Abstract
The morphology, external coloration as well as the life span of a kleptoplastic mollusc, Elysia nigrocapitata, was affected by its algal diet. Among algal diets, Chaetomorpha moniligera was the best for growth but not for animal longevity. TEM studies showed several distinctive layers composed of different cell types in sectioned parapodia. The chloroplast-containing digestive cells were located beneath the layer of vacuolated cells. The digestive cells contained 10–15 chloroplasts, in varying states of intactness, and several nuclei. Chloroplasts were not enclosed by any membranous structure in the host cytosol. Chlorophyll a fluorometry showed that the photosynthetic activity of kleptoplasts in E. nigrocapitata could be maintained for a long time only when animals were kept in the dark. The photosynthetic activity of kleptoplasts lasted 3–4 days when the animals were exposed to continuous illumination of 200 μmol photons m−2 s−1. These results suggested that the contribution of kleptoplasts to the survival of the animals might be minimal if the chloroplasts are not sequestered continuously. Cox I, 16S rDNA, and 28S rDNA sequence data have been obtained in order to phylogenetically place the new species of Elysia found in Korea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of animals to perform photosynthesis through the formation of symbiotic associations with intact unicellular algae or cyanobacteria appears to be limited to aquatic environments and to the phyla Mollusca (giant clams, nudibranchs), Porifera (sponges), Cnidaria (corals, anemones, and hydra), Acoelomorpha (flatworms), and Chordata (ascidians) (reviewed in Rumpho et al. 2011). In a unique adaptation leading to photosynthesis by animals, several sacoglossan molluscs (sea slugs) have evolved the ability to retain functional chloroplasts (kleptoplasts) from their algal food (e.g., Evertsen and Johnsen 2009; Händeler et al. 2009; Yamamoto et al. 2009; Klochkova et al. 2010).
In the sacoglossan genus Elysia (Opisthobranchia, Gastropoda), 89 species were described to date. Elysia spp. are generally reported to feed on Ulvophyceaen algae, and several species were suggested to incorporate algal chloroplasts inside their cells. The incorporated chloroplasts are functional for days to months, depending on the mollusc species. Until recently, only three species were reported to perform greatly prolonged retention of chloroplast activity, including Elysia chlorotica Gould, Elysia crispata Mørch, and Elysia timida Risso (Händeler et al. 2009; Wägele et al. 2011), while in other investigated Elysia spp., the chloroplast retention was short term. Transmission electron microscope observations revealed that intact algal chloroplasts were present in digestive glandular system of several Elysia spp. (e.g., Trench et al. 1969; Hawes and Cobb 1980; Rumpho et al. 2000; Wägele et al. 2011).
Elysia nigrocapitata Baba was first described from Japan (Baba 1957) and was also listed from Hong Kong (Jensen 1998) and Korea (Klochkova et al. 2010). Although this mollusc was described over 50 years ago and is currently included in numerous lists of fauna available in the Internet, the information on its morphology is very limited and has not been amended since first description in 1957. To our knowledge, the anatomy of this species was never an object of study, and there was no study on development of this species in captivity. We found one locality on the southern coast of Korea where animals corresponding well to the description of E. nigrocapitata were abundant in autumn and winter seasons. We maintained two generations of field-collected animals of E. nigrocapitata under laboratory conditions for over 2 years. Our previous study reported the food algae for this mollusc and also showed that this species could maintain the acquired chloroplasts photosynthetically functional for a long time (Klochkova et al. 2010). In animals starved for 1.5 months while being maintained in 12:12-h light/dark cycles with 30 μmol photons m−2 s−1, the F v /F m value was 0.53–0.67, which represented 66.2–83.7 % of the chlorophyll fluorescence values in intact coenocytic green algal thalli (Klochkova et al. 2010). E. nigrocapitata was able to endure starvation for up to 5 months under reduced light condition. In this study, we performed transmission electron microscopic observations and in vivo chlorophyll a fluorescence measurements to gain insight into structure and function of kleptoplasts of this photosynthetic mollusc.
Materials and methods
Sea slug collection and maintenance in the laboratory
During the course of this study, we used animals of Elysia nigrocapitata collected from the same collecting site in Wando, Korea, in 2009 and 2010, that is, two generations of animals (Table 1). We established a natural 10 × 10 m quadrat by marking each corner and observed the site every 2 weeks from July 2009 to July 2011, until the area was compromised because some construction started there in August 2011. The area was composed of soft substrate made up of small stones, sand, and silt. Dominating seaweeds in the area during all seasons were green algae Ulva spp., Bryopsis plumosa (Hudson) Agardh, Codium fragile (Suringar) Hariot, and Cladophora spp. Sea slug E. nigrocapitata is a seasonal species (Klochkova et al. 2010), thus most animals were collected in October in each year. Some animals were kept in IMR medium (Klochkova et al. 2006) in 9 × 5-cm Petri dishes at 20 °C in 12:12-h light/dark cycles with 15 μmol photons m−2 s−1 provided by cool-white fluorescent bulbs. Some animals were kept in aquariums measuring 150 cm long, 60 cm wide, and 60 cm high. The aquariums were filled with filtered seawater and constantly aerated. The aquariums received natural sunlight, and the seawater temperature was maintained at 20 °C. During the course of this study, we collected and observed over 400 animals.
Feeding experiment
The food algae for E. nigrocapitata, their collection site or source, and temperature for laboratory culture are given in Table 2. Before the feeding experiments, all algae were grown in IMR medium in 9 × 5-cm Petri dishes in 12:12-h light/dark cycles with 15 μmol photons m−2 s−1. While performing feeding experiments, each algal thallus was left until completely consumed by the mollusc. Thereafter, the culture dishes and medium were freshly replaced and new algal thalli were provided to the sea slugs. The feeding experiments were performed at 20 °C in 12:12-h light/dark cycles with 15 μmol photons m−2 s−1. During the starvation condition, the animals were maintained at the same culture conditions, but not provided with any algal food. All experiments were performed twice (in years 2009 and 2010), and for each trial (i.e., 5 food algae and starvation condition), we used over 20 experimental animals.
In vivo chlorophyll a fluorescence measurement
To observe whether the algal chloroplast is functional in the animal cells, the photosynthetic efficiency of the animals was measured under two different light regimes using a PAM fluorometer (Walz, Effeltrich, Germany). Twenty healthy animals of E. nigrocapitata collected from the field were pre-incubated and fed on B. plumosa in the laboratory at 20 °C in 12:12-h light/dark cycles with 15 μmol photons m−2 s−1 for 2 weeks. Thereafter, the animals were randomly separated into two experimental groups; one group was put under continuous dark condition (group 1) and the other was exposed to continuous illumination at 200 μmol photons m−2 s−1 without dark cycle (group 2). The reason for the continuous illumination was to remove any daily rhythm of photosynthesis in order to show any effect of light exposure time on the retention of kleptoplastic photosynthesis under extreme light condition. The maximum quantum yield of PSII (F v /F m ) was measured daily using Diving-PAM fluorometer. The fiber optic was placed on the opened parapodia within 5 mm distance from the dorsal surface. Fluorescence data were obtained after fluorescence signal reached stability. The maximum quantum yield of PSII (F v /F m ) was calculated as F v /F m = (F m − F o )/F m , where F m and F o represented steady-state fluorescence and maximum fluorescence after 10 min of dark acclimation, respectively. Also, Imaging-PAM (Walz, Effeltrich, Germany) was used to obtain fluorescence images of kleptoplasts at day 0 and day 3 after starvation.
Electron microscopic observations
For this experiment, we used animals of E. nigrocapitata maintained at 20 °C in 12:12-h light/dark cycles with 15 μmol photons m−2 s−1 and fed on Chaetomorpha moniligera. The animals were fixed in 1× PBS buffer containing 2.5 % glutaraldehyde at 4 °C for 6 h. Glutaraldehyde was then rinsed out with PBS buffer, and the parapodia were sliced off and postfixed with 1 % osmium tetroxide containing 1 % potassium ferrocyanide (K4Fe(CN)6·3H2O) at 4 °C for 2 h. Thereafter, the tissues were rinsed out with PBS buffer and were dehydrated in a graded ethanol series with 10 % increments (each step was 20 min), embedded in Spurr’s epoxy resin and polymerized overnight in a 72 °C oven (Polysciences Inc., USA). Thin sections stained with 4 % uranyl acetate for 1 h and lead citrate (Reynolds 1963) for 10 min were viewed and photographed on a Hitachi H-300 transmission electron microscope.
Phylogenetic analysis
Genomic DNA was isolated using i-genomic CTB DNA Extraction Mini Kit following the manufacturer’s instructions (Intron Biotech, Seoul, Korea). Polymerase chain reaction (PCR) was performed using primers listed in Table S1, with an initial denaturation at 95 °C for 3 min, followed by 35 cycles of amplification (denaturation at 95 °C for 20 s, annealing at 55 °C for 30 s and extension at 72 °C for 1 min for 16S rDNA and Cox I, and 1.5 min for 28S rDNA) with a final extension at 72 °C for 10 min. Amplified PCR products were purified using a gel extraction kit (Qiagen, Valencia, CA, USA) and cloned into a T-easy vector (Promega, Madison, WI, USA) to determine DNA sequences. The phylogenetic analyses were performed using sequences obtained from GenBank (NCBI 2011). The DNA sequences were aligned using ClustalW version 1.6 matrix (European Bioinformatics Institute, Cambridge, UK) at 50 % of delay divergent cutoff, and the obtained alignments were manually refined. Maximum parsimony (MP) analyses were performed using Windows-based program, MEGA version 4.1 (Tamura et al. 2007; Kumar et al. 2008). The MP analyses were tested for robustness by bootstrapping with 1,000 replications.
The sequences determined in this study have been deposited in GenBank under accession numbers JX524802 (16S rDNA), JX524803 (28S rDNA), and JX524804 (Cox I).
Results
Morphology and anatomy
The animals ranged in color from light to very dark green according to algal diet (Fig. 1). When starved, they became pale grayish-yellow in color (data not shown). Parapodia were leaf-like when flattened (Fig. 1b), with a wide black band along the ventral (i.e., reverse side) edges (Fig. 1a) and thin white (Fig. 1c, g) or sometimes dirty white (Fig. 1d–f) colored band along the dorsal (i.e., upper side) edges. In some animals, however, the black band was faded or absent, especially in those that underwent prolonged starvation and re-feeding cycles (Fig. 1c). Numerous tiny white spots were present on the ventral side of parapodia (Fig. 1d). The head was black, while the rhinophores and areas around eyespots were white. The black coloration pattern on head and neck varied slightly in different individuals (Fig. 1a–c, e). White or sometimes yellowish spot was present on the neck area (Fig. 1d–f), but in some animals, it was absent (Fig. 1c, g).
Morphology of parapodia-bearing plakobranchid sacoglossan Elysia nigrocapitata and its veligers. In the animals shown in a–c, e, the black coloration on the neck was extensive (a, e) or reduced (b, c). a, b Animals fed on Chaetomorpha moniligera. Note the absence of a white spot on the neck and broad black band on the ventral surface of parapodia edges (a, arrow). b Dorsal view of the mollusc with unfolded parapodia. c Animal that underwent prolonged starvation and re-feeding cycles using C. moniligera as diet. Note that the black band is almost absent (arrow), the white spot on the neck is not seen well, and the black coloration on the neck is reduced (g is enlarged image from c). d–f Animal fed on Bryopsis plumosa. Note the smaller body size, different color, tiny white spots on the ventral surface of parapodia (d, arrowhead), and white spot on the neck (e, f, arrows). h A shell with two egg clutches of E. nigrocapitata on its surface. i, j Through-focus images showing morphology of hatched planktonic veligers
For transmission electron microscopic studies (Figs. 2, 3, 4, 5), only parapodia were cut off from the animal’s body after fixation in glutaraldehyde. A schematic diagram of particular cell layers seen in transverse section through the parapodium is shown in Fig. 6. Several distinctive layers composed of different cell types were seen in sectioned parapodia with TEM. The dorsal surface of parapodia was covered with cilia and microvilli, and the ventral side was covered with microvilli and had a thick layer of mucus (Figs. 2, S1). The cilia surface was smooth (Figs. 2b, S1A-B), and cilia roots were striated (Fig. 2a). Small electron-dense (black-colored) globules were present beneath the membrane on the ventral side of parapodia (Figs. 2d, S1C). In some epidermal cells, an extensive membrane network of the rough endoplasmic reticulum’s cisternae associated with the nuclei was well seen (Fig. 3d).
Elysia nigrocapitata. Cross-sections (a, b, d, e) and tangential section (c) through the parapodia. a, b, c The dorsal surface of parapodia was covered with cilia and microvilli. a Cilia roots were striated. b Enlarged longitudinal section of the cilium (arrows point to the wavy surface). c Tangential section showing cell outlines in the epidermal layer (arrows). d The ventral surface of parapodia was covered with microvilli. d, e Beneath parapodia dorsal and ventral surfaces was a layer of uninucleate cells. cl cilia; ch channel; cr cilium root; lp lipid globule; mv microvilli; ER endoplasmic reticulum; rER rough endoplasmic reticulum; v vacuole
Elysia nigrocapitata. Cross-sections through the parapodia showed that beneath the epidermal layer composed of a row of uninucleate cells was a layer of vacuolated cells. a Some vacuoles contained what looked like a material enclosed by a vesicle (arrows). b Vacuolated cells were different in size. c Enlarged bilayer of vacuole. d In some other observed cell types, abundant endoplasmic reticulum was seen. lp lipid globule; n nucleus; rER rough endoplasmic reticulum; v vacuole
Elysia nigrocapitata. a Chloroplast-containing cell in sea slug fed on Chaetomorpha moniligera. Note that the number of nuclei (arrows) and chloroplasts do not correspond. b Enlarged image of two chloroplasts and two mitochondria enclosed by a membrane (cytoplast, shown with black arrows). However, inside this membrane, another membrane is seen (white dashed arrows). Also, another membranous structure is seen close to the chloroplasts (black arrowheads). c chloroplast; dc degenerating chloroplast; n nucleus; asterisks mitochondria; white arrowhead lysosome
Elysia nigrocapitata. a, b Enlarged images of the chloroplast-containing cell, showing intact chloroplasts with starches and plastoglobule lipid bodies and degenerating chloroplast with broken membrane bilayer, dissociated thylakoids, and degenerating pyrenoid. c Enlarged image of mitochondria, showing their structure. Arrowheads point to mitochondria; c chloroplast; dc degenerating chloroplast; cl cilia; mv microvilli; n nucleus; rER rough endoplasmic reticulum (arrow)
The epidermal layer was composed of a row of uninucleate cells (Fig. 3a). Beneath these epidermal cells was a layer of cells with large vacuoles that occupied almost all cell contents (Fig. 3a, b). The vacuoles were bounded by a membrane bilayer (Fig. 3c). In some cells, several smaller vacuoles were present instead of one large vacuole. These vacuolated cells were uninucleate, ellipsoidal, or spherical in shape, 9–24 μm in size, contained several mitochondria and 1 or 2 electron-dense (black-colored) globules. They also had many small vesicles pushed to the cell’s periphery, and some vacuoles contained what looked like a material enclosed by a vesicle and engulfed inside (Fig. 3a). In this epidermal layer, many lipid droplets of various sizes were scattered (Figs. 2d, 3b).
The chloroplast-containing cells (digestive cells) were located beneath the layer of vacuolated cells (Fig. 4a). The digestive cells contained from 3 to 10–15 chloroplasts, numerous mitochondria, and several nuclei (3 nuclei were seen in one section). The number of nuclei did not correspond to the number of chloroplasts within the cell. Many chloroplasts looked intact and contained aligned thylakoids, pyrenoid surrounded with starch grains, and plastoglobule lipid bodies (Figs. 4, 5). The chloroplasts were sometimes surrounded by a sheath of endoplasmic reticulum (Fig. 5b). Some chloroplasts showed signs of degeneration, such as broken membrane bilayer, dissociated thylakoids, and degenerated pyrenoid (Fig. 5a). The presence or absence of cytoplast and phagosome enclosing incorporated chloroplasts was not clearly confirmed. Cytoplasm of digestive cells indeed contained numerous membranous structures, which at times enclosed chloroplasts with mitochondria (Fig. 4b). However, in many sections, these membranous structures were not distinguished; rather chloroplasts were naked in the host cytosol (Fig. 5a). The lysosomes were contained in the digestive cells as well.
Diet
When animals were collected from the field, they were found to feed on Cladophora sakaii (multicellular multinucleate green alga with a siphonocladous level of organization) and occasionally on B. plumosa (unicellular multinucleate green alga with a siphonous level of organization). In the laboratory, the animals were also introduced to feed on Chaetomorpha moniligera (thereafter C. moniligera) and Phyllodictyon orientale (multicellular multinucleate green algae with a siphonocladous level of organization).
Interestingly, depending on diet, the size of E. nigrocapitata ranged from 3 to 30 mm in length (when fully extended). When the adult animals (14–17 mm in length) were starved for 3–5 months after being brought from the field, they reduced to 2–3 mm in length (wet weight approximately 1.5–2 mg) in approximately 2 months and completely lost green coloration.
The body size could reversibly change 7–10 times in the same animals, depending on feeding conditions. For example, the size could reduce from 30 mm (when fed on C. moniligera) to 3 mm in approximately 2–3 months (when starved) and back to 25 mm in 3–4 weeks (when feeding on C. moniligera was restored). However, the size of animals reached only 5–13 mm (max.) in length when they were continuously fed on Bryopsis spp. The animals continuously fed with P. orientale showed much reduced size (3–5 mm in length).
Although we could not find any C. moniligera growing in the habitat where we collected E. nigrocapitata, it was its favorite diet during the whole period of culture, because the largest size (30 mm in length and 10 mm in width, wet weight approximately 188 mg) was measured only when animals were continuously fed on this alga. Moreover, only those animals that were fed on C. moniligera and C. sakaii reached body size necessary to be able to deposit egg masses.
When four species of green algae were given together, the animals consumed all C. moniligera first; then, after several days of starvation, they began to eat C. sakaii, Bryopsis spp. or P. orientale. Often the animals fed with these algae stopped eating and had intervals of self-induced starvation, while they ate C. moniligera continuously as long as it was available. When field-collected animals were fed on C. moniligera, they increased in size from 2–3 to 20–30 mm in 3–4 weeks. However, in many cases, the life span of animals continuously supplied with C. moniligera for the whole time period after being brought from the field was from 3 to 8 months; while animals fed on Bryopsis spp. and P. orientale could live for 12–14 months.
Reproduction
Elysia nigrocapitata cross-copulate. The body size of reproductive animals was 17 and more mm in length, which was achievable only when they were fed with C. moniligera or C. sakaii. The egg deposition was observed very rarely (in less than 5 % of all collected animals). In the laboratory, the same animal of E. nigrocapitata could produce egg clutches 3–5 times. After that, the animals did not die and continued to live for 1–2 months without producing any more eggs. The egg masses were light yellow-colored because of the pigmented embryos and free of algal chloroplasts (Fig. 1h). E. nigrocapitata laid their fertilized eggs in circles and preferably on a spherical surface, such as the empty shells (Fig. 1h), balloon-like thalli of Valonia or gametophytes of Derbesia that we kept in the aquarium together with the animals. Individual eggs were 176 μm long and 128 μm wide (in the broadest part). Planktonic veligers (Fig. 1i, j) hatched within 8 days following egg deposition (in 20 °C). We were able to cultivate trochophore larvae for 20 days after hatching. During this time, the larvae underwent several physical changes and were fed on a plankton species, Isochrysis galbana Parke. However, attempts to proceed to a next developmental stage and grow post-metamorphic larvae were unsuccessful so far. The trochophore larvae stopped eating I. galbana, and we were unable to find another suitable microalgae or macroalgae to prolong their survival in the laboratory.
Our observations for 2 years showed that in the field, E. nigrocapitata usually appeared in the first week of October. It was absent before October and after April (Table 1). All animals collected in October were tiny, 2–3 mm in lengths, indicating that they just metamorphosed from larvae and were so-called babies (Table 1). However, the size of animals collected after October was 7–17 mm in length, indicating that they were adults. The population number also dropped after October, since not all small animals were able to survive. From May to September, we could not find a single animal in the field. Although it is still early to conclude, we suggest that in our collecting site, the development of trochophore larvae from the eggs occurred during warm months (from May to September) when numerous phytoplankton species were available, which allowed larvae to feed. The final stage when post-metamorphic larvae attached to macroalgal food and developed into miniature animals probably occurred in September to first week of October. By that time, numerous Cladophora and Bryopsis thalli were available in the field, which allowed newly emerged slugs to feed and gradually reach the large body size necessary to produce eggs. The egg masses were laid somewhere before May, since no animals were found after that, before the second field generation of tiny slugs appeared in next October, repeating the same cycle.
Molecular phylogeny
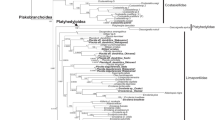
The molecular phylogeny of E. nigrocapitata based on 16S rDNA (Fig. S2), 28S rDNA (Fig. 7), and Cox I (Fig. S3) gene sequencing was performed in order to verify our specimens. At present, sequence data on E. nigrocapitata available in the GenBank are very limited. Analysis of 16S rDNA showed that our specimens from Korea had affinity with another population of E. nigrocapitata from Okinawa, Japan (DQ480175, Bass 2006). The 28S rDNA data are not currently available for any other population of E. nigrocapitata; the closest species to our specimens was E. atroviridis collected from the same locality in Korea. On Cox I gene analysis, the most related species was a population of E. nigrocapitata from Okinawa, Japan (DQ471226-228, Bass 2006).
In vivo chlorophyll a fluorescence assay
The retention time of functional kleptoplasts was directly connected with the light condition. The range of maximum quantum yield (F v /F m ) was 0.49–0.66 in the well-fed animals exposed to a high light of 200 μmol photons m−2 s−1 (Fig. 8a). When the animals were starved for algal food, the F v /F m value decreased. As starvation continued, the F v /F m value fell to 0.06–0.16 after 5 days, indicative of severe high light-induced photoinhibition of photosynthesis, which occurred under such circumstances. After the food resupply (day 5), the F v /F m increased to 0.36 ± 0.17 within 3 days (Fig. 8a).
The effect of light exposure on kleptoplastic photosynthesis in Elysia nigrocapitata. a Decrease and recovery of the maximum quantum yield of PSII (F v/F m) during the starvation (day 0–5) and re-feeding (after day 5) measured in animals exposed to dark condition and 200 μmol photons m−2 s−1. b Sample images from Imaging-PAM showing minimum fluorescence (F o), maximum fluorescence (F m), and F v/F m at day 0 and day 3 in animals exposed to 200 μmol photons m−2 s−1
As expected, high F v /F m values (0.64 ± 0.02) were measured when the animals were kept in the dark, and remained essentially unchanged even when the animals were starved for an extended period (Fig. 8a), suggesting that the photosynthetic machineries in the chloroplasts were intact and potentially fully functional.
With the fluorescence images obtained from Imaging-PAM, heterogeneity of fluorescence intensity on different parts of the mollusc’s body was found (Fig. 8b). The parapodia showed the higher minimum/initial fluorescence (F o ) than the other part, but the maximum quantum yield (F v /F m ) of the whole animal body showed a similar value to the well-fed E. nigrocapitata (day 0). Also, maximum fluorescence (F m ) relatively decreased after the animals were starved for 3 days, compared to the initial day, which led to reduction of F v /F m in more starved sea slugs (Fig. 8b). Under the dark conditions, the F v /F m value was stable and not affected by the food availability.
Discussion
The morphological and anatomical characteristics of E. nigrocapitata were studied in detail for the first time. The life span as well as the morphology of the animals depended on the algal diet. While the animals grew best and reached the largest size when fed constantly on C. moniligera, their life span was shorter (3–8 months) compared to the animals fed on Bryopsis spp. and P. orientale (12–14 months). Thus, although diet was the most important parameter affecting the size, reproduction ability, and kleptoplastic activity of E. nigrocapitata, the choice of most favored algal diet, C. moniligera, and life longevity of animals on that diet did not correlate. Good algal diet for the growth of E. nigrocapitata may not provide the most compatible kleptoplasts for longer survival during starvation.
The morphological characteristics and the molecular data based on the 16S rDNA and Cox I gene showed that our specimens could be identified as E. nigrocapitata. Phylogenetic analysis of three gene regions supported monophyly for the genus Elysia, which corresponded well with the results of Händeler et al. (2009). Parapodia-bearing Elysia species were separated from the cerata-bearing Placida species.
Some morphological characteristics of the animals (i.e., external coloration, body size, presence/absence of black band along the reverse side edges, presence/absence of white or yellowish spot on the neck area) were affected by their algal diet. When the animals were fed with Bryopsis, the body color became dark green, but it changed to bright green when the algal diet was changed to C. moniligera. The ramification of digestive gland on parapodia became more evident, and the yellowish spot on the neck area was brighter when the animals were fed on C. moniligera. The black band along the ventral side edges of parapodia, one of the key characters of this species, varied depending on algal diet and nutritional state of the animals.
The digestive gland extended throughout the entire body of the slug and was highly ramified. The presence or absence of a cytoplast—a membrane surrounding sequestered plastids including cytoplasm and even mitochondria (Grant and Howard 1980; Grant and Borowitzka 1984)—was not clearly confirmed in the digestive cells. Also, whether the chloroplasts inside the digestive cells were enveloped in a phagosome was not clearly confirmed, since some functional chloroplasts were surrounded by what looked like phagosome (or cytoplast), while many functional chloroplasts were naked in the host cytosol. We also observed chloroplasts surrounded by a sheath of endoplasmic reticulum. In many elysiid sacoglossans, the chloroplasts remain in primary phagosomal vacuoles (Mondy and Pierce 2003; Evertsen and Johnsen 2009). In E. timida, no cytoplasts or phagosomes surrounding sequestered chloroplasts were shown; rather chloroplasts were naked in the host cytosol and sometimes even oppressed upon slug nuclei (Wägele et al. 2011). In the case of E. nigrocapitata, we observed nuclei in a very close proximity to a chloroplast membrane, but a thin layer of host cytoplasm was always present between the chloroplast and nucleus. The digestive cells contained several nuclei, which were morphologically undistinguishable from other animal nuclei present in other cell types in E. nigrocapitata. Also, in our specimens, the layer of digestive cells was localized beneath the distinct layer of uninucleate vacuolated cells located beneath the epidermis, whereas in other kleptoplastic sea slugs, the digestive system was just one cell layer beneath the epidermis (Rumpho et al. 2000).
The algae that have been identified as food for E. nigrocapitata include 5 genera of Chlorophyta, that is, Bryopsis Lamouroux, Derbesia Solier, Chaetomorpha Kützing, Cladophora Kützing, and Phyllodyction Gray (Klochkova et al. 2010). However, the sources of diet range may be broader and include other species of the multinucleate green algae. In the field, the animals fed on softer plants like C. sakaii and Bryopsis spp., but did not consume the hard thick-walled plant Cladophora japonica Yamada growing abundantly nearby. As observed, only large animals with body length over 17 mm were able to deposit eggs. In our experiments, such body length developed only after feeding on large amounts of C. sakaii or C. moniligera. It is noteworthy that the most favored food alga, C. moniligera, did not grow anywhere in Wando where E. nigrocapitata was collected, and was introduced to the sea slugs in the laboratory.
To date, only four sacoglossan species are known to perform long-term maintenance of acquired chloroplasts, including E. chlorotica, E. timida, E. crispata, and Plakobranchus ocellatus van Hasselt, while many other sacoglossans have short-term chloroplast retention (e.g., Händeler et al. 2009; Yamamoto et al. 2009; Klochkova et al. 2010; Wägele et al. 2011). A concern exists, however, whether kleptoplast functionality was assumed rather than demonstrated in some sacoglossan molluscs (Händeler et al. 2009). For instance, Hinde and Smith (1972) reported that chloroplast retention in Elysia viridis Montagu was functional for at least 3 months, based on a long surviving slug (out of 39 specimens), but they also mentioned 96 % loss of the slug’s body mass over the experimental period. Evertsen and Johnsen (2009) reported that functional chloroplasts from Codium fragile (Suringar) Hariot in the digestive cells of E. viridis were always kept within the phagosome and had a capacity to maintain photosynthesis for 5–9 months; however, they demonstrated measurements of ΦPSII values only until 80 days of starvation and did not specify the starvation period in case of animals used for TEM preparations (Evertsen and Johnsen 2009). They also reported 33–49 % of the wet weight loss in E. viridis starved for 73 days. Moreover, most kleptoplastic sea slugs showed a rapid decrease in photosynthetic functions under light when they were not provided with food. Our current observation that photosynthetic kleptoplasts obtained from food algae were very unstable inside the animal cells agrees with previous reports (Evertsen and Johnsen 2009; Händeler et al. 2009; Serôdio et al. 2010; Vieira et al. 2009). Our previous study showed that the photosynthetic activity of kleptoplasts could be maintained for a long time when the animals were kept in low light intensity (Klochkova et al. 2010). However, the light intensity often exceeds 2,000 μmol photons m−2 s−1 in the natural habitat. To observe how long the kleptoplasts would function in more extreme conditions, the animals were kept in continuous illumination of 200 μmol photons m−2 s−1. The photosynthetic activity lasted only for 3–4 days. These results suggest that the kleptoplasts may not properly function in the natural habitat for a long time without recruit of new chloroplasts. Therefore, if the sea slugs are not provided with an algal diet, the portion of energy generated from photosynthesis would not be sufficient for the long-term survival of the animals.
In the field, E. nigrocapitata exhibits behavior that minimizes exposure to high solar irradiation, which may prolong the activity of functional chloroplasts. The parapodia were folded and the animals were often embedded in a soft muddy substrate and hardly noticeable from the outside. However, the animals have to be exposed to the light to perform photosynthesis. It is hard to imagine that the animal could survive in the field with sequestered algal chloroplasts for 5–6 months until new algal thalli become available. Further physiological studies using enough number of specimens are necessary to evaluate the exact contribution of kleptoplastic photosynthesis to the survival of these animals.
It has been suggested that algal nuclear genes might have been laterally transferred to Elysia spp. to maintain functional kleptoplasts, and several convincing pieces of evidence of lateral gene transfer were reported focusing on the family of light harvesting genes (vcp) (Pierce et al. 2007), psbO (Rumpho et al. 2008), prk (Rumpho et al. 2009) and chlorophyll biosynthesis genes (Pierce et al. 2009; Schwartz et al. 2010). In these cases, the genes were PCR-amplified from E. chlorotica adult DNA and usually aposymbiotic sea slug egg or veliger DNA. However, more recent studies on the large ESTs of sea slugs obtained from pyrosequencing method challenged the above hypothesis (Rumpho et al. 2011; Pelletreau et al. 2011). No algal-derived nuclear-encoded gene was found in the large ESTs of photosynthetic sacoglossans so far. Similar results were reported by Wägele et al. (2011) for E. timida and P. ocellatus based on partial transcriptome analysis. At least 95 % of the predicted proteins had top hits to Metazoa, and also 20 ESTs of potential foreign origin, derived from different prokaryotes, eukaryotes, and viruses; several plastid-derived transcripts primarily from V. litorea were identified in sea slugs; however, none of them had a direct involvement in photosynthesis (Pelletreau et al. 2011). Thus, we return to the original question: How do the kleptoplasts function inside the sea slug’s body? The explanation of long-term survivability of kleptoplastic sea slugs under starvation awaits a more exhaustive analysis or may not be attributed solely to the kleptoplasts. The sharp decrease in photosynthetic activity of kleptoplasts of E. nigrocapitata under strong light condition implies that the animal cell could not provide supplies to the degenerating kleptoplasts to maintain their function. It is noteworthy that chloroplasts of some Ulvophyceaen algae and Xanthophyceaen V. litoralis, which constitute diet for kleptoplastic sea slugs, possess a remarkable ability to survive in vitro (e.g., Kim et al. 2001; Klotchkova et al. 2003; Green et al. 2005).
Most studies on the kleptoplast photosynthesis in sacoglossan molluscs relied on the chlorophyll a fluorescence measurement because it was difficult to measure the photosynthesis of kleptoplasts through the oxygen evolution and carbon uptake approaches. Limited information was published with standard photosynthesis measurement methods (Green et al. 2000; Rumpho et al. 2000). In this study, chlorophyll a fluorometry was used to estimate photosynthetic activity of symbiont chloroplast during the starvation and re-feeding conditions. Our specimens of E. nigrocapitata survived for 5 months without food; however, this did not seem to happen solely due to the ability to live on photoautotrophic CO2 fixation by kleptoplasts. We presume that some other unknown mechanisms might also be involved in long-term survival of this species during starvation, in addition to CO2 fixation by kleptoplasts. During starvation, the loss of body mass was dramatic in all animals (93 % after 5 months) and the green coloration faded completely after approximately 3 months. Our TEM studies also showed that some chloroplasts in the digestive cells showed signs of degeneration soon after they were consumed. Perhaps a large part of the food source for long-term survival of these molluscs was in fact part of themselves, whereby degrading part of animal’s body mass as an energy source for long-term survival under food shortage or otherwise unfavorable environmental conditions. If this is the case, the long-term survival of the molluscs under starvation has nothing to do with their kleptoplasts. More precise ecological evaluation on the contribution of kleptoplasts to survival of the animal is essential to understand this intriguing phenomenon.
References
Baba K (1957) The species of the genus Elysia from Japan. Publ Seto Mar Biol Lab 6:69–74
Bass AL (2006) Evolutionary genetics of the family Placobranchidae (Mollusca: Gastropoda: Opisthobranchia: Sacoglossa). PhD thesis, University of South Florida, p 143
Evertsen J, Johnsen G (2009) In vivo and in vitro differences in chloroplast functionality in the two north Atlantic sacoglossans (Gastropoda, Opisthobranchia) Placida dendritica and Elysia viridis. Mar Biol 156:847–859
Grant BR, Borowitzka MA (1984) The chloroplasts of giant-celled and coenocytic algae: biochemistry and structure. Bot Rev 50:267–307
Grant BR, Howard RJ (1980) Kinetics of C-14 distribution during photosynthesis by chloroplast preparations isolated from the siphonous alga Caulerpa simpliciuscula. Plant Physiol 66:29–33
Green BJ, Li W-Y, Manhart JR, Fox TC, Summer EJ, Kenney RA, Pierce SK, Rumpho ME (2000) Mollusc-algal chloroplast endosymbiosis. Photosynthesis, thylakoid protein maintenance, and chloroplast gene expression continue for many months in the absence of the algal nucleus. Plant Physiol 124:331–342
Green BJ, Fox TC, Manhart JR, Rumpho ME (2005) Stability of isolated chromophytic algal chloroplasts that participate in a unique molluscan/algal endosymbiosis. Symbiosis 40:31–40
Händeler K, Grzymbowski YP, Krug PJ, Wägele H (2009) Functional chloroplasts in metazoan cells: a unique evolutionary strategy in animal life. Front Zool 6:28
Hawes CR, Cobb AH (1980) The effects of starvation on the symbiotic chloroplasts in Elysia viridis: a fine structural study. New Phytol 84:375–379
Hinde R, Smith DC (1972) Persistence of functional chloroplasts in Elysia viridis (Opisthobranchia, Sacoglossa). Nat New Biol 239:30–31
Jensen KR (1998) Zoogeographic affinities of Hong Kong Opisthobranchia (Mollusca, Gastropoda). In: Morton B (ed) The marine biology of the South China Sea. Hong Kong University Press, Hong Kong, pp 43–55
Kim GH, Klotchkova TA, Kang Y-M (2001) Life without a cell membrane: regeneration of protoplasts from disintegrated cells of the marine green alga Bryopsis plumosa. J Cell Sci 114:2009–2014
Klochkova TA, Kang SH, Cho GY, Pueschel CM, West JA, Kim GH (2006) Biology of a terrestrial green alga, Chlorococcum sp. (Chlorococcales, Chlorophyta), collected from the Miruksazi stupa in Korea. Phycologia 45:349–358
Klochkova TA, Han JW, Kim JH, Kim KY, Kim GH (2010) Feeding specificity and photosynthetic activity of Korean sacoglossan mollusks. Algae 2:217–227
Klotchkova TA, Chah O-K, West JA, Kim GH (2003) Cytochemical and ultrastructural studies on protoplast formation from disintegrated cells of a marine green alga Chaetomorpha aerea (Chlorophyta). Eur J Phycol 38:205–216
Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinf 9:299–306
Mondy WL, Pierce SK (2003) Apoptotic-like morphology is associated with annual synchronized death in kleptoplastic sea slugs (Elysia chlorotica). Invert Biol 122:126–137
NCBI (2011) GenBank. http://www.ncbi.nlm.nih.gov. Accessed 10 Dec 2011
Pelletreau KN, Bhattacharya D, Price DC, Worful JM, Moustafa A, Rumpho ME (2011) Sea slug kleptoplasty and plastid maintenance in a metazoan. Plant Physiol 155:1561–1565
Pierce SK, Curtis NE, Hanten JJ, Boerner SL, Schwartz JA (2007) Transfer, integration and expression of functional nuclear genes between multicellular species. Symbiosis 43:57–64
Pierce SK, Curtis NE, Schwartz JA (2009) Chlorophyll a synthesis by an animal using transferred algal nuclear genes. Symbiosis 49:121–131
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–213
Rumpho ME, Summer EJ, Manhart JR (2000) Solar-powered sea slugs. Mollusc/algal chloroplast symbiosis. Plant Physiol 123:29–39
Rumpho ME, Worful J, Lee J, Kannan K, Tyler MS, Bhattacharya D, Moustafa A, Manhart JR (2008) Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica. Proc Natl Acad Sci USA 105:17867–17871
Rumpho ME, Pochareddy S, Worful JM, Summer EJ, Bhattacharya D, Pelletreau KN, Tyler MS, Lee J, Manhart JR, Soule KM (2009) Molecular characterization of the Calvin cycle enzyme phosphoribulokinase in the stramenopile alga Vaucheria litorea and the plastid hosting mollusc Elysia chlorotica. Mol Plant 2:1384–1396
Rumpho ME, Pelletreau KN, Moustafa A, Bhattacharya D (2011) The making of a photosynthetic animal. J Exp Biol 214:303–311
Schwartz JA, Curtis NE, Pierce SK (2010) Using algal transcriptome sequences to identify transferred genes in the sea slug, Elysia chlorotica. Evol Biol 37:29–37
Serôdio J, Pereira S, Furtado J, Silva R, Coelho H, Calado R (2010) In vivo quantification of kleptoplastic chlorophyll a content in the “solar-powered” sea slug Elysia viridis using optical methods: spectral reflectance analysis and PAM fluorometry. Photochem Photobiol Sci 9:68–77
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Trench RK, Greene RW, Bystrom BG (1969) Chloroplasts as functional organelles in animal tissue. J Cell Biol 42:404–417
Vieira S, Calado R, Coelho H, Serôdio J (2009) Effects of light exposure on the retention of kleptoplastic photosynthetic activity in the sacoglossan mollusc Elysia viridis. Mar Biol 156:1007–1020
Wägele H, Deusch O, Händeler K, Martin R, Schmitt V, Christa G, Pinzger B, Gould SB, Dagan T, Klussmann-Kolb A, Martin W (2011) Transcriptomic evidence that longevity of acquired plastids in the photosynthetic slugs Elysia timida and Plakobrachus ocellatus does not entail lateral transfer of algal nuclear genes. Mol Biol Evol 28:699–706
West JA (2012) Master culture list. http://www.botany.unimelb.edu.au/West. Accessed 14 May 2012
Yamamoto YY, Yusa Y, Yamamoto S, Hirano Y, Hirano Y, Motomura T, Tanemura T, Obokata J (2009) Identification of photosynthetic sacoglossans from Japan. Endocytobiosis Cell Res 19:112–119
Acknowledgments
We thank Dr. Taizo Motomura (Hokkaido University, Japan) for kindly allowing us to use the TEM facilities. Dr. Qiang Hu (Arizona State University, USA) and Dr. Nina G. Klochkova (Kamchatka State Technical University, Russia) provided very useful consultations on the subject. This study was partially funded by National Research Foundation of Korea (NRF 20120006718) to G.H. Kim. This research was supported by a grant from Extreme Genomics Program Funded by Ministry of Land, Transport and Maritime Affairs of Korean Government to G.H. Kim.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kühl.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Klochkova, T.A., Han, J.W., Chah, KH. et al. Morphology, molecular phylogeny and photosynthetic activity of the sacoglossan mollusc, Elysia nigrocapitata, from Korea. Mar Biol 160, 155–168 (2013). https://doi.org/10.1007/s00227-012-2074-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2074-7