Abstract
The photosynthetic functionality in chloroplasts in the two sacoglossan molluscs Placida dendritica and Elysia viridis from the Trondheim fjord in Norway was studied. P. dendritica and E. viridis with no functional chloroplasts in their digestive system were introduced to the green macroalgae Codium fragile. Our results showed that P. dendritica was not able to retain functional (photosynthetic) chloroplasts. Transmission electron microscopy (TEM) showed that chloroplasts were directly digested when phagocytosed into the digestive cells. Four stages of chloroplast degradation were observed. A corresponding operational quantum yield of chl a fluorescence (ΦPSII ~ 0) indicated autofluorescence, and the presence of highly degraded chl a supported these observations. In contrast, E. viridis was able to retain functional chloroplasts. For this species it took only 1 week for the chloroplasts inside the digestive cells to acquire the same ΦPSII and light utilisation coefficient (α) as C. fragile kept under the same light conditions. Data for 8 days showed a 2–6-fold increase in the maximum photosynthetic rate (Pmax) and light saturation index (Ek) relative to C. fragile. This increase in available light was probably caused by a reduced package effect in the digestive gland of E. viridis relative to C. fragile, resulting in a partial photoacclimation response by reducing the turnover time of electrons (τ). Isolated pigments from C. fragile compared to E. viridis showed the same levels of photosynthetic pigments (chl a and b, neoxanthin, violaxanthin, siphonaxanthin, siphonein and β,ε-carotene) relative to μg chl a (w:w), indicating that the chloroplasts in E. viridis did not synthesise any new pigments. After 73 days of starvation, it was estimated that chloroplasts in E. viridis were able to stay photosynthetic 5–9 months relative to the size of the slugs, corresponding to an RFC of level 8 (a retention ability to retain functional chloroplasts (RFC) for more than 3 months). The reduction in ΦPSII, Pmax and α as a function of time was caused by a reduction in chloroplast health and number (chloroplast thylakoid membranes and PSII are degraded). These observations therefore conclude that chloroplasts from C. fragile cannot divide or synthesise new pigments when retained by E. viridis, but are able to partially photoacclimate by decreasing τ as a response to more light. This study also points to the importance of siphonaxanthin and siphonein as chemotaxonomic markers for the identification of algal sources of functional chloroplasts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Placida dendritica (Alder & Hancock, 1843) and Elysia viridis (Montagu, 1804) are two sacoglossan molluscs (Gastropoda, Opisthobranchia) commonly found associated with the coenocytic green macroalgae Codium fragile (Suringar) Hariot, 1889 (Ulvophyceae, Bryopsidales) in north Atlantic waters (Trowbridge 2002). Sacoglossans have a specific feeding habit feeding almost exclusively on the cytoplasm from coenocytic (multinucleate cells lacking transverse cell walls: e.g. chlorophyte macroalgae of the Bryopsidales, and xanthophyte macroalgae like Vaucheria) and siphonous (multinucleate cells with transverse cell walls: e.g. chlorophytes of the Siphonocladales, and rhodophytes like Griffithsia) macroalgae (Jensen 1980, 1997; DeWreede 2006). Some sacoglossans are also able to retain functional chloroplasts in the digestive system for several months, and eight different levels of retention have been described for sacoglossans retaining non-functional chloroplasts (level 1–3) to retaining functional chloroplasts from less than 1 day up to 9 months (level 4–8: Clark et al. 1990; Evertsen et al. 2007). This ability to retain functional chloroplasts (RFC) refers to how well a given sacoglossan is able to retain chloroplasts in a functional state in their digestive cells as a function of time (Evertsen et al. 2007).
Chloroplasts have been observed in the digestive cells of P. dendritica (Taylor 1968; McLean 1976) and it has been suggested that these chloroplasts are functional for a short time (Taylor 1968; Greene and Muscatine 1972; Hinde 1980).
On the other hand, E. viridis has been observed to retain chloroplasts inside its digestive cells (Taylor 1968; Trench et al. 1973a; Hawes 1979; Hawes and Cobb 1980; Hinde 1980; Trench 1980; Williams and Cobb 1989) and the chloroplasts have been observed to be functional up to 3 months (Hinde and Smith 1972; Trench et al. 1973b). Observations of whether or not chloroplasts inside the digestive cells of E. viridis divide are inconclusive (Trench and Olhorst 1976). It has been observed that the chloroplasts in E. viridis do not synthesise any photosynthetic chlorophylls, galactolipids or proteins (Trench and Smith 1970; Trench et al. 1973b), indicating that chloroplast division does not take place. As far as we know, no studies yet have pursued the pigment composition, chemotaxonomy (specific pigment tracers), functionality (RFC, ΦPSII, photosynthetic and photoprotective pigments), degradation and photoacclimation status of the chloroplasts inside the sacoglossan digestive system. This is necessary if we want to understand which factors govern the functionality of the chloroplasts when retained by sacoglossans. Photoacclimation is responsible for minimising variations in growth rate in fluctuating light, and can be attributed to three major physiological changes in chloroplasts (Falkowski 1980): active changes in the amount and ratios of photosynthetic and photoprotective pigments; changes in photosynthetic parameters; and changes in enzymatic activities involved in photosynthesis and respiration. Photoacclimation is thus the sum of compensation mechanisms, which allow the chloroplasts to work under a wide range of irradiances at nearly the same optimal cell growth rate, in this regard chloroplast division rate.
This study investigates the functionality of chloroplasts being ingested by P. dendritica and E. viridis feeding on the same green macroalgae C. fragile. The in vivo method is based on pulse amplitude modulated fluorometry measurements of living sacoglossans using a diving-PAM. Previous investigations by Wägele and Johnsen (2001), Burghardt et al. (2005) and Evertsen et al. (2007) have shown that the diving-PAM can be used to investigate photosynthetic activity of chloroplasts and zooxanthellae in opisthobranchs. The diving-PAM measures the quantum yield of chlorophyll a (chl a) fluorescence from reaction centres (RCPSII) in photosystem II (PSII; Eqs. 1 and 2), indicating the fraction of functional chloroplasts related to the quantum yield of electrons generated relative to the amount of photons absorbed. For green macroalgae, most in vivo fluorescence is emitted by chl a in PSII. When chloroplasts are acclimated in darkness, the chl a fluorescence emitted from chloroplasts is minimal when all functional RCPSII are open (F0). However, under saturation light conditions, the chl a fluorescence increases until it reaches a maximum, Fm, when all RCPSII are closed (Eqs. 1 and 2). The fraction of open RCPSII is given as the operational (dark acclimated chloroplasts) and maximum (in actinic light) quantum yield of PSII estimated, as defined by Butler (1978) and Dau (1994) with annotations as suggested by van Kooten and Snel (1990).
A decline in ΦPSII may be a result of RCPSII becoming photosynthetically incompetent due either to inefficient excitation transfer from light harvesting complexes (LHC) to RCPSII, impairment of primary photochemistry or disruption of electron transport between Q and PQ (Kroon et al. 1993). The initial ΦIIe, measured on the day of collection, can be divided by the reduction of ΦIIe day−1 to estimate a time period in days in which the chloroplasts are functional inside the sacoglossans (Evertsen et al. 2007).
ΦPSII as a function of the incident irradiance (E, μmol photons m−2 s−1) is defined as the relative electron transfer rate (ETR = ΦPSII·E = (mol e− mol photons−1) μmol photons m−2 s−1). Since ETR here denotes how many electrons are generated relative to the amount of photons absorbed as a function of the incident irradiance, ETR can be used to create photosynthesis versus irradiance (P versus E) curves to obtain information on the photoacclimational state of the chloroplasts. P versus E curve fitting of ETR versus E (Eq. 3) was done according to Webb et al. (1974).
α, the light utilisation coefficient, is related to light absorbed by PSII, \( \bar{a}_{\text{PSII}} \) (m−2 mg chl a−1; Johnsen and Sakshaug 2007) and is spectrally dependent (α = ΦPSII·\( \bar{a}_{\text{PSII}} \)). Pmax, the maximum photosynthetic rate, is dependent on the concentration of chl a in the photosynthetic unit (PSU) (q) and the minimum turnover time for processing photons (τ) (Dubinsky et al. 1986), thus obtaining no spectral dependency (Pmax = ΦPSII/qτ). Ek is the ratio between Pmax/α and is denoted as the light saturation parameter (μmol photons m−2 s−1; (Kroon et al. 1993; Sakshaug et al. 1997).
The isolation of pigments using high precision liquid chromatography (HPLC) can be used to check for taxon specific pigment markers (Jeffrey et al. 1997; Johnsen and Sakshaug 2007), the functionality of pigments in the sacoglossans food source (coenocytic macroalgae) and in the sacoglossan digestive cells with regard to photosynthetic and photoprotective pigments, and their corresponding degradation status. The amount of pigment relative to the amount of chl a (w:w) can be used to compare the photoacclimation status in the chloroplasts in their natural cell environment in the macroalgae relative to the chloroplasts in the digestive cells of the sacoglossans. Significant differences in ratios of pigment relative to chl a, can be indicative of photoacclimation (Rodriguez et al. 2006).
Light microscopy studies can be used to investigate the organisation of the digestive gland of the sacoglossans. A high degree of ramification of the digestive gland tubules has been proposed as a prior condition for the ability to retain functional chloroplasts in the Sacoglossa (Jensen 1997). TEM methodology can further be used to investigate the contents of the digestive cells, type of organelles and how many functional and degraded chloroplasts are present. This will tell us something about the conditions in the digestive cells that the chloroplasts are exposed to. It has been suggested that chloroplasts from C. fragile in the digestive cells of E. viridis are either directly exposed to the digestive cell cytoplasm, or contained within a phagosome (Trench 1980). The structural integrity of the chloroplasts in the digestive cells of the sacoglossans will therefore be investigated with regard to the phagosome, chloroplast double membrane, thylakoid membranes and starch grains.
Materials and methods
E. viridis and P. dendritica were collected by SCUBA-diving at Mausundvær, Frøya (63°52′30 N, 08°38′36E) at the Trøndelag coast of Norway in April 2005. No C. fragile was found at the sampling site at 5–7 m depth, and was therefore later obtained from Tautra (63°34′00 N, 10°36′52E) in the Trondheimsfjord. The algae and slugs were brought back to the laboratory and kept in running seawater aquaria similar to in situ conditions at 11°C and under light conditions at 30 μmol photons m−2 s−1 at the surface of the aquaria (no background light) under 6 h day length (09:00–15:00 hours) for all experiments.
Two experiments were conducted to investigate the functionality of C. fragile chloroplasts in the digestive cells of P. dendritica and E. viridis. Experiment 1 investigated functionality of chloroplasts from C. fragile as function of being retained in empty (containing no functional chloroplasts before the experiment) digestive cells of P. dendritica and E. viridis. In vivo measurements of the P versus E parameters Pmax, α and Ek were conducted as a function of 8 days using eight different irradiance levels (0, 5, 10, 25, 40, 70, 145 and 520 μmol photons m−2 s−1) for each curve. In vitro investigation of chloroplast functionality was conducted through HPLC pigment extraction and histology using LM and TEM.
Experiment 2 investigated the photosynthetic activity of chloroplasts from C. fragile in starving E. viridis. In vivo measurements of P versus E curves were conducted every second or third day for 73 days for E. viridis only. To achieve this 15 different irradiance levels (0, 3, 6, 13, 25, 40, 60, 90, 120, 190, 260, 350, 540, 740 and 1,020 μmol photons m−2 s−1) were used to create the P versus E curves. ΦPSII, Pmax, α and Ek were then investigated as a function of time. The wet weight of each slug was measured five times and presented as a mean wet weight to the nearest 0.01 g for all experiments.
The fluorescence induction curve was measured with a diving-PAM (Heinz Waltz GmbH, Germany) using a weak non-actinic (non-photosynthetic) probe flash (at 0.15 μmol photons m−2 s−1, obtained from a light emitting diode (LED at 650 nm) using a pulse modulated probe light sending pulses at a frequency of 0.6 KHz), measuring F0 and F0′ (Eqs. 1, 2). A change from F0 and F0′ to Fm and Fm′ was induced by a saturating flash with a peak irradiance of ~10,000 μmol photons m−2 s−1 of 0.8 s duration with the probe light (Halogen, white light) obtaining data at a frequency of 20 KHz, corresponding to the plateau level P of the Kautsky curve (see Govindjee 1995) before non-photochemical quenching processes start to reduce the chl a fluorescence (Kromkamp and Forster 2003).
P versus E curves were obtained by placing individual sacoglossans and pieces of C. fragile into separate 24 ml glass vials, with seawater, placed next to each other in a white styrofoam box for even light distribution. Pieces of black cardboard were placed between the vials during PAM measurements to prevent the PAM flash from influencing the light acclimation of the adjacent slugs. A slide projector with slides having different layers of plankton-net as neutral density filters was used to create different irradiance levels. The spectral irradiance from each slide was checked with a RAMSES ACC underwater spectroradiometer at 350–800 nm (Trios GmbH, Germany) and showed similar spectra. E(PAR) during experiments was determined from inside the vials with the PAR 2π light collector (model Diving-LS, Heinz Waltz GmbH, Germany) attached to the diving-PAM. Prior to P versus E curve experiments, all organisms were dark acclimated for 15 min. Exposure to subsequent irradiance levels was 2 min incubation time. After each P versus E experiment, all organisms were again dark acclimated for 15 min to check that ΦPSII recovered, where the ΦPSII after experiments was on average 13% lower than the initial ΦPSII values. The P versus E curves were plotted in Sigmaplot version 10.0 to estimate the photosynthetic parameters Pmax and α using Eq. 3.
For pigment analysis, all samples were prepared under subdued light to avoid pigment degradation. The slugs were immediately dried with some paper cloth to avoid excess water reducing extraction efficiency. Correspondingly, the C. fragile tissue was thoroughly squeezed without rupturing it to get as much water as possible out of the tissue. Pigment extraction was done in glass tubes with 2–4 ml pre-cooled methanol bubbled with N2 to avoid oxidation. The tissue was ground with a glass rod allowing 24 h of extraction in darkness at 4°C. After extraction, the samples were filtered through sterile syringes equipped with a 0.45 μm Millipore filter to remove debris, and 150 μl extract was then injected into the HPLC system (Hewlett-Packard HPLC Series 1100) equipped with a quaternary pump system and diode array detector, using the protocol of Rodriguez et al. (2006). Chlorophylls and carotenoids were detected by absorbance at 420, 440, 460 and 480 nm and identified by an absorbance detector (350–750 nm with 1.2 nm spectral resolution). HPLC calibration was performed using chl a and β,β-carotene standards from SIGMA (Aldrich, UK) and own chlorophyll and carotenoid standards (chl b, chl c1, violaxanthin, neoxanthin, siphonaxanthin and siphonein). The extinction coefficient of 250 L g−1 cm−1 at 445 nm in acetone for β,β-carotene (Jeffrey et al. 1997) was used to quantify siphonaxanthin and siphonein, adjusting for differences in molecular weight between pigments according to Johnsen and Sakshaug (1993).
For light microscopy (LM), the slugs were preserved in 4% final concentration of 39% formaldehyde (Merck, Germany) diluted in seawater. After 24 h the slugs were dried in increasing concentrations of ethanol from 70% for 24 h, followed by two 1 h washes in 80%, and three 1 h washes in 90% and 1 h in 100% ethanol. They were then embedded whole in Technovit 7100 (Heraeus Kulzer GmbH, Germany), and cut into 2.5 μm thick sections with a powered microtome (Autocut model 1140) and stained with toluidine blue (Merck, Germany), before being examined with a light microscope (Zeiss Axiovert 200).
For the transmission electron microscopy studies, tissue samples from the slugs were cut into 1 mm3 pieces and immersed in a final concentration of 2% glutaraldehyde buffered with 0.1 M phosphate buffer (pH 7.3) for 12 h at 4°C. The samples were then washed in 0.1 M phosphate buffer once, and then postfixed in a final concentration of 2% osmium tetroxide (OsO4) in 0.1 M phosphate buffer for 1 h. After fixation, the samples were rinsed in 0.1 M phosphate buffer and dehydrated in increasing concentrations of ethanol at 30, 50, 70 and 96% twice each at 5 min intervals and in 100% ethanol with molecular sieve three times at 5 min intervals. Infiltration started with 1 h embedding in 1:1 solution of Epon (Hexion, Amsterdam, Netherlands) and 100% ethanol, followed by two immersions for 1 h in pure Epon at room temperature (20°C). The samples were then embedded in pure Epon and polymerised for 24 h at 60°C and stabilised for 24 h at room temperature (20°C). Thin sections of 70 nm were cut with a diamond knife in a Leica EMU C6 ultramicrotome and collected on 200 mesh copper grids. The samples were then poststained for 25 min with a final concentration of 2% uranyl acetate (UO2(OCOCH3)22H2O) in 50% ethanol, and for 5 min in a final concentration of 0.1% aqueous lead citrate (Pb(C6H5O7)23H2O) following the protocols of Reynolds (1963). The samples were then observed and photographed in a Jeol NEM-1011 electron microscope.
Results
Experiment 1, in vivo results
One week after in situ collection with no C. fragile available, both E. viridis and P. dendritica showed autofluorescence (ΦPSII = 0). This indicated that no functional chloroplasts were present inside the digestive cells of the slugs. After this, three specimens of P. dendritica and E. viridis were put in an aquarium containing C. fragile and allowed feeding for 8 days.
The mean wet weight of P. dendritica at the start was 0.095 g, and 0.105 g at the end of the experiment. Correspondingly, E. viridis weighed 0.17 g at the start and 0.19 g at the end of the experiment, with an increase in wet weight of 9.5% for P. dendritica and 10.5% for E. viridis, relative to the initial wet weight.
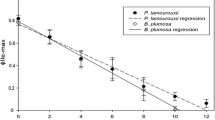
P. dendritica did not show any photosynthetic responses throughout the whole experiment (ΦPSII = 0 for all days, indicating autofluorescence; Fig. 1; Table 1).
E. viridis displayed a gradual increase in ΦPSII values over the first 4 days of the experiment (40% lower than C. fragile), and similar values as C. fragile on the last 2 days of the experiment (Fig. 1; Table 1).
For the whole experimental period, α in E. viridis was similar to that in C. fragile. However, Pmax and Ek in E. viridis varied significantly compared to C. fragile (Table 1), with values ranging from two to six times higher than that in C. fragile for the whole experiment.
Experiment 1, in vitro results
The extraction of pigments from C. fragile, E. viridis and P. dendritica (Table 2), concluding experiment 1, showed the presence of both photosynthetic chlorophylls and carotenoids in all samples. In C. fragile, only photosynthetic pigments were found: chl a and b, and the carotenoids siphonaxanthin and siphonein, violaxanthin, neoxhanthin and β,ε-carotene. The percentage of chl b to chl a (w:w) for both slugs was similar to C. fragile, ranging from 45 to 47%. However, there was 8% chl a-like and 31% phaeophorbide a pigments in P. dendritica, and 12% phaeophytin a in E. viridis, indicating a degradation of chlorophylls. The presence of chl c-like pigment in both slugs was not found in C. fragile, indicating the remains of phaeophytes (brown algae) in the slugs.
Of the photosynthetic carotenoids present in C. fragile, neoxanthin was absent in P. dendritica. However, the percent violaxanthin and β, ε-carotene content relative to chl a (w:w) was 4 and 15% higher in P. dendritica than in C. fragile. E. viridis contained all the photosynthetic pigments found in C. fragile, with only the percentage of neoxanthin and siphonein relative to chl a were 4 and 6% higher in E. viridis compared to C. fragile.
The LM sections of P. dendritica showed that the digestive gland extends into the cerata and into extensions on the dorsal side of the animal (Fig. 2a–c). This seemed to be the only extensions of the digestive gland from the gut and no other ramifications of the digestive gland tubules were observed. The digestive gland tubule in a cerata occupied the whole volume with a very wide lumen.
a The cerata-bearing sacoglossan Placida dendritica. The square denotes where the light microscope sections were taken. b Light microscope sections showing the digestive gland (dg) extending dorsally and into the cerata. c Closeup of the tip of the ceratum, indicated by the square in (b) showing the digestive cells lining the inside of the lumen (lu)
The LM sections through the parapodium of E. viridis were perforated with narrow digestive gland tubules (Fig. 3a–c). This showed that the digestive gland extended throughout the entire body of the slug and was highly ramified.
a The parapodia-bearing plakobranchid sacoglossan Elysia viridis. The square indicates the light microscopy sections cut from the parapodium. b A light microscopy section through the parapodium of E. viridis which clearly shows the digestive gland tubules (dgt) perforating the most of the tissue. c A closeup of the square indicated in (b) showing the digestive gland tubues as doughnut-shaped bodies surrounding a lumen
TEM sections of digestive cells in the cerata of P. dendritica showed degraded chloroplasts in several stages of digestion (chlp 1–4 in Fig. 4a, b). Some chloroplasts seemed structurally compact with a phagosome membrane surrounding the chloroplast (chlp1), indicating the first stage of degradation. Chlp1-chloroplasts are characterised by the dense stacking of the thylakoid membranes so that they cannot be discerned from the chloroplast double membrane and the intact starch grain. As degradation of the chloroplasts progressed (chlp2), the phagosome membrane, chloroplast double membrane, and the thylakoid membranes disintegrated. The starch grain began to break up. At level 3 (chlp3), the breaking up of the starch grain doubled the size of the “degrading chloroplast vacuole”. Fragments of the phagosome membrane, chloroplast double membrane and thylakoid membranes could still be seen dispersed around the edges of the vacuole. The last stage of degradation appeared to be electron dense material occupying the vacuole with undifferentiated material dispersed around the edges (chlp4), leaving what seemed to be an empty vacuole. These observations showed that in P. dendritica, the phagosome membrane, chloroplast double membrane, thylakoid membranes and starch grain degraded simultaneously leaving only empty vacuoles. No plastoglobuli were observed in any of the degraded chloroplasts in the digestive cells of P. dendritica.
a The digestive cells in Placida dendritica with microvilli (mv) showing retained degraded chloroplasts (dgc). b A close up of the degraded chloroplasts reveals four stages of degradation (chlp1–4): chlp1 are chloroplasts surrounded by an intact phagosome membrane (arrow), thylakoid membranes are not distinct surrounding a starch grain; chlp2 also have a phagosome membrane, but thylakoid membranes are beginning to disintegrate and the starch grain is still visible; chlp3 are only the remnants of the starch surrounded by fragments of the thylakoide membranes; chlp4 are totally disintegrated chloroplasts appearing as electron dense (empty) vacuoles
The TEM images of the digestive glands in E. viridis consisted mostly of intact chloroplasts, but in some of the chloroplasts, the phagosome membrane and the chloroplast double membrane appeared to have burst open, exposing the thylakoid membranes to the digestive cell cytoplasm (Fig. 5a). We could see that the phagosome membrane and the thylakoid membranes were degraded, leaving only the chloroplast double membrane with a more or less intact starch grain inside it (dgc in Fig. 5a and b). All intact chloroplasts examined in this study were surrounded by a phagosome and had a spherical shape with distinct thylakoid membranes, and in most the starch grains was visible and plastoglobuli were present (Fig. 5a, b). The digestive cell contained apart from 46–57% chloroplasts, a digestive cell nucleus, mitochondria, digestive vacuoles and lots of ribosomes dispersed throughout the digestive cell cytoplasm (Fig. 5a).
a Digestive cell in Elysia viridis with intact chloropasts (ic), broken chloroplasts exposed to the digestive cell cytoplasm (bc), digested chloroplasts where only the chloroplast membrane and starch grain (sg) are left (dgc), a nucleus (nu) and lumen (lu). The square indicates the close up of chloroplasts shown in (b). The estimated area covered by chloroplast ranging from 2 to 2.5 μm in diameter is 110–137 μm2 covered by the 35 chloroplasts counted in this digestive cell. The area of this digestive cell is estimated to be 240 μm2. The area covered by the chloroplasts amounts to 46–57% of the digestive cell. b Close-up of intact chloroplasts surrounded by the phagosome membrane (phm) and digested chloroplasts with a starch grain (sg) and a double chloroplast membrane (cdm). Note the presence of distinct thylakoid membranes (tm) and plastoglobuli (pg) in the intact chloroplasts. The cytoplasm surrounding the chloroplasts is filled with ribosomes (rb), some mitochondria (m) and vacuoles (va)
Experiment 2, in vivo results
Three individuals of E. viridis were kept without the possibility of feeding on C. fragile and ingesting fresh chloroplasts. The starving conditions as a function of time resulted in a loss of 33–49% wet weight in the largest to the smallest slugs during 73 days of starvation (Fig. 6a).
Experiment 2, starving experiment with Elysia viridis removed from Codium fragile, showing ΦPSII and change in wet weight as a function of days. a The initial wet weight in the smallest slug (Elysia1) was 0.242 g, and 0.268 and 0.280 g in the two larger slugs (Elysia2 and3), giving a daily decrease in wet weight of 0.0015, 0.0016 and 0.0012 g day−1 respectively, from the smallest to the largest slug. b The daily decrease in ΦPSII in the three specimens of E. viridis is calculated to 0.0032 ΦPSII day−1 for the smallest slug (Elysia1) and 0.0052 ΦPSII day−1 for the two larger slugs (Elysia2 and 3). The respective RFC values for each specimen are then estimated to 155 days, for the smallest slug based on an initial ΦPSII of 0.797 mol e− mol photons−1, 261 and 273 days for the two larger slugs estimated from initial ΦPSII of 0.826 and 0.855 mol e− mol photons−1, respectively
The photosynthetic activity also decreased as a function 73 days of starvation and size of the slugs. First of all ΦPSII decreased 28–29% for the two larger slugs, and by 47% for the smallest slug (Fig. 6b). In this regard, an RFC of 155 days could be estimated for the smallest slug, and an RFC of 261 and 273 days for the larger slugs.
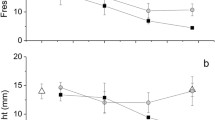
Secondly, plotting the photosynthetic parameters as a function of time gave a decrease in Pmax of 49–74% from the smallest to the largest slug (Fig. 7a), and a decrease in α of 70 to 20–29% from the smallest to the largest slugs (Fig. 7b). However, Ek as a function of time varied differently (Fig. 7c), where the smallest slug showed Ek to increase by 68%, and decrease by 22–57% in the two larger slugs.
Experiment 2, starving experiment with Elysia viridis removed from Codium fragile, showing the P versus E paramters Pmax, α and Ek as a function of days. a The daily decrease in Pmax is calculated to 1.73, 2.20 and 1.92 ((mol e− mol photons−1)(μmol photons m−2 s−1)) day−1 from the smallest (Elysia1) to the smallest slug (Elysia3). b The daily decrease in α is calculated to 0.00960, 0 0028 and 0.0040 ((mol e− mol photons−1)(μmol photons m−2 s−1)2) day−1 from the smallest (Elysia1) to the two larger slugs (Elysia2 and 3). c In the smallest of the three specimens (Elysia1), the Ek increased by 1.27 μmol photons m−2 s−1 day−1, whilst for the two larger specimens Ek decreased by 2.25 and 1.07 μmol photons m−2 s−1 day−1 (Elysia2 and 3)
Discussion
Placida dendritica
In experiment 1, the in vivo results indicate that the chloroplasts in the digestive cells of P. dendritica feeding on C. fragile are not functional (ΦPSII = 0; Fig. 1; Table 1). This is in contrast to Taylor (1968), Greene and Muscatine (1972) and Hinde (1980) who observed photosynthetic 14C incorporation in P. dendritica. In this regard, Greene and Muscatine explained a low uptake of 14C in chloroplasts from C. fragile in P. dendritica as a result of passive uptake of 14C in slugs (which they defined as “heterotrophic fixation”) and not by photosynthesis. In fact, structural damage to the chloroplasts in P. dendritica were observed within minutes after ingestion by McLean (1976), suggesting a quick loss of photosynthetic activity. McLeans’s observations still show structurally intact chloroplasts when they are in the process of being phagocytosed into the digestive cell. The thylakoid membranes are still clearly visible and it is easy to discern their organisation into layers. However, as soon as the chloroplasts are truly incorporated into the digestive cell, the thylakoid membrane structure becomes indistinct. The two-first degradation stages described in this study (chlp 1 and 2 in Fig. 4b) are therefore also found in McLean’s study, as well as in Hinde (1980) and Taylor (1968).
The pigment composition in P. dendritica compared to the food source suggested a mix of photosynthetic and degraded chloroplasts (Table 2). However, the presence of highly degraded chlorophylls in P. dendritica, with 31% of phaeophorbide a and 8% of chl a-like pigment relative to chl a, indicates that chloroplasts are digested as soon as they enter the digestive cells. The digestive gland branches into each ceratum, where it appears wrinkled but seems to occupy the ceratum as a single tubule (Fig. 2b). In this regard, long main ducts and rather wide lumina of the digestive gland tubules are associated with reduced functional kleptoplasty (Clark et al. 1990). The presence of functional chloroplasts were observed by means of 14C uptake in related limapontid species like Hermaea bifida and Costasiella lilianae (Kremer and Schmitz 1976; Clark et al. 1981) and by ΦPSII measurements in Ercolania kencolesi (Grzymbowski et al. 2007). The observation of 14C uptake in H. bifida and C. lilianae needs further study, since Green and Muscatine (1972) suggested that the presence of 14C in P. dendritica was caused by passive uptake of 14C; in H. bifida, 14C assimilation rate in the algae was estimated to be 1,000 times higher than in the slug; and in E. kencolesi, ΦPSII values were lower than 0.2 mol e− mol photons−1 in all measurements (Grzymbowski et al. 2007), indicating that low ΦPSII values are attributed to degraded chloroplasts. This implies that most limapontid sacoglossans can be designated to RFC level 1–3 retention of non-functional chloroplasts (Clark et al. 1990; Evertsen et al. 2007).
Elysia viridis
During the 8 days that E. viridis was allowed to feed on C. fragile, photosynthetic activity appeared as a gradual increase and stabilisation of ΦPSII and α values (Table 1). This is probably a result of E. viridis filling the digestive cells with functional chloroplasts (Fig. 5a, b). The similarity of ΦPSII and α between E. viridis and C. fragile indicates that the photoacclimation status of the chloroplasts in the digestive cells of E. viridis is the same as for chloroplasts in C. fragile. However, Pmax and Ek varied greatly in E. viridis and two to six times higher than in C. fragile (Table 1). Since α was constant as a function of time in E. viridis from the first day, Ek varies as a function of Pmax. The variations indicate a high turnover of chloroplasts when E. viridis feeds on C. fragile and reflects the photoacclimation status of C. fragile. Gallop et al. (1980) observed that feeding animals replaced 75% of their chloroplasts during a 9-day period, while starving animals only lost 15% in the same period. This implies that with the constant replacement of chloroplasts throughout the whole digestive gland system, the threshold where all RCPSII are saturated with photons will also vary as a function of [functional chloroplasts] in the digestive system. The two to six times higher values of Pmax and Ek also indicate that the chloroplasts in the digestive cells of E. viridis receive more light than chloroplasts in C. fragile, e.g. as high light acclimation. We find this to be unlikely since chloroplasts in the slugs need to divide to actively photoacclimate (Cran and Possingham 1974). Instead, the higher Ek values probably reflects the morphological adaptations in plakobranchiid (=Elysiidae) sacoglossans, where an increased branching of the digestive gland provides larger surface areas for retention of photosynthetic chloroplasts (Jensen 1997). This implies that the package effect, which is an effect of intracellular self-shading dependent on cell size and shape, cellular pigment composition, chloroplast size, shape, number and morphology and thylakoid stacking (Johnsen et al. 1994), is less for chloroplasts in E. viridis than for chloroplasts in C. fragile, inducing higher Ek values in the former. Since both α and Pmax comprise ΦPSII, the former parameter cannot explain the increase in Pmax and Ek. Since the pigment composition remains unchanged, α and q should not change either. The increase in Ek may therefore be attributed to an increase of τ, leading to a decreased turnover time of e−, indicating that chloroplasts in the digestive cells of sacoglossans experience higher irradiances than in the food algae. The LM images of the parapodium of an E. viridis support these results, where we observed that the digestive gland tubules are evenly distributed throughout the slug tissue (Fig. 3b).
The amount of photosynthetic pigments relative to chl a (w:w) of the chloroplasts in E. viridis were overall the same as for C. fragile, which support the observations for ΦPSII that all light harvesting pigments are present compared to the food source, and that the photosynthetic chloroplasts in E. viridis do not photoacclimate with regard to synthesising new pigments, because of arrested chloroplast division in the digestive cells (Table 2). The presence of 12% pheophytin a relative to chl a in E. viridis indicates that some degradation of chloroplasts is present, reflecting the turnover of up to 75% of the chloroplast as observed by Gallop et al. (1980).
The degradation of the C. fragile chloroplasts in E. viridis seems different from P. dendritca. In P. dendritica, the phagosome membrane, chloroplast double membrane, thylakoid membranes and starch grain disintegrate at the same rates (Fig. 4b). In E. viridis, only the phagosome and thylakoid membranes seem to be degraded, leaving only the chloroplast double membrane containing an intact starch grain (Fig. 5b). These observations may solve the confusion whether or not a phagosome membrane envelops functional chloroplast (Trench et al. 1973b; Hawes 1979; Hawes and Cobb 1980), where the former suggested that the phagosome membrane is re-absorbed so that functional chloroplasts lie free in the cytoplasm of the digestive cell. What we see, is in fact only damaged chloroplasts exposed to the cytoplasm of the digestive cell, leading to degradation. The functional chloroplasts are still enveloped in the phagosome in this study. In this regard these cytoplasm-exposed and degraded chloroplasts are related to the turnover of chloroplasts in feeding E. viridis explained by Gallop et al. (1980). Plastoglobuli were present inside most of the chloroplasts (Fig. 5c). They function as lipid storage sites outside the thylakoid membranes and contain the lipophilic quinones functioning as the oxidation–reduction catalysts in the photochemically active thylakoid membranes (Tevini and Steinmüller 1985), indicating that the thylakoid membranes of the chloroplasts are not repaired, but show a sign of beginning senescence. This indicates that the synthesis of thylakoid membranes in the chloroplasts is arrested, which is supported by Hawes and Cobb (1980) who observed an increase in the number of plastoglobuli per chloroplast in the digestive cells of E. viridis after 28 days of starvation.
In experiment 2, the starving experiment with E. viridis removed from C. fragile for 73 days, the observations indicate an arrested chloroplast division and no active photoacclimation in the chloroplasts. The decline in ΦPSII (Fig. 6a) can be related to chloroplast health status (Bjørkmann and Demmig 1987), as a result of a reduction in the number of functional RCPSII of the PSU. A lack of functional D1, one of the reaction centre proteins of PSII, is related to a decrease in ΦPSII when the PSU is not repaired (Vasilikiotis and Melis 1994). This is also supported by the decrease of α in our results (Fig. 7b). Since the chloroplasts are not able to rebuild their membranes, repair damaged PSUs or synthesise new pigments, the chloroplasts will degrade as function of time. This is also correlated with the decrease in Pmax in our study (Fig. 7a), which is related to the chl a content in the PSU and the enzymatic processes related to the electron transport chain (Dubinsky et al. 1986). The varying responses in Ek (Fig. 7c) may be attributed to Pmax and α whose decrease is related to a reduction in PSII functionality (non-functional D1, arrested pigment synthesis and PSU repair) and declining enzyme activity caused by arrested chloroplast division. Related studies have observed that chloroplasts from C. fragile in the digestive cells of E. viridis cannot synthesise chl a, galactolipids or membrane proteins (Trench and Smith 1970; Trench et al. 1973b; Trench and Olhorst 1976). This implies that chloroplasts from C. fragile, when sucked out of the algal cell environment and phagocytosed into the digestive cells of E. viridis, are not able to renew the thylakoid membranes or replace pigments. Hawes and Cobb (1980) observed in their experiments, on the effects on starvation of the chloroplasts from C. fragile in E. viridis, still intact chloroplasts present in the digestive cells of E. viridis after 40 days of starvation, displaying increased swelling and disintegration of the thylakoid membranes and indicating a progressive degradation of the chloroplasts.
The gradual degradation and overall decrease of 28–47% in ΦPSII of the chloroplasts in E. viridis can also be linked to the loss of 33–49% wet weight in this study (Fig. 6a, b). The two larger slugs with relatively more chloroplasts per animal lost only 33% of their wet weight and had an estimated RFC up to 9 months. The smaller slug lost 49% of its wet weight and had an estimated RFC of up to 5 months. Our observations on the changes in weight in starving E. viridis are similar to Hinde and Smith (1975) observations where E. viridis fed on C. fragile lost 40% of their initial weight after 10 weeks of starvation.
The estimated RFC values of 5–9 months for E. viridis in this study corresponds to level 8, characterised by retention of functional chloroplasts for more than 3 months according to Evertsen et al. (2007). In this regard, our results far exceed previous studies on RFC for E. viridis. Hinde and Smith (1975, 1972) observed continuous CO2 fixation for up to 3 months in E. viridis using 14C assimilation methods. But, there are some important differences in the experimental setup that might explain the discrepancies: in this study the slugs were kept under irradiances at 30 μmol photons m−2 s−1 at 11°C. In Hinde and Smith (1975, 1972) the sacoglossans were kept at 18°C and 440 μmol photons m−2 s−1, inducing high light conditions that cause a relatively higher respiration to photosynthetic rates compared to our cool and low light conditions. High light conditions result in a rapid turnover of the D1 protein in RCPSII, which plays a major role in maintaining PSII integrity in high light (Franklin and Larkum 1997).
The presence of siphonaxanthin and siphonein in green macroalgae is variable; however, among the Ulvophyceae, siphonaxanthin and siphonein are present in the Bryopsidales (=Caulerpales) no matter their depth or habitat distribution (Yokohama 1981). For the other ulvophyceaean taxa, siphonaxanthin, but not siphonein, is present in the Ulvales, Siphonocladales and Cladophorales in deep water or shaded habitats. Siphonaxanthin and siphonein lack in the Dasycladales. For details considering the phylogeny of the ulvophyceaean taxa, confer Lam and Zechman (2006), Hayden and Waaland (2002), Leliaert et al. (2007) and Zechman (2003). The presence of siphonaxanthin and siphonein together in a sacoglossan with photosynthetic chloroplasts can therefore be used as chemotaxonomic markers to indicate Bryopsidales as a food source. This is important since sacoglossans are not only reported to feed on coenocytic and siphonous green algae, but also on red and brown algae and seagrasses (Jensen 1980), and only a handful of sacoglossans species with photosynthetic chloroplasts have been associated with a chloroplast donor algae (Clark et al. 1990; Evertsen et al. 2007).
Conclusion
The chloroplasts from C. fragile seem to be retained differently in P. dendritica compared to E. viridis. In P. dendritica, the membranes of the phagosomes, chloroplast, thylakoides and starch grain are degraded as soon as the chloroplasts are phagocytosed into the digestive cells. In E. viridis, chloroplasts exposed to the digestive cell cytoplasm are all in the process of being degraded, but the chloroplast double membrane and the starch grain remain throughout degradation. These differences reflect that the “robustness” that has been described for coenocytic chlorophytes (Grant and Borowitzka 1984) may not be sufficient to explain the retention of functional chloroplasts in the Sacoglossa. Instead it appears that the adaptations in the digestive system of the sacoglossans render the slugs capable of keeping the chloroplasts in a functional state. Functional chloroplasts from C. fragile in the digestive cells of E. viridis are always kept within the phagosome and have a capacity to maintain photosynthesis for 5–9 months. Even though the chloroplasts are able to maintain photosynthesis for long time periods, this study indicates that chloroplast division is arrested and that the chloroplasts are not able to photoacclimate regarding pigment synthesis, but may partially be able to adjust their turnover time of electrons (τ) to higher irradiance levels. This implies that the functionality of retained chloroplasts in various sacoglossans must also be investigated with regard to synthesis of pigments, lipids, proteins, nucleic acids and starch formation. In this regard, it has been observed that the sacoglossan Elysia chlorotica from the east coast of North America which feeds on the siphonous xanthophyte Vaucheria litorea, retains chloroplasts that not only have a photosynthetic capacity up to 9 months, but which are also capable of synthesising several photosynthetic proteins like the carbon fixating enzyme RuBisCO, D1, D2, and CP43 core complexes of PSII, and electron transport chain proteins like cyt f and others (Pierce et al. 1996; Mujer et al. 1996; Green et al. 2000). This demonstrates that chloroplasts have different functional capabilities depending on which algae the sacoglossan has collected chloroplasts from, and on the type of sacoglossan. Chemotaxonomic markers, like siphonaxanthin and siphonein used in this study, are therefore useful when identifying the origin of the functional chloroplasts found in scoglossans.
References
Bjørkmann O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 k among vascular plants of diverse origins. Planta 170:489–504
Burghardt I, Evertsen J, Johnsen G, Wägele H (2005) Solar powered seaslugs: mutualistic symbiosis of aeolid Nudibranchia (Mollusca, Gastropoda, Opisthobranchia) with Symbiodinium. Symbiosis 38:227–250
Butler WL (1978) Energy distribution in the photochemical apparatus of photosynthesis. Annu Rev Plant Physiol 29:345–378
Clark KB, Jensen KR, Stirts HM, Fermin C (1981) Chloroplast symbiosis in a non-elysiid mollusc, Costasiella lilianae Marcus (Hermaeidae: Ascoglossa (=Sacoglossa): effects of temperature, light intensity and starvation on carbon fixation rate. Biol Bull 160:43–54
Clark KB, Jensen KR, Stirts HM (1990) Survey for functional kleptoplasty among west Atlantic Ascoglossa (=Sacoglossa) (Mollusca: Opisthobrachia). Veliger 33(4):339–345
Cran DG, Possingham JV (1974) The effect of cell age on chloroplast structure and chlorophyll in cultured spinach leaf discs. Protoplasma 79:197–213
Dau H (1994) Molecular mechanisms and quantitative models of variable photosystem II fluorescence. Photochem Photobiol 60:1–23
DeWreede R (2006) Biomechanical properties of coenocytic algae (Chlorophyta, Caulerpales). Sci Asia Suppl 1:57–62
Dubinsky Z, Falkowski PG, Wyman K (1986) Light harvesting and utilization by phytoplankton. Plant Cell Physiol 27:1335–1349
Evertsen J, Burghardt I, Johnsen G, Wägele H (2007) Retention of functional chloroplasts in some sacoglossans from the Indo-Pacific and Mediterranean. Mar Biol 151:2159–2166
Falkowski PG (1980) Primary production in the sea. Plenum, New York
Franklin LA, Larkum AWD (1997) Multiple strategies for a high light existence in a tropical marine macroalgae. Photosynth Res 53:149–159
Gallop A, Bartrop J, Smith DC (1980) The biology of chloroplast acquisition by Elysia viridis. Proc R Soc Lond B 207:335–349
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 22:131–160
Grant BR, Borowitzka MA (1984) Changes in structure of isolated chloroplasts of Codium fragile and Caulerpa filiformis in response to osmotic shock and detergent treatment. Protoplasma 120:155–164
Green BJ, Li W-Y, Manhart JR, Fox TC, Summer EL, Kennedy RA, Pierce SK, Rumpho ME (2000) Mollusc–algal chloroplast endosymbiosis: photosynthesis, thylakoid protein maintenance, and chloroplast gene expression continue for many months in the absence of the algal nucleus. Plant Physiol 124:331–342
Greene RW, Muscatine L (1972) Symbiosis in sacoglossan opisthobranchs: photosynthetic products of animal–chloroplast associations. Mar Biol 14:253–259
Grzymbowski Y, Stemmer K, Wägele H (2007) On a new Ercolania Trinchese, 1872 (Opisthobranchia, Sacoglossa, Limapontiidae) living within Boergesenia Feldmann, 1950 (Cladophorales), with notes on anatomy, histology and biology. Zootaxa 1577:3–16
Hawes CR (1979) Ultrastructural aspects of the symbiosis between algal chloroplasts and Elysia viridis. New Phytol 83(2):445
Hawes CR, Cobb AH (1980) The effects of starvation on the symbiotic chloroplasts of Elysia viridis: a fine structural study. New Phytol 84:375–379
Hayden HS, Waaland JR (2002) Phylogenetic systematis of the Ulvaceae (Ulvales, Ulvophyceae) using chloroplast and nuclear DNS sequences. J Phycol 38:1200–1212
Hinde R (1980) Chloroplast ‘‘symbiosis’’ in sacoglossans molluscs. In: Schwemmler W, Schenk HEA (eds) Endocytobiology, endosymbiosis and cell biology. Proceedings of the international colloquium on endosymbiosis and cell research, Tubingen, Germany, Walter de Gruyter, pp 729–736
Hinde R, Smih DC (1975) The role of photosynthesis in the nutrition of the mollusc Elysia viridis. Biol J Linn Soc 7:161–171
Hinde R, Smith DC (1972) Persistence of functional chloroplasts in Elysia viridis (Opisthobranchia, Sacoglossa). Nature 239:30–31
Jeffrey SW, Mantoura RFC, Bjørnland T (1997) Data for the identification of 47 key phytoplankton pigments. In: Jeffrey SW, Mantoura RFC, Wright SW (eds) Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO, Paris, pp 449–559
Jensen KR (1980) A review of sacoglossan diets, with comparative notes on radular and buccal anatomy. Mal Rev 15:55–77
Jensen KR (1997) Evolution of the Sacoglossa (Mollusca, Opisthobranchia) and the ecological associations with their food plants. Evol Ecol 11:301–335
Johnsen G, Sakshaug E (1993) Optical characteristics and photoadaptive responses in the toxic and bloom-forming dinoflagellates Gyrodinium auroleum, Gymnodinium galatheanum, and two strains of Prorocentrum minimum. J Phycol 29:627–642
Johnsen G, Sakshaug E (2007) Bio-optical characteristics of PSII and PSI in 33 species (13 pigment groups) of marine phytoplankton, and the relevance for pulse amplitude modulated and fast repetition rate fluorometry. J Phycol 43:1236–1251
Johnsen G, Nelson NB, Jovine RV, Prezelin BB (1994) Chromoprotein- and pigment-dependent modelling of spectral light absorption in two dinoflagellates, Prorocentrum minimum and Heterocapsa pygmaea. Mar Ecol Prog Series 114:245–258
Kremer BP, Schmitz K (1976) Aspects of 14CO2 fixation by endosymbiotic rhodoplasts by the marine opisthobranchiate Hermaea bifida. Mar Biol 34:313–316
Kromkamp JC, Forster RM (2003) Use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. Eur J Phycol 28(2):103–112
Kroon B, Prezelin BB, Schofield O (1993) Chromatic regulation of quantum yields for photosystem II charge separation, oxygen evolution, and carbon fixation in Heterocapsa pygmaea (Pyrrophyta). J Phycol 29:453–462
Lam DW, Zechman FW (2006) Phylogenetic analyses of the Bryopsidales (Ulvophyceae, Chlorophyta) based on RUBISCO large sub-unit sequences. J Phycol 42:669–678
Leliaert F, De Clerk O, Verbruggen H, Boedker C, Coppejans E (2007) Molecular phylogeny of the Siphonocladales (Chlorophyta: Cladophorophyceae). Mol Phylogenet Evol 44:1237–1256
McLean N (1976) Phagocytose of chloroplasts in Placida dendritica (Gastropoda: Sacoglossa). J Exp Zool 197(3):321–329
Mujer CV, Andrews DL, Manhart JR, Pierce SK, Rumpho ME (1996) Chloroplast genes are expressed during intracellular symbiotic association of Vaucheria litorea plastids with the sea slug Elysia chlorotica. Proc Natl Acad Sci USA 93:12333–12338
Pierce SKR, Biron W, Rumpho ME (1996) Endosymbiotic chloroplasts in molluscan cells contain proteins synthesized after plastid capture. J Exp Biol 199:2323–2330
Reynolds ES (1963) The use of lead citrate at high pH as an electronopaque stain in electron microscopy. J Cell Biol 17:208–212
Rodriguez F, Chauton M, Johnsen G, Andresen K, Olsen LM, Zapata M (2006) Photoacclimation in phytoplankton: implications for biomass estimates, pigment functionality and chemotaxonomy. Mar Biol 148:963–971
Sakshaug E, Bricaud A, Dandonneau Y, Falkowski PG, Kiefer DA, Legendre L, Morel A, Parslow J, Takahashi M (1997) Parameters of photosynthesis: definitions, theory and interpretations of results. J Plankton Res 19(11):1637–1670
Taylor DL (1968) Chloroplasts as symbiotic organelles in the digestive gland of Elysia viridis (Gastropoda:Opisthobranchia). J Mar Biol Assoc UK 48:1–15
Tevini M, Steinmüller D (1985) Composition and function of plastoglobuli. II. Lipid composition of leaves and plastoglobuli during beach leaf senescence. Planta 163:91–96
Trench RK (1980) Uptake, retention and function of chloroplasts in animal cells. In: Schwemmler W, Schenk HEA (eds) Endocytobiology, endosymbiosis and cell biology. Proceedings of the international colloquium on endosymbiosis and cell research. Tubingen, Germany, Walter de Gruyter, pp 703–727
Trench RK, Olhorst S (1976) The stability of chloroplasts from siphonaceous algae in symbiosis with sacoglossan molluscs. New Phytol 76:99–109
Trench RK, Smith DC (1970) Synthesis of pigment in symbiotic chloroplasts. Nature 227:196–197
Trench RK, Boyle JE, Smith DC (1973a) The association between chloroplasts of Codium fragile and the mollusc Elysia viridis. III. Movement of photosynthetically fixed 14C in tissues of intact living E. viridis and in Tridachia crispata. Proc R Soc Lond B 185:453–464
Trench RK, Boyle JE, Smith DC (1973b) The association between chloroplasts of Codium fragile and the mollusc Elysia viridis. II. Chloroplast ultrastructure and photosynthetic carbon fixation in E. viridis. Proc R Soc Lond B 184:63–81
Trowbridge CD (2002) Coexistence of introduced and native congeneric algae: Codium fragile and C. tomentosum on Irish rocky intertidal shores. J Mar Biol Assoc UK 81:931–937
Vasilikiotis C, Melis A (1994) Photosystem II reaction centre damage and repair cycle: chloroplast acclimation strategy to irradiance stress. Proc Natl Acad Sci USA 91:7222–7226
van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Wägele H, Johnsen G (2001) Observations on the histology and photosynthetic performance of ‘‘solar powered’’ opisthobranchs (Mollusca, Gastropoda, Opisthobranchia) containing symbiotic chloroplasts or zooxanthellae. Org Divers Evol 1:193–210
Webb WL, Newton M, Starr D (1974) Carbon dioxide exchange of Alnus rubra: a mathematical model. Ecologia 17:281–291
Williams ML, Cobb AH (1989) Isolation of functional chloroplasts from the sacoglossan mollusc Elysia viridis Montague. New Phytol 113(2):153–160
Yokohama Y (1981) Distribution of the green light-absorbing pigments siphonaxanthin and siphonein in marine green algae. Bot Mar 24:637–640
Zechman FW (2003) Phylogeny of the Dasycladales (Chlorophyta, Ulvophyceae) based on analyses od RUBISCO large sub-unit (rbcL) gene sequences. J Phycol 39:819–827
Acknowledgments
We would like to thank Torkild Bakken and Anita Kaltenborn for field assistance collecting sacoglossans and algae, Heike Wägele and Ingo Burghardt for assistance with the light microscopy sections at Spezielle Zoologie, Ruhr-Universität Bochum, Germany, and Kåre Tvedt and Linh Huoang at the Department of Laboratory Medicine, NTNU, for practical assistance with the TEM sections, and Kjersti Andresen at Trondhjem Biological Station, for HPLC pigment isolation. This study was supported by the Norwegian Research Council to J. Evertsen (NFR 153790/120). All experiments comply with the current laws of the country in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kühl.
Rights and permissions
About this article
Cite this article
Evertsen, J., Johnsen, G. In vivo and in vitro differences in chloroplast functionality in the two north Atlantic sacoglossans (Gastropoda, Opisthobranchia) Placida dendritica and Elysia viridis . Mar Biol 156, 847–859 (2009). https://doi.org/10.1007/s00227-009-1128-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1128-y