Season 2. During behavioural observations, we recorded mating duration, defined as the time from when the male began to grasp the female to the time when he left the female. The presence of spermatangia on the female body was checked for after copulation and the places where males attached spermatangia were recorded. If spermatangia were not found, we judged that the male had failed to pass the female the spermatangia, and the trial data were not used. We also recorded the elapsed time between the end of copulation and the beginning of buccal mass elongation, and whether the spermatangia picking was successful. Moreover, the duration of buccal mass elongation was recorded in Season 2.
All squids used for the observation were fixed in Bouin’s solution after the experiment, and the dorsal mantle length (DML) was measured to the nearest 0.01 mm. To assess whether females consumed spermatangia during the picking behaviour, we made sections of the stomach in four cases in which we observed swallowing of spermatangia (see Exp. 2 for detailed methodology). Twenty and eight trials were conducted with virgin and copulated females, respectively, between 26 December 2008 and 18 February 2009 during Season 1. In total, 28 females (mean DML ± SD = 11.33 ± 0.98 mm) and 28 males (9.51 ± 0.91 mm) were used. In Season 2, 32 trials were conducted with copulated females between 16 April and 12 March 2009. In total, 32 females (11.89 ± 1.61 mm) and 32 males (8.65 ± 0.95 mm) were used. As the males in Season 1 were significantly larger than those in Season 2 (Student’s t test: t 57 = 3.59; p < 0.001), we did not pool the data for copulated females from Seasons 1 and 2.
Experiment 2: examination of sperm transfer to the seminal receptacle
To test whether females stored sperm from spermatangia to the seminal receptacle using picking behaviour, 34 virgin females in Season 1 (not used for behavioural observations) were experimentally copulated once and fixed in Bouin’s solution under five different conditions: (1) nine females were fixed within 30 s after copulation, (2) 14 females that picked up spermatangia were fixed 10 min after copulation, and of the 11 females that did not show buccal mass elongation, (3) nine, (4) one and (5) one females were fixed at 10 min, 30 min and 2 h, respectively, after copulation (Fig. 1). We defined fixing conditions (1) and (2) as “Soon after copulation” and “Picking”, respectively; (3), (4) and (5) were defined as “No picking”. In this experiment, we confirmed that all females that elongated their buccal mass did so soon after copulation and picked up spermatangia around 5 min after copulation, and thus, a 10-min observation time was sufficient to assess whether females attempted to pick up the spermatangia. Each male was used repeatedly in two or three trials.
The seminal receptacles of all 34 fixed samples (and 4 stomach samples from Exp. 1) were embedded in paraffin wax, and serial sections were cut at 8 μm. All sections were stained with haematoxylin and eosin by standard methods, and they were observed under a microscope and photographed with a digital camera (VB-7010; Keyence, Chiba, Japan). The area of sperm stored in the seminal receptacle was imaged and measured using ImageJ software (NIH, Bethesda, MD, USA), and the sperm volume was calculated by multiplying the total sperm area summed over the serial section thickness.
Results
Experiment 1: copulatory and postcopulatory behaviour
Males copulated with females immediately after the partition was removed. The mating duration in virgin females (mean ± SD = 4.45 ± 1.91 s) did not differ significantly from that in copulated females (4.65 ± 2.70 s; Student’s t test: t 81 = 0.39; p = 0.69). No significant difference was observed between copulated females in Seasons 1 (5.31 ± 3.11 s) and 2 (4.49 ± 2.62 s; Student’s t test: t 11 = 0.73; p = 0.48). Spermatangia were attached to the arm base on the ventral side in 15 copulations with virgin females and in 28 copulations with copulated females, but others were attached on the dorsal side of the arm base or at the tip of the arms. All females blew water into the spermatangia from their funnel immediately after copulation, and we confirmed that one to three spermatangia and the cases of empty spermatophores were dropped from the body in 11 copulations with virgin females and 20 copulations with copulated females.
Eleven virgin females and seven copulated females elongated their buccal mass within 5 min after the copulation, but nine virgin females and one copulated female did not elongate before the trials ended (at 30 min) in Season 1. In contrast, 28 copulated females elongated their buccal mass within 5 min after copulation, but four copulated females did not elongate before the end of the trial in Season 2. This shows that females elongated their buccal mass within 5 min, if they did so at all. The elongating behaviour was observed more frequently in copulated females than in virgin females (Fisher’s exact test: n = 60, p < 0.01; Table 1), and season did not affect the frequency of such behaviours by copulated females (Fisher’s exact test: n = 40, p = 0.73; Table 1).
When elongation occurred, females elongated their buccal mass towards and appeared to search all arm bases (Fig. 2a and supplementary material video N1), irrespective of whether spermatangia were attached there (Fig. 2b). Females frequently failed to pick up spermatangia, and the spermatangium removal probability was about 50 % (Table 2). This probability did not differ significantly between virgin and copulated females (Fisher’s exact test: n = 46, p = 0.79; Table 2) or between copulated females in Seasons 1 and 2 (Fisher’s exact test: n = 35, p = 0.82; Table 2). Although 21 females stopped elongating their buccal mass within 10 min, the duration of the elongation differed among females, and some females elongated their buccal mass for around 30 min (Fig. 3). Females stopped elongating the buccal mass even if spermatangia remained on the body and vice versa.
After females picked up a spermatangium with their buccal mass, they showed two patterns of behaviour. One was spermatangium-blowing behaviour. Three virgin and two copulated females picked up the spermatangium from their body, then retracted their buccal mass, and blew it away by jetting water using their funnel (Fig. 4 and Supplementary Material video N2). The other was spermatangium-eating behaviour, whereby the spermatangium was sucked and removed from her body. Fifteen copulated females ate the spermatangium via the elongating behaviour in Season 2 experiments. We could observe the spermatangium passing through the oesophagus because of the squids’ transparent bodies. The spermatangium and spermatozoa were confirmed in the stomach in all fixed females (Fig. 5). When females picked up spermatangia, whole spermatangia were completely removed from the body by blowing or eating, and no pieces remained in any observations.
Experiment 2: sperm storage in the seminal receptacle
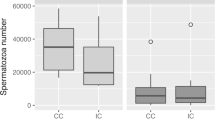
No female fixed immediately after copulation had sperm in her seminal receptacles (Table 3). While some squids picked the spermatangium and others did not, this behaviour was apparently not related to the transference of the spermatangium to the seminal receptacle (Fisher’s exact test: n = 23, p = 0.98). Furthermore, no significant difference in sperm volume was observed in the seminal receptacle between those showing spermatangium picking (n = 14) and those not showing buccal mass elongation (n = 9; Mann–Whitney U test, U = 67, p = 0.81; Fig. 6). Finally, even in females that did not pick up spermatangia, the sperm volume stored in the seminal receptacle increased with time after copulation (Spearman’s rank correlation: r s = 0.76, n = 9, p < 0.05; Fig. 6).
Box plots of sperm volume in the seminal receptacle. Sample numbers were 14 at Picking (10 min), 9 at No Picking (10 min), 1 at No Picking (30 min) and 1 at No Picking (2 h). Preservation times after copulation are shown by the numbers in round brackets. The boxes represent the 25–75th percentiles, with the average shown as a solid line within the boxes
Discussion
Sperm volume in the seminal receptacle did not increase with the picking of the spermatangium. In this study, we used seminal receptacles preserved 5 min after the picking behaviour to examine whether sperm was transported by this behaviour. As the seminal receptacles of I. paradoxus were located in a superficial site on the ventral regions of the buccal membranes surrounding the buccal mass (Sato et al. 2010), it would take little time to transfer sperm. Therefore, after 5 min, the sperm would have already reached the seminal receptacle. Spermatangia have not been confirmed in either the buccal mass or the seminal receptacle through historical observations (Sato, personal observation), and female squids are not considered to maintain spermatangia in the buccal mass and slowly transfer sperm to the seminal receptacle. Moreover, we observed that the picking behaviour was used for the removal and eating the spermatangium. These results suggest that female I. paradoxus did not transfer sperm from the spermatangium into the seminal receptacles via this behaviour.
Sperm may be transferred to the seminal receptacle through sperm actively swimming in seawater from the spermatangium in I. paradoxus. We confirmed that sperm were released from the spermatangium immediately after completing the spermatophoric reaction and that they were active in seawater in I. paradoxus (Sato, unpublished data). Moreover, the morphological study of the seminal receptacle would support this hypothesis. Many mucus cells are distributed at the seminal receptacle in some decapod cephalopod species (Drew 1911; van Oordt 1938; Lumkong 1992; Hanlon et al. 1999; Naud et al. 2005). This cell type may secrete a chemical substance to attract sperm. Drew (1919) noted that spermatozoa are directed, evidently by ciliary action, into the seminal receptacle in L. pealei. In I. paradoxus, multiple vacuoles, instead of mucus cells, are distributed in the bottom region of each sac in the seminal receptacle, and sperm in the receptacle were facing the sac bottom (Sato et al. 2010). “Picking” and “No Picking” females fixed at 10 min after copulation had no or few sperm stored in the seminal receptacle in this study, but females fixed at 0.5 and 2 h after copulation had many sperm in their seminal receptacles. Increasing sperm volume in the seminal receptacle with time may also support the hypothesis that sperm swim in seawater to reach the seminal receptacle.
The observed spermatangium-removing behaviour by eating and blowing may be used for postcopulatory sexual selection in I. paradoxus. In the field cricket, a male attaches his spermatophore to the opening of the female spawning duct, and the sperm in the spermatophore are then transferred to the sperm storage organ (spermathecae) (Alexander and Otte 1967; Sakaluk 1984). Sperm number in the spermathecae increases with spermatophore attachment time (Sakaluk 1984) and male paternity also increases with time (Sakaluk and Eggert 1996). Female crickets remove the spermatophore soon after copulating with unpreferred males. When the females copulate with preferred males, however, they do not remove the spermatophore from the body and store many sperm into their spermathecae (Bussiere et al. 2006). Similar to these cricket studies, female Japanese pygmy squids may remove the spermatangium before completing sperm transfer into their seminal receptacle if they copulate with unpreferred males. In the Caribbean reef squid (S. sepioidea), a female grab spermatangia deposited by a male and then tuck the sperm into the seminal receptacle or remove after copulation (Moynihan and Arcadio 1982). Male cuttlefish remove spermatangia of rival males during copulation (e.g. Hanlon et al. 1999; Naud et al. 2004; Wada et al. 2005, 2006, 2010). Additionally, females may ingest the spermatangium to gain nutrients. Female crickets feed on the spermatophores attached to their body (Sakaluk and Eggert 1996). In simultaneously hermaphroditic opisthobranchs and pulmonates, females digest stored sperm and absorb them as nutritional fluids in the female duct (Bauer 1998). This behaviour may be also used for cleaning the body. In marine animals, crustaceans have been reported to conduct body grooming (e.g. Bauer 1978; Fleischer et al. 1992; Becker and Wahl 1996). Although decapod cephalopods are not known to conduct body grooming, cephalopods may perform this behaviour to keep clean.
Copulated females more frequently exhibited buccal mass elongation than virgin females, suggesting that this behaviour may function as a method of CFC. If picking behaviour is used for gaining nutrients or cleaning the body, no difference would be expected between the frequency of buccal mass elongation in virgin and copulated females. The mating duration, which would relate to the sperm volume transferred by males to females, did not differ significantly between virgin and copulated females or between copulated females in Seasons 1 and 2. Moreover, the buccal mass elongation and sperm removal rates did not differ significantly between copulated females in Seasons 1 and 2. Nevertheless, most copulated females tried to pick up spermatangia. This result is consistent with our prediction that copulated females choose their mates more strictly based on the sequential mate choice hypothesis (Halliday 1983). Sequential mate choice may occur in female I. paradoxus. Although we do not know which trait(s) affect female squid preference, because male squids show neither direct competition with other males nor courtship behaviour (Kasugai 2000; Sato et al. 2010), body size may be related to the preference because the DML differed between squids in Seasons 1 and 2. However, it is possible that copulated females attempt to remove frequently spermatangia because they may be more sensitive than virgin females. Copulated females may also need more nutrients than virgin females. To exam whether the sperm removal behaviour work for CFC, it needs further investigation of the relationship.
Female squids frequently failed to pick up spermatangia and continued buccal mass elongation even if all spermatangia were removed from the body. They also elongated their buccal mass to locations where no spermatangium was attached. These results suggest that females do not know where and how many spermatangia have been attached correctly.
Typically, females did not use their arms, but used their buccal mass to remove spermatangia. In most copulations, spermatangia were attached at the arm base. It may be difficult for squids to remove spermatangia attached at the arm base using their arms. This removal behaviour of the spermatangium may also be a characteristic of the pygmy squid, which can elongate its buccal mass (Kasugai 2001).
In conclusion, the reason for spermatangia picking behaviour after copulation by female squids was apparently not to store sperm in the seminal receptacle. The present results did not support the idea that the female can transfer sperm directly to the seminal receptacle. Sperm transference in I. paradoxus may be conducted by the sperm actively swimming. Furthermore, this behaviour is considered to have been used as a means of postcopulatory female choice.
References
Alexander RD, Otte D (1967) The evolution of genitalia and mating behavior in crickets (Gryllidae) and other orthoptera. Misc Publ Mus Zool Univ Mich 133:1–62
Bakker TCM, Milinski M (1991) Sequential female choice and the previous male effect in sticklebacks. Behav Ecol Sociobiol 29(3):205–210
Bauer RT (1978) Antifouling adaptations of caridean shrimps—cleaning of antennal flagellum and general body grooming. Mar Biol 49:69–82
Bauer B (1998) Sperm competition in molluscs. In: Birkhead TR, Moller AP (eds) Sperm competition and sexual selection. Academic Press, London, pp 255–305
Becker K, Wahl M (1996) Behaviour patterns as natural antifouling mechanisms of tropical marine crabs. J Exp Mar Biol Ecol 203(2):245–258. doi:10.1016/0022-0981(96)02575-0
Buresch KC, Maxwell MR, Cox MR, Hanlon RT (2009) Temporal dynamics of mating and paternity in the squid Loligo pealeii. Mar Ecol Prog Ser 387:197–203
Bussiere LF, Hunt J, Jennions MD, Brooks R (2006) Sexual conflict and cryptic female choice in the black field cricket, Teleogryllus commodus. Evolution 60(4):792–800
Drew GA (1911) Sexual activities of the squid, Loligo pealii (Les.). 1. Copulation, egg laying and fertilization. J Morphol 22:327–359
Drew GA (1919) Sexual activities of the squid, Loligo pealii (Les.) 2. The spermatophore; its structure, ejaculation and formation. J Morphol 32:379–436
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton
Fedina TY, Lewis SM (2007) Female mate choice across mating stages and between sequential mates in flour beetles. J Evol Biol 20(6):2138–2143. doi:10.1111/j.1420-9101.2007.01432.x
Fleischer J, Grell M, Hoeg JT, Olesen J (1992) Morphology of grooming limbs in species of Petrolisthes and Pachycheles (Crustacea, Decapoda, Anomura, Porcellanidae)—a scanning electron-microscopy study. Mar Biol 113(3):425–435. doi:10.1007/bf00349168
Gabor CR, Halliday TR (1997) Sequential mate choice by multiply mating smooth newts: females become more choosy. Behav Ecol 8(2):162–166
Halliday TR (1983) The study of mate choice. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 3–32
Hanlon RT, Messenger JB (1996) Cephalopod behaviour. Cambridge University Press, Cambridge
Hanlon RT, Ament SA, Gabr H (1999) Behavioral aspects of sperm competition in cuttlefish, Sepia officinalis (Sepioidea: Cephalopoda). Mar Biol 134(4):719–728
Hanlon RT, Smale MJ, Sauer WHH (2002) The mating system of the squid Loligo vulgaris reynaudii (Cephalopoda, Mollusca) off South Africa: fighting, guarding, sneaking, mating and egg laying behavior. Bull Mar Sci 71(1):331–345
Ikeda Y, Sakurai Y, Shimazaki K (1993) Fertilizing capacity of squid (Todarodes pacificus) spermatozoa collected from various sperm storage sites, with special reference to the role of gelatinous substance from oviducal gland in fertilization and embryonic development. Invertebr Reprod Dev 23:39–44
Iwata Y, Sakurai Y (2007) Threshold dimorphism in ejaculate characteristics in the squid Loligo bleekeri. Mar Ecol Prog Ser 345:141–146
Iwata Y, Munehara H, Sakurai Y (2005) Dependence of paternity rates on alternative reproductive behaviors in the squid Loligo bleekeri. Mar Ecol Prog Ser 298:219–228
Iwata Y, Shaw P, Fujiwara E, Shiba K, Kakiuchi Y, Hirohashi N (2011) Why small males have big sperm: dimorphic squid sperm linked to alternative mating behaviours. BMC Evol Biol 11:9. doi:10.1186/1471-2148-11-236
Izzo AS, Gray DA (2011) Heterospecific courtship and sequential mate choice in sister species of field crickets. Anim Behav 81(1):259–264. doi:10.1016/j.anbehav.2010.10.015
Kasugai T (2000) Reproductive behavior of the pygmy cuttlefish Idiosepius paradoxus in an aquarium. Venus 59(1):37–44
Kasugai T (2001) Feeding behaviour of the Japanese pygmy cuttlefish Idiosepius paradoxus (Cephalopoda: Idiosepiidae) in captivity: evidence for external digestion? J Mar Biol Assoc UK 81:979–981
Kasugai T, Segawa S (2005) Life cycle of the Japanese pygmy squid Idiosepius pardoxus (Cephalopoda: Idiosepiidae) in the zostera beds of the temperate coast of central honshu, Japan. Phuket Mar Biol Cent Res Bull 66:249–258
Lu CC, Dunning MC (1998) Subclass Coleoidea. In: Beesley PL, Ross GJB, Wells A (eds) Fauna of Australia, vol 5. CSIRO, Melbourne, pp 499–563
Lumkong A (1992) A histological study of the accessory reproductive-organs of female Loligo Forbesi (Cephalopoda, Loliginidae). J Zool 226:469–490
Marian JEAR (2012) Spermatophoric reaction reappraised: novel insights into the functioning of the loliginid spermatophore based on Doryteuthis plei (Mollusca: Cephalopoda). J Morphol 273(3):248–278
Moynihan M (1983) Notes on the behavior of Idiosepius Pygmaeus (Cephalopoda, Idiosepiidae). Behaviour 85:42–57
Moynihan M, Arcadio FR (1982) The behavior and natural history of the Caribbean reef squid, sepioteuthis sepioidea, vol 25. Advances in ethology. Verlag Paul Parey, Berlin, Hamburg
Nabhitabhata J (1998) Distinctive behaviour of thai pygmy squid, Idiosepius thailandicus Chotiyaputta, Okutani & Chaitiamvong, 1991. Phuket Mar Biol Cent Special Publ 18(1):25–40
Nabhitabhata J, Suwanamala J (2008) Reproductive behaviour and cross-mating of two closely related pygmy squids Idiosepius biserialis and Idiosepius thailandicus (Cephalopoda: Idiosepiidae). J Mar Biol Assoc UK 88(5):987–993
Natsukari Y (1970) Egg-laying behavior, embryonic development and hatched larva of the pygmy cuttlefish, Idiosepius pygmaeus paradoxus Ortmann. Contrib Fish Exp Stn Nagasaki Univ 30:15–29
Naud MJ, Hanlon RT, Hall KC, Shaw PW, Havenhand JN (2004) Behavioural and genetic assessment of reproductive success in a spawning aggregation of the Australian giant cuttlefish, Sepia apama. Anim Behav 67:1043–1050
Naud MJ, Shaw PW, Hanlon RT, Havenhand JN (2005) Evidence for biased use of sperm sources in wild female giant cuttlefish (Sepia apama). Proc R Soc Lond B 272(1567):1047–1051
Nesis KN, Katugin ON, Ratnikov AV (2002) Pygmy cuttlefish Idiosepius paradoxus (Ortmann, 1888) (Cephalopoda)—first record of Idiosepiidae in Russian seas. Ruthenica 12(1):81–84
Nigmatullin CM, Laptikhovsky VV, Moustahfid H (2002) Brief review on the ecology in the North African population of arrow squid Todarodes sagittatus (Cephalopoda: Ommastrephidae). Bull Mar Sci 71(2):581–590
Pitcher TE, Neff BD, Rodd FH, Rowe L (2003) Multiple mating and sequential mate choice in guppies: females trade up. Proc R Soc Lond B 270(1524):1623–1629
Sakaluk SK (1984) Male crickets feed females to ensure complete sperm transfer. Science 223(4636):609–610
Sakaluk SK, Eggert AK (1996) Female control of sperm transfer and intraspecific variation in sperm precedence: antecedents to the evolution of a courtship food gift. Evolution 50(2):694–703
Sasaki M (1923) On an adhering habit of a pygmy cuttlefish, Idiosepius pygmaeus steenstrup. Annot Zool Jpn 10(21):209–213
Sato N, Kasugai T, Munehara H (2008) Estimated life span of the Japanese pygmy squid, Idiosepius paradoxus from statolith growth increments. J Mar Biol Assoc UK 88(2):391–394
Sato N, Kasugai T, Ikeda Y, Munehara H (2010) Structure of the seminal receptacle and sperm storage in the Japanese pygmy squid. J Zool 282(3):151–156. doi:10.1111/j.1469-7998.2010.00733.x
Takahama H, Kinoshita T, Sato M, Sasaki F (1991) Fine-structure of the spermatophores and their ejaculated forms, sperm reservoirs, of the Japanese common squid, Todarodes Pacificus. J Morphol 207(3):241–251
Thornhill R (1983) Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am Nat 122(6):765–788
van Oordt GJ (1938) The spermatheca of Loligo vulgaris. 1 T. Structure of the spermatheca and function of its unicellular glands. Q J Microsc Sci 80:593–600
Wada T, Takegaki T, Mori T, Natsukari Y (2005) Sperm displacement behavior of the cuttlefish Sepia esculenta (Cephalopoda: Sepiidae). J Ethol 23(2):85–92. doi:10.1007/s10164-005-0146-6
Wada T, Takegaki T, Mori T, Natsukari Y (2006) Reproductive behavior of the Japanese spineless cuttlefish Sepiella japonica. Venus 65(3):221–228
Wada T, Takegaki T, Mori T, Natsukari Y (2010) Sperm removal, ejaculation and their behavioural interaction in male cuttlefish in response to female mating history. Anim Behav 79(3):613–619. doi:10.1016/j.anbehav.2009.12.004
Acknowledgments
We thank S. Awata for critical comments on the manuscript. We also thank T. Wada and three anonymous referees for their helpful comments. This study was supported financially by the Mikimoto Fund for Marine Ecology (to NS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Picking behaviour of spermatangia by a female. The female elongated its buccal mass (mouth) to the spermatangia. Supplementary material 1 (WMV 6490 kb)
A female picked up some spermatangia using the buccal mass and blew them away by jetting water from the funnel. The latter part of the video shows a slow-motion replay of the blowing behaviour. Supplementary material 2 (WMV 5615 kb)
Rights and permissions
About this article
Cite this article
Sato, N., Kasugai, T. & Munehara, H. Sperm transfer or spermatangia removal: postcopulatory behaviour of picking up spermatangium by female Japanese pygmy squid. Mar Biol 160, 553–561 (2013). https://doi.org/10.1007/s00227-012-2112-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2112-5