Abstract
Brooding, embryonic and larval development, and the influence of environmental and biological factors in tidepool habitats were studied in the sea star, Anasterias minuta, at various sites along ~220 km of the Patagonian coast. This species has a benthic, lecithotrophic development that includes eight distinct developmental stages. A larval organ, the connection cord, is developed from a small preoral lobe at early stages of development and becomes larger and thinner at advanced stages. Fecundity and average egg size increased with female body size. The regression of log egg number to log sea-star size and weight at different sites had a slope significantly less than 3.0, resulting in negative allometry and indicating that brood capacity was limited in large females. Development was generally synchronous among sites, but varied within each brood at advanced stages, with more developed brooded larvae located at the periphery of the brood mass. Brooding was synchronous among various populations at different years and spatial scales, and extended over a period of 8 months. The highest proportion of brooding females occurred during May and June (austral winter). Juveniles were released mainly during September. The likelihood of finding brooding sea stars decreased with increasing sea water temperature, tidal height, and wave exposure, and increased with increasing body size. Both body size of brooding females and brooding rate were higher in the infralittoral fringe than at midlittoral levels. A revision of the current model of brooding behavior and development among forcipulate sea stars is given.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Echinoderms exhibit a great diversity of life-history strategies and some of the most widely divergent reproductive modes among invertebrates (McEdward and Miner 2001). Although relatively rare, brooding is known to occur in all classes of echinoderms (Chia and Walker 1991) and is of interest as a derived reproductive strategy (Lawrence and Herrera 2000). The complete range of echinoderm life-history patterns occurs in the Asteroidea (McEdward and Miner 2001): broadcast spawning and production of planktotrophic or pelagic lecithotrophic larvae are widespread. However, brooding species with non-pelagic lecithotrophic embryos are less common (Hyman 1955; McClary and Mladenov 1990; Chia and Walker 1991; McEdward and Miner 2001).
Brood protection in invertebrates is often related to small adult size (Strathmann and Strathmann 1982) and usually results in lecithotrophic and abbreviated development of the embryo. Brooding in sea stars is associated with high and mid-latitudes, and is almost absent in tropical and subtropical species. Most studies have been carried out in north temperate/polar waters (Leptasterias spp.), on sub-Antarctic and Antarctic species, or in southern South America and Australia (Pearse et al. 2009). Brooding strategies vary among species: some are intraovarian brooders (e.g. Byrne 1996, 2005) while others have special brood chambers, and embryos are retained at different places such as on the aboral surface among spines, inside an aboral nidamental chamber, cardiac stomach, mouth, or under the body of the female (e.g. Lieberkind 1920; Himmelman et al. 1982; Chia and Walker 1991). Embryos generally rely on the maternal reserves in large, yolky eggs for nutrition and lack a pelagic phase, which limits larval dispersal (McEdward and Janies 1993; Lawrence and Herrera 2000; Villinski et al. 2002; Raff and Byrne 2006; Prowse et al. 2008).
Brood protection in the order Forcipulatida occurs in nine genera from temperate and polar regions: Anasterias, Cryptasterias, Diplasterias, Granaster, Leptasterias, Lysasterias, Neosmilaster, Notasterias, and Smilasterias (Philippi 1870; Perrier 1891; Ludwig 1903; Koehler 1906; Macbride and Simpson 1908; Verrill 1914; Fisher 1940; Bosch and Pearse 1990; O’Loughlin and O’Hara 1990; Pearse and Bosch 1994; Pearse et al. 2009). Oral brooding is prevalent, but benthic and gastric brooding also occurs in some genera. Anasterias is a genus of brooding sea stars with a circumpolar distribution, including several species that are widely distributed in the sub-Antarctic region (Philippi 1870; Perrier 1891; Fisher 1940; Bernasconi 1964; Clark and Downey 1992; Pearse and Bosch 1994). Development of embryos has not previously been described for the genus, but a connection cord between the brooded juvenile and the central egg mass was observed in A. varia (=Asteracanthion varium) (Philippi 1870), A. antarctica (=Asterias spirabilis) (Perrier 1891), and A. rupicola (Blankley and Branch 1984). Anasterias minuta occurs along the Patagonian coast of Argentina, including Tierra del Fuego and the Malvinas Islands (Bernasconi 1964). Its depth range is from the midlittoral zone to 80 m, although it is most common in the intertidal (Bernasconi 1964; Hernández and Tablado 1985; Gil and Zaixso 2008).
Many aspects of the life-history of A. minuta remain poorly understood. However, its broad geographic distribution provides an opportunity to not only examine variability in reproductive traits, but also to determine the influence of environmental conditions. The goals of the present study were to (1) describe the embryonic and larval development, brooding process, and temporal pattern of brooding for A. minuta at multiple sites in Patagonia; (2) evaluate fecundity, egg loss and determine whether allometric constraints on brooding space limit the fecundity of large females; and (3) examine the influence of environmental and biological factors on brooding activity.
Materials and methods
Study areas
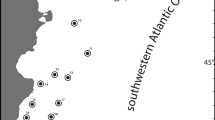
The study was conducted at three sites on the east coast of Patagonia (Fig. 1): from May, 1997, to November, 1998, at Punta Maqueda (PM; 46°01′S; 67°01′W); and from January to December, 2006, at PM, Caleta Cordova Norte (CCN; 45°43′S; 67°22′W), and the Foca Peninsula (FP; 47°45′S; 65°50′W). The substratum at all three sites consists of hard bedrock platforms with numerous, extensive tidepools and rocks that provide shelter for sea stars during low tides (Gil and Zaixso 2008). Tides are semi-diurnal, with mean and maximum fluctuations ~4 and ~5.8 m, respectively. The three sites differ in wave exposure: FP is an exposed site, with heavy surf breaking onto or just below the intertidal; PM is a moderately exposed site; and CCN is a semi-protected site within a small bay. Salinity is ~32.4 at FP, and 33.3–33.9 at CCN and PM (Zaixso, unpublished data). Purple mussels (Perumytilus purpuratus) are common at all three sites, particularly at midlittoral levels. A. minuta occurs in the intertidal and sublittoral zones. In the intertidal region, sea stars occurred beneath mussel hummocks, in crevices, or underneath rocks in tidepools (Gil and Zaixso 2007, 2008). The hydrography and biota were described in detail by Zaixso (1975), Zaixso and Pastor (1977), and Gil and Zaixso (2008).
Field surveys and laboratory analyses
Brooding activity of A. minuta was examined monthly at all sites in tidepools habitats. All individuals present under rocks in tidepools were examined for the presence of a brood, and the radius (R) from the center of the disc to the tip of the longest arm was measured (±0.1 mm). During 2006 surveys, tidal height was also recorded for each sea star. Altogether, 6,770 observations were recorded. Sea water temperature was recorded monthly at each site.
Fecundity and embryonic development were studied at PM during 1997–1998, and at CCN during 2006. A minimum of 15 brooding females were collected each month, and the radius of each was recorded. Each brood mass was removed and weighed (±0.01 g), and the minimum and maximum diameters of the entire egg mass were measured using digital calipers (±0.01 mm). Total brood volume was calculated using the formula for an oblate spheroid, as in Turner and Lawrence (1979):
where v = volume (mm3), dl = maximum diameter, and d2 = minimum diameter. Brood volume and weight were calculated for CCN only during May and July, when a cohesive brood mass was present. The eggs, embryos and brooded larvae were separated from the brood mass, categorized into eight developmental stages (see “Results”), counted, and their diameters were measured using an ocular micrometer. Microscopic modifications of the preoral lobe were examined using differential interference contrast microscopy. Within each brood, abortive eggs or non-fertilized eggs were identified by the absence of segmentation and differential coloration (bright red—see ESM 2). These eggs were counted.
Sex ratios and size at first brooding were assessed at the three sites during June and July, 2006, by randomly collecting ~200 sea stars. Sex was determined in the laboratory by visual inspection of the gonad (Salvat 1985). This sampling was done at the same time but in an area remote from the brooding surveys.
Data analysis
Sex ratios at each site were calculated for four body size categories (15–20, 20.1–27, 27.1–34, and 34.1–60 mm), and the statistical significance of differences from the expected 1:1 ratio was determined using goodness-of-fit (G) tests for pooled data and for body size subcategories (Sokal and Rohlf 1995). Sex ratios across different size classes and sites were also compared using G tests (Sokal and Rohlf 1995). Differences on body size between sexes and shore zones (infralittoral fringe, <1.20 m; and low midlittoral, 1.20–2.50 m) were compared using factorial analysis of variance.
The arm length (R) at which 50% of the females at each site were brooding was estimated using a logistic regression model for binary responses, fitted to the data by maximum-likelihood procedures with STATISTICA 6.0 software. Body size of brooding females was compared among zones and sites using ANOVA. The proportion of brooding sea stars (±95% CI) with an R > 15 mm was calculated for each month and shore zone. G tests were then performed to test for differences in the proportion of brooding females across shore zones for each sampling month.
A multiple logistic regression model was used to test the influence of environmental and biological variables on the brooding activity of A. minuta. A binary response variable (presence or absence of brood) was expressed as a linear combination of sea water temperature, tidal height, and sea-star size through a logit link function (Agresti 2007). Wave exposure was included as an ordinal categorical variable: semi-protection = 0, moderately exposure = 1, and exposure = 2. The interactive effect of exposure and sea-star size was also included in the model. The continuous variable body size was centered in order to reduce the correlation between a multiplicative term and its component variables (Aiken and West 1991). A forward stepping variable selection procedure included all main effects variables and the interaction term. Overall model fit was assessed with the likelihood ratio goodness-of-fit test, and individual parameters were tested using a Wald χ2 test (Hosmer and Lemeshow 2000). Correlations between explanatory variables were calculated to evaluate multicollinearity between variables in the regression model.
Allometric relationships between arm length (R) and brood volume, brood weight, and fecundity were described by linear regressions obtained from log transformation of the power curve, Y = a × Xb. The slope indicating isometry depends on the dimensions of the dependent and independent variables (Gould 1966; Hines 1982). For example, if X is a linear measure (e.g., R) and Y is a volume or volume approximation (e.g., egg number), a slope of 3 indicates isometry (see Gould 1966; Litulo 2005).
To evaluate variation in fecundity among sites and during the brooding period, brood sizes of females collected during April–May were compared with those collected in July, using a two-way GLM analysis of covariance (ANCOVA), with time and site as main effects, and arm length as a covariate. A homogeneity slope test was carried out to determine whether potential brood loss between periods and sites varied with female size. The number of abortive eggs per brood was also compared between brood periods using a one-way ANCOVA with arm length as a covariate.
ANOVAs and ANCOVAs were performed using STATISTICA 6.0 software. Homogeneity tests showed no significant differences between slopes (P > 0.13 in all cases). Assumptions of homoscedasticity and normality were tested with Cochran’s C test and Shapiro–Wilk test, respectively. Unless otherwise indicated, error terms are SD. Statistical significance of all tests was accepted at P < 0.05.
Results
Brooding behavior and embryonic development
Anasterias minuta spawns through ventral gonopores into a brood chamber, which is formed by arching the central disc, while tube feet at the distal end of the arms hold on to the substratum. Tube feet are also used to hold and protect the brood mass. In a few cases (~0.7%, N = 350), one arm was held below the egg mass to protect the brood. The brood mass was usually free of debris.
Brooding individuals occurred in the intertidal and sublittoral zones at all three study sites. Sea stars were rarely seen on exposed rock surfaces. At FP and CCN, brooding females did not feed (N = 207 and 502, respectively). Out of 636 observations at PM during 2006, two brooding sea stars were feeding in October during the final phase of brooding.
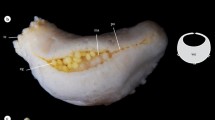
The progeny of 290 brooding A. minuta were classified into eight developmental stages (Fig. 2, ESM 1). Stage I (mean diameter = 1.81 ± 0.27 mm, N = 279) includes eggs to early embryos (segmentation and gastrulation stages). Spherical, bright orange/yellow eggs are aggregated by their fertilization membranes and surrounded by whitish mucus, forming a uniform, cohesive brood mass. Fertilized eggs have a distinct membrane in which the egg floats freely. Stage II (1.88 ± 0.16 mm, N = 49) begins to exhibit oral-aboral symmetry: the embryo develops an external fold on the oral side that forms a disc with a central depressed area. The disc is usually oriented toward the inside of the brood mass and represents the preoral lobe and the primordia of the connection cord. No distinctive brachiolar arms are visible. Brooded larvae are closely interlinked by this structure and surrounded by mucus that has become denser and red. In Stage III (1.85 ± 0.17 mm, N = 38), five primordial ambulacra (primary hydrocoel lobes) are visible through the body wall as small whitish spots, and the body is slightly pentagonal. The aboral side is dome-shaped and has an internal region of yolk platelets and/or lipid granules. The connection cord is larger and wider at the distal end, which is attached to an amorphous mass of red aborted eggs and mucus at the center of the brood. Yolk platelets and lipid inclusions are visible inside the connection cord. At this stage, the brood mass begins to break down, with brooded larvae separated as individuals or in small groups. The surrounding mucus is reddish and very dense. In Stage IV (R:1.32 ± 0.10 mm, N = 20), arms begin to emerge on the oral side, with rounded ends and developing tube feet. At this and subsequent stages, the siblings are oriented with their mouth toward the center of the brood mass (ESM 2). In Stage V (R:1.23 ± 0.15 mm, N = 33), the incipient brooded juvenile appears as a biconvex sea star. Arms have rounded ends and two rows of prominent tube feet. A sunken area indicates where the mouth will open. Sections of endoskeleton appear in the aboral region, forming white plates with a poorly defined central disc. In stage VI, brooded juveniles (R:1.15 ± 0.16 mm, N = 78) have five distinct arms. Ossicle development is visible on the aboral edge of the disc and arms, which have a spine on both sides of the distal end. Stage VII (R:1.23 ± 0.16 mm, N = 155) is characterized by further development of the endoskeleton and a pair of ocelli at the tip of each arm. The ambulacral groove is visible, connected to the perioral ring from which a long and thin connection cord emerges. The adambulacral plates develop large spines, and spines surround the ocellii at the ends of the arms. Stage VIII (R:1.39 ± 0.15 mm, N = 238) exhibits marked changes in the endoskeleton and podia, and the appearance of the madreporic plate surrounded by spines. The connection cord is long and thin, and linked to the juvenile at the interradial area between arms A and C (Carpenter system; Hyman 1955). The mouth opens to the exterior, and pyloric caeca are visible within each arm. Spines are conspicuous throughout the body. Brooded juveniles are farther apart and, when released from the mother, float and adhere to the aquaria.
When juveniles reach Stage VIII and are released, an amorphous mass of reddish mucus remains at the center of the brood, with atrophied eggs or embryos with connection cords (ESM 2). An overall mean prevalence of 7.9 ± 4.9% (N = 59) of abortive eggs was recorded in the brood masses. The number of abortive eggs per brood ranged from 1 to 34 (adjusted mean = 8.86 ± 7.21 eggs, N = 59) and was linearly related to female body size (P < 0.005). The size-adjusted mean number of abortive eggs did not vary among brood periods (ANCOVA, F1,56 = 1.20, P = 0.50).
Differential interference contrast microscopy revealed that the connection cord has constrictions throughout its length (ESM 3) and is connected to the amorphous central mass by an adhesive disc that sometimes has a lobed appearance (Fig. 3a). The connection cord contains many loose fibrous strands, yolk platelets, and lipid droplets, especially at intermediate stages of development (Fig. 3b). The wall of the connection cord is a thick epithelium, composed of long, thin cells covered by a cuticle.
The monthly distribution of developmental stages was variable for different years and sites but a general temporal pattern could be seen (Fig. 4). Stage I occurred from April/May to August, with maximum frequency during May and June. Development through stages IV–VI was relatively rapid, occurring mainly during 2 months. Advanced stages (VII) begin to appear during July/August and peaked in September. The release of juveniles occurred mainly during August–September. Variability in developmental stage within the same brood occurred at intermediate and advanced stages, with more advanced stages located at the periphery of the brood mass.
Sex ratio, body size, and first maturity
Sex ratios were not significantly different from 1:1 (G test, P = 0.53, N = 1,121) and were homogeneous within and across all sites and size classes (R × C test, P > 0.56 in all cases) (ESM 4). Over all sites, zones, seasons, and sexes, maximum arm length (R) ranged from 6 to 69 mm and averaged 26.7 mm (±8.81, N = 1,170; ESM 5). The effects of sex, zone, and sex × zone on body size were not significant (P > 0.28 in all cases). Brooding females ranged from 8 to 58 mm. Size at which 50% of the females were brooding (R50) differed among sites and was highest at FP (Table 1, ESM 6). Body size of brooding females was consistently greater in the infralittoral fringe than at higher tidal heights at the three sites. The body size difference was marked for the FP population, where body size of brooding females was even lower at midlittoral shore zones (Table 1).
Fecundity
The number of eggs/embryos per brood ranged from 11 to 475 and averaged 124 ± 78 (N = 249). Both mean egg number and egg diameter increased significantly with female arm length (Table 2). Fecundity decreased between brood periods (April–May vs. July) and differed between sites: females at CCN had higher fecundity than females at PM (ESM 7). The log values of egg number, brood volume, and brood weight increased significantly with the log of maximum maternal arm length (Table 2). The slopes of these relationships were significantly less than 3, indicating negative allometry; i.e., large females have proportionally fewer eggs, smaller brood volume, and lower brood weight than smaller females.
Seasonality and factors affecting brooding
Brooding sea stars were found from March or April through October or November, with minor variation among sites and zones (Fig. 5). Brooding sea stars were found in mid-midlittoral zone only at PM; no brooding sea star was found above that level at any of the study sites. The proportions of brooding females in the infralittoral fringe of PM and CCN remained higher than in the midlittoral zones during the mid and late brood period (Fig. 5).
Monthly and vertical variations on the proportion of brooding females (with R ≥ 15 mm, N ~ 240–300 per month) and sea water temperatures at different years and multiple sites in Patagonia. (+) in PM (1998) indicate the presence of brooding females at mid-midlittoral. Error bars denotes 0.95 interval confidence. *P < 0.05; **P < 0.01; ***P < 0.001; ns: P > 0.05
Results of the logistic regression model indicated that sea water temperature, tidal height, body size, wave exposure, and exposure × body size interaction were significant predictors of brooding (Table 3; log-likelihood ratio Chi-square = 509.1, P < 0.001). A large majority (82.2%, N = 6,770) of individuals were correctly categorized by this model. Correlation coefficients between explanatory variables did not exceed 0.85, indicating non-significant multicollinearity between variables (ESM 8). Overall, the likelihood of finding brooding sea stars decreased with increasing seawater temperature (Fig. 5), tidal height, and wave exposure, and increased with increasing sea-star body size. In all but the smallest sea stars, the likelihood of finding brooding females decreased with increasing wave exposure (Fig. 6).
Discussion
Brooding behavior and embryonic development in Anasterias minuta
Oral brooding has been observed in all species of Anasterias studied to date, including A. minuta (Philippi 1870; Smith 1876; Perrier 1891; Arnaud 1974; Simpson 1982; Blankley and Branch 1984; Salvat 1985; Gil and Zaixso 2007). The present study found that brooding behavior includes the development of an external oral brood chamber and the formation of a brood mass that is strongly cohesive, especially during the early stages of development.
Cohesive properties are common, but not universal, among eggs and embryos of brooding sea stars (e.g., Osterud 1918; Komatsu et al. 1979; Byrne 1992; Haesaerts et al. 2006; Mercier and Hamel 2008; Naughton and O’Hara 2009). The eggs of A. minuta were strongly aggregated by their fertilization membranes and surrounded by mucus. The mucus could improve oxygen supply to inner embryos via passive transport, as observed in other marine invertebrates (Strathmann and Strathmann 1995). The embryos initially attached via the fertilization and mucous membranes, and later by a modified brachiolar complex that acted as a connection cord.
Embryonic development within the brood masses of A. minuta was moderately asynchronous, especially during intermediate development. Siblings on the surface of the brood mass were more developed, while those inside the brood mass were often retarded or abortive. Retardation of centrally located embryos was reported in other invertebrates, including brooding echinoderms (Bosch and Slattery 1999; Gil et al. 2009), and attributed to oxygen limitation (Strathmann and Strathmann 1995; Cohen and Strathmann 1996; Fernández et al. 2003) or adelphophagy (Bosch and Slattery 1999). In fact, Kamel et al. (2010) found evidence that developing siblings compete for oxygen within egg masses. However, in A. minuta, separation of siblings (occurring initially at Stage III) at advanced stages of development would contribute to adequate gas exchange.
Sea stars exhibit a diversity of larval forms such as bipinnaria, brachiolaria, yolky brachiolaria, barrel-shaped larva, yolky non-brachiolaria larva, and non-larval mesogen with direct development (McEdward and Miner 2001). Most brooded larvae possess the typical larval settlement structures (McEdward and Janies 1997). Reduction or modifications of these structures such as number and size of brachiolar arms have been reported previously, from a tripod-larva (e.g. Leptasterias hexactis) to the presence of small brachiolar lobes at the end of a long connection cord (e.g., Leptasterias groenlandica) (Lieberkind 1920; Haesaerts et al. 2006). The typical benthic tripod brachiolaria observed in Aquilonastra minor (=Asterina minor), Leptasterias aequalis, L. hexactis, L. polaris, Parvulastra exigua, and Tosia neossia (Chia 1968; Komatsu et al. 1979; Byrne 1995; Hamel and Mercier 1995; Bingham et al. 2004; Naughton and O’Hara 2009) were not observed in A. minuta and have never been recorded among oral brooding forcipulate sea stars from the southern hemisphere. The development of A. minuta includes a non-feeding, lecithotrophic, modified brachiolaria. The disc structure formed during early development, and the subsequent formation of a single connection cord emerging from the interradius, corresponds to the modified preoral lobe of a brachiolaria. The main function of the fixing disc in brachiolaria is to cement the larva to the substratum during metamorphosis (Haesaerts et al. 2006). The connection cord in A. minuta holds the brooded larvae together, even at advanced stages of development. The presence of preoral lobe modifications as single cord structures has been cited previously. In this regard, Philippi (1870) was the first to describe the presence of an “umbilical cord” in the juveniles of the sea star Anasterias varia (=Asteracanthion varium). The presence of a connection cord in the brooding species was then described for Anasterias antarctica (=Asterias spirabilis) (Perrier 1891), Lysasterias chiropora and Lysasterias belgicae (Ludwig 1903), Lysasterias perrieri (=Anasterias tenera) (Koehler 1906), Diplasterias brandti (Macbride and Simpson 1908), Leptasterias groenlandica (Lieberkind 1920), Leptasterias arctica (Fisher 1930), Diplasterias octoradiata (Fisher 1940), Leptasterias tenera (Hendler and Franz 1982), Anasterias rupicola (Blankley and Branch 1984), Neosmilaster georgianus and Diplasterias meridionalis (Bosch and Slattery 1999). The variation on larval anatomy in brachiolaria larvae observed in the development of forcipulate brooding sea stars (e.g., tripod brachiolaria, a cord with minute brachiolar arms and single connection cord) might also indicate independent origins of lecithotrophy in this group or be the result of different evolutionary age. With the exception of L. arctica, L. groenlandica, and L. tenera, all single connection cords were described in sub-Antarctic or Antarctic species. Moreover, no record of egg laying or benthic substrate brooding in sub-Antarctic or Antarctic forcipulate sea stars was reported to date. Byrne (1995) suggested that egg laying was an integral step in the sequence of reproductive and developmental changes that led to the evolution of brooding in the Asteroidea. These observed hemispheric asymmetries regarding postural behaviors and embryonic patterns might account to a difference in the recent evolutionary history and age of the two polar regions (Clarke and Crame 2010).
Attachment of embryos to an amorphous central mass in the center of the brood is common among sea stars having connections cords, such as: Anasterias antarctica (Perrier 1891), Notasterias armata (Clark 1962), Anasterias rupicola (Blankley and Branch 1984), Anasterias mawsoni (Simpson 1982), Lysasterias chiropora and Lysasterias belgicae (Ludwig 1903), Lysasterias perrieri (Koehler 1906), Diplasterias octoradiata (Fisher 1940), Diplasterias meridionalis and Diplasterias brucei (Bosch and Slattery 1999), Leptasterias arctica (Fisher 1930), Leptasterias tenera (Hendler and Franz 1982) and Neosmilaster georgianus (Bosch and Slattery 1999). In A. minuta, this amorphous tissue was present after Stage III and appeared to be associated with aborted eggs or abnormal embryos and remnants of fertilization membranes. In other species, a similar tissue was described as the tangled ends of larval organs (Fisher 1930) or as fertilization membranes that co-adhered after hatching (Hendler and Franz 1982). The connection cord has a number of possible functions in addition to retention of juveniles until development is complete. The increasing size of developing siblings, and the presence of yolk platelets and lipid inclusions in the connection cord, may indicate translocation of nutritive reserves from aborted eggs and abnormal embryos in the center of the brood mass. Vitellogenic droplets in the connection cord were also observed in A. antarctica (Perrier 1891), L. perrieri (Koehler 1906), and N. georgianus (Bosch and Slattery 1999). Ingestion of nurse eggs, malformed embryos, or cannibalism of siblings has been suggested or observed for some echinoderms with internal or external broodcare (e.g., Notocrinus virilis (Mortensen 1920); Leptosynapta clarki (Everingham 1961); Ophionotus hexactis (Turner and Dearborn 1979); Parvulastra vivipara, Parvulastra parvivipara, Cryptasterina hystera (Byrne 1996, 2005)). Brooded juveniles of Pteraster militaris may also ingest particulate organic matter derived from abortive embryos (McClary and Mladenov 1990). In N. georgianus, Bosch and Slattery (1999) observed remnants of embryos or atrophied brood members in the center of the brood and interpreted these interactions as a form of cannibalism or adelphophagia supporting the increase in weight observed in embryonic development. Other potential uptake of nutrients that could account for the observed increase in size of brooded juveniles in A. minuta might be absorption of dissolved nutrients from sea water which has been documented for many echinoderms at various stages of development (e.g., Strathmann 1975; Meyer and Manahan 2009).
The presence of isolated discs within the amorphous central mass at advanced stages of development suggests that juveniles are released via rupture of the connection cord. The cord may then aid in passive transport and dispersal of floating juveniles after liberation from the mother, and/or enhance retention in nearby suitable habitats, e.g., via entanglement in coralline turfs in the low intertidal. Other marine invertebrates juveniles are known to use filaments as a mechanism of dispersal (e.g., Lane et al. 1985; Baker and Mann 1997), and the floating behavior of sea-star juveniles, as a mean of dispersal, is known to occur in Patiriella exigua (Byrne 1995), Cryptasterina pentagona and Aquilonastra minor (Soliman and Nojima 1984; Chen and Chen 1992).
Spatial and temporal patterns of brooding in Anasterias minuta
Results of the present study showed that spawning events and brooding have similar seasonality from year to year, and are largely synchronous among A. minuta populations in central Patagonia. Major spawning events, evidenced by the presence of eggs in the brood, occurred from April to May, and brooding occurred over the subsequent eight-month period, in agreement with previous observations (Salvat 1985; Gil and Zaixso 2007). Minor differences among sites might be attributable to differences in sea water temperature or severity of winter conditions: maximum brooding rate occurred during May at the southernmost site (FP) and in June at more northerly sites. Winter and early spring brooding is common in brooding sea stars (Hayashi 1943; Niesen 1973; O’Brien 1976; Boivin et al. 1986; O’Loughlin and O’Hara 1990; Hamel and Mercier 1995; Komatsu et al. 2006; Eernisse et al. 2010), including other Anasterias species (Blankley and Branch 1984; Simpson 1982). Spawning in other brooding sea stars was thought to be triggered by a seasonal decline in sea water temperature (O’Brien 1976; Hamel and Mercier 1995). However, summer brooding was found in A. mawsoni (Simpson 1982) in the Macquarie islands, and the lack of seasonality is suggested for Diplasterias brucei in the Southern Ocean (Bosch and Pearse 1990). Brooding during the coldest months in cold temperate regions may minimize energy expenditure and allow release of juveniles under favorable environmental conditions during spring (O’Brien 1976; Boivin et al. 1986; Hamel and Mercier 1995).
Factors affecting brooding and fecundity of Anasterias minuta
Environmental harshness at intertidal rocky shores of Patagonia might change A. minuta reproduction parameters among populations by affecting the brooding activity, causing the observed between-site variations, particularly those regarding brooding incidence. The significance of physical stress on intertidal brooding sea stars has been evaluated but only regarding reproductive output (Menge 1974; George 1994a, b). In addition to sea water temperature, results of the present study indicated that tidal height, wave exposure, body size, and the exposure × body size interaction were significant predictors of brooding in A. minuta. No brooding sea stars were found in the high midlittoral. The proportion of brooding females in the mid-midlittoral (only found in PM) decreased markedly, compared with the lower tidal levels, during the mid- and late-brood period. Brooding activity increased in the low midlittoral and infralittoral fringe. Increasing physical stress with increasing tidal height probably accounts for this gradient. The intertidal rocky shores of Patagonia are harsh environments with extreme temperatures and strong westerly winds, subjecting the biota to thermal and desiccation stress (Bertness et al. 2006). At the study sites, intertidal sea stars were located mainly in crevices, underneath mussel beds (hummocks), or under rocks in tidepools (Gil and Zaixso 2007, 2008), providing some protection from physical stress. However, the significance of brooding on midlittoral hummocks habitats was not included in this paper.
Wave exposure affects organisms through physical disturbance and dislodgement, flux of nutrients or food and propagules, and sedimentation, as well as by modifying thermal and desiccation stress (e.g., Menge 1976; Vadas et al. 1990; Bertness et al. 1992; Metaxas and Scheibling 1993; Lindegarth and Gamfeldt 2005; Prowse and Pile 2005). The inverse relationship between brooding in large A. minuta females and wave exposure found in the present study, probably reflects the high potential for dislodgement or brood loss, especially at exposed sites, such as FP. The arched brooding posture of large females must increase their susceptibility to waves. The dislodgment and movement of rocks and sea stars is a potential cause of mortality or brood loss in females by affecting common sea stars refuges, reducing foraging activity and reproductive success. Among brooding sea stars, physical disturbance, morphological damage, and mortality were also observed in Leptasterias hexactis (Niesen 1973; Menge 1974).
Information regarding fecundity among oral brooding in forcipulate sea stars is scarce and often incomplete (but see Menge 1974; George 1994a, b; Bingham et al. 2004). Within Anasterias, fragmentary information is available for some species (Simpson 1982; Blankley and Branch 1984). Figure 7 shows the relation between maximum fecundity and egg size among studied species. The fecundity in A. minuta has similar ranges than other Anasterias species. Fecundity and egg size are usually inversely related among invertebrates (Vance 1973), and this also applies to forcipulate brooding sea stars (Fig. 7). Gastric brooding sea stars share low fecundity and a low egg size comparable with Leptasterias species having tripod brachiolaria. Compared to other genera of brooding sea stars, fecundity in A. minuta is lower than brooding Leptasterias (Fig. 7) which has a smaller egg size but higher than Lysasterias, Diplasterias, and Notasterias species being the last reported as having an exceptionally large egg among brooders (McClintock and Pearse 1986). The tripod benthic brachiolaria characteristic of some Leptasterias species seems related to a small egg size, while sea stars having connection cords are characterized by a larger egg size and a reduced fecundity. However, this general pattern needs to be tested for other Antarctic species.
Relation between maximum fecundity and egg size among studied brooding forcipulate sea stars. Tbr tripod brachiolaria, redTbr reduced tripod brachiolaria, CC connection cord, CCm connection cord with minute brachiolar arms. References 1 Himmelman et al. 1982; Hamel and Mercier 1995; 2 Chia 1966; 3 George 1994b; 4 Bingham et al. 2004; 5 O’Loughlin and O’Hara 1990; Komatsu et al. 2006; 6 Worley et al. 1977; 7 Fisher 1930; 8 Blankley and Branch 1984; 9 Lawrence et al. 1984; 10 Simpson 1982; 11 this study; 12 Ludwig 1903; 13 Bosch and Pearse 1990
In A. minuta, fecundity increased significantly with maternal size. Female size also explained ~50% of the variation in egg size. Production of larger, more energy rich, embryos by large females has been noted for other species of brooding sea stars (George 1994a; Bingham et al. 2004). Previous studies on brooding invertebrates suggested that allometric constraints limit the brooding space and fecundity of large individuals (Strathmann and Strathmann 1982; Strathmann et al. 1984). In A. minuta, the allometric coefficient was significantly below 3, suggesting that fecundity of large females is indeed constrained by the limited brood area under the mouth. Loss of embryos, due to oxygen limitation, adelphophagy, or mechanical removal by waves probably compensates to some extent for embryonic growth. Brood loss has been reported in other marine brooding echinoderms, including Leptasterias species (Niesen 1973; Menge 1974; Hendler 1979; Hamel and Mercier 1995; Sewell 1996; Bingham et al. 2004; Gil et al. 2009). Embryos of L. aequalis species have a differential loss of smaller individuals since a relatively smaller brachiolar surface area is available for contact with the rest of the brood mass and the female sea star (Bingham et al. 2004). The modification of the brachiolar complex as a connection cord in A. minuta might prevent, to some extent, the loss of individual eggs, embryos or larvae by mechanical forces. The persistence of this cord at advanced stages of development in A. minuta could also allow for an extended brood period in time. Strong wave action could be responsible for the loss of the entire brood mass, as could be happening in the most exposed wave site studied here as indicated by the low incidence of brooding in FP.
The observation in the present study of only two brooding A. minuta feeding out of 1,345 observed agreed with results of previous studies (Gil and Zaixso 2007, 2008). Lack of feeding was noted in brooding females of other sea-star species (e.g., Perrier 1891; Chia 1966; Himmelman et al. 1982; Raymond et al. 2004; Bosch and Slattery 1999), with few exceptions (Blankley and Branch 1984). Brooding females of L. polaris might have slower somatic growth, compared with males, due to the repeated use of energetic reserves and lack of feeding, resulting in a male-skewed sex ratio in large individuals (Raymond et al. 2004). In A. minuta, however, sex ratios were equal among all size classes. As the proportion of males feeding in winter was low (0.7–1.5%; Gil and Zaixso 2007), summer feeding must largely support growth of both sexes. In fact, higher fecundity was found at the semi-protected site, which is also characterized by a higher prevalence of feeding sea stars during summer (Gil and Zaixso 2008). However, interannual differences on fecundity between 1997–1998 and 2006 could also be responsible for the observed differences between sites.
The present study found that differences in reproductive output among populations of A. minuta could be attributed to site-specific environmental conditions, as well as to the size structure of the population. Future studies should examine the mechanisms of these relationships, for example, the impact of wave exposure on brood loss and the relationship between reproductive output and maternal body size, especially between sea-star populations that experience variations in prey availability or habitat quality in order to discern the degree to which reproductive allometries and mean values of reproductive output shift in response to variation in energy supply.
References
Agresti A (2007) An introduction to categorical data analysis, 2nd edn. Wiley, New York
Aiken LS, West SG (1991) Multiple regression: testing and interpreting interactions. Newbury Park, Sage
Arnaud PM (1974) Contribution à la bionomie marine benthique des regions antarctiques et subantarctiques. Tethys 6:465–556
Baker P, Mann R (1997) The postlarval phase of bivalve mollusks: a review of functional ecology and new records of postlarval drifting of Chesapeake Bay bivalves. Bull Mar Sci 61:409–430
Bernasconi I (1964) Distribución geográfica de los Equinoideos y Asteroideos de la extremidad austral de Sudamérica. Boletín del Instituto de Biología Marina (Mar del Plata) 7:43–50
Bertness MD, Gaines SD, Stephens EG, Yund PO (1992) Components of recruitment in populations of the acorn barnacle Semibalanus balanoides (Linnaeus). J Exp Mar Biol Ecol 156:199–215
Bertness MD, Crain CM, Silliman BR, Bazterrica MC, Reyna V, Hildago F, Farina JK (2006) The community structure of western Atlantic Patagonian rocky shores. Ecol Monogr 76:439–460
Bingham BL, Giles K, Jaeckle W (2004) Variability of broods of the seastar Leptasterias aequalis. Can J Zool 82:457–463. doi:10.1139/Z04-009
Blankley WO, Branch GM (1984) Co-operative prey capture and unusual brooding habits of Anasterias rupicola (Verrill) (Asteroidea) at sub-Antarctic Marion Island. Mar Ecol Prog Ser 20:171–176
Boivin YY, Larrivée D, Himmelman JH (1986) Reproductive cycle of the subarctic brooding asteroid Leptasterias polaris. Mar Biol 92:329–337. doi:10.1007/BF00392673
Bosch I, Pearse JS (1990) Developmental types of shallow-water asteroids of McMurdo Sound, Antarctica. Mar Biol 104:41–46. doi:10.1007/BF01313155
Bosch I, Slattery M (1999) Costs of extended brood protection in the Antarctic sea star, Neosmilaster georgianus (Echinodermata: Asteroidea). Mar Biol 134:449–459. doi:10.1007/s002270050561
Byrne M (1992) Reproduction of sympatric populations of Patiriella gunnii, P. calcar and P. exigua in New South Wales, asterinid seastars with direct development. Mar Biol 114:297–316. doi:10.1007/BF00349533
Byrne M (1995) Changes in larval morphology in the evolution of benthic development by Patiriella exigua (Asteroidea: Asterinidae), a comparison with the larvae of Patiriella species with planktonic development. Biol Bull 188:293–305
Byrne M (1996) Viviparity and intragonadal cannibalism in the diminuitive sea stars Patiriella vivipara and P. parvivipara (Family Asterinidae). Mar Biol 125:551–567. doi:10.1007/BF00353268
Byrne M (2005) Viviparity in the sea star Cryptasterina hystera (Asterinidae)—conserved and modified features in reproduction and development. Biol Bull 208:81–91. doi:10.2307/3593116
Chen BY, Chen CP (1992) Reproductive cycle, larval development, juvenile growth and population dynamics of biology of Patiriella pseudoexigua (Echinodermata: Asteroidea) in Taiwan. Mar Biol 113:271–280
Chia FS (1966) Brooding behavior of a six rayed starfish, Leptasterias hexactis. Biol Bull 130:304–315
Chia FS (1968) The embryology of a brooding starfish, Leptasterias hexactis. Acta Zool Stockh 49:321–364
Chia FS, Walker CW (1991) Echinodermata: Asteroidea. In: Giese A, Pearse J, Pearse V (eds) Reproduction of marine invertebrates, vol VI. Boxwood Press, California, pp 301–353
Clark HES (1962) The fauna of the Ross Sea. Part 3, Asteroidea. Bull NZ Dep Scient Ind Res 151:1–84
Clark A, Downey M (1992) Starfish of the Atlantic. An illustrated key. Koeltz Scientific Book, Cambridge
Clarke A, Crame JA (2010) Evolutionary dynamics at high latitudes: Speciation and extinction in polar marine faunas. Phil Trans R Soc B 365:3655–3666. doi:10.1098/rstb.2010.0270
Cohen C, Strathmann R (1996) Embryos at the edge of tolerance: effects of environment and structure of egg masses on supply of oxygen to embryos. Biol Bull 190:8–15
Eernisse DJ, Strathmann MF, Strathmann RR (2010) Henricia pumila sp. nov.: A brooding seastar (Asteroidea) from the coastal northeastern Pacific. Zootaxa 2329:22–36
Everingham JW (1961) The intra-ovarian embryology of Leptosynapta clarki. MS Thesis, University of Washington, Seattle, USA
Fernández M, Ruiz-Tagle N, Cifuentes S, Pörtner HO, Arntz W (2003) Oxygen-dependent asynchrony of embryonic development in embryo masses of brachyuran crabs. Mar Biol 42:559–565. doi:10.1007/s00227-002-0965-8
Fisher WK (1930) Asteroidea of the North Pacific and Adjacent Waters, Pt. 3: Forcipulata. Bull US Nat Mus 76:1–356
Fisher WK (1940) Asteroidea. Discov Rep 20:1–305
George SB (1994a) Population differences in maternal size and offspring quality for Leptasterias epichlora (Brandt) (Echinodermata: Asteroidea). J Exp Mar Biol Ecol 175:121–131. doi:10.1016/0022-0981(94)90179-1
George SB (1994b) The Leptasterias (Echinodermata: Asteroidea) species complex: variation in reproductive investment. Mar Ecol Prog Ser 109:95–98
Gil DG, Zaixso HE (2007) The relation between feeding and reproduction in Anasterias minuta (Asteroidea: Forcipulata). Mar Biol Res 3:256–264. doi:10.1080/17451000701472035
Gil DG, Zaixso HE (2008) Feeding ecology of the sub-Antarctic sea star Anasterias minuta within tide pools in Patagonia, Argentina. Rev Biol Trop 56:311–328
Gil DG, Zaixso HE, Tolosano JA (2009) Brooding of the sub-Antarctic heart urchin, Abatus cavernosus (Spatangoida: Schizasteridae), in southern Patagonia. Mar Biol 156:1647–1657. doi:10.1007/s00227-009-1200-7
Gould SJ (1966) Allometry and size in ontogeny and phylogeny. Biol Rev Camb Philos Soc 41:587–640
Haesaerts D, Jangoux M, Flammang P (2006) Adaptations to benthic development: functional morphology of the attachment complex of the brachiolaria larva in the sea star Asterina gibbosa. Biol Bull 211:172–182. doi:10.2307/4134591
Hamel JF, Mercier A (1995) Prespawning behavior, spawning, and development of the brooding starfish Leptasterias polaris. Biol Bull 188:32–45
Hayashi R (1943) Contributions to the classification of the sea-stars of Japan. II. Forcipulata, with the note on the relationships between the skeletal structure and respiratory organs of the sea-stars. J Fac Sci Hokkaido Univ Ser 6 Zool 8:13–281
Hendler G (1979) Sex-reversal and viviparity in Ophiolepis kieri, n. sp., with notes on viviparous brittlestars from the Caribbean (Echinodermata: Ophiuroidea). Proc Biol Soc Wash 92:783–795
Hendler G, Franz DR (1982) The biology of a brooding seastar, Leptasterias tenera, in Block Island sound. Biol Bull 162:273–289
Hernández DA, Tablado A (1985) Asteroidea de Puerto Deseado (Santa Cruz, Argentina). Contribución No104. CENPAT, Argentina
Himmelman JH, Lavergne Y, Cardinal A, Martel G, Jalbert P (1982) Brooding behaviour of the Northern sea star Leptasterias polaris. Mar Biol 68:235–240. doi:10.1007/BF00409590
Hines AH (1982) Allometric constraints and variables of reproductive effort in brachyuran crabs. Mar Biol 69:309–320. doi:10.1007/BF00397496
Hosmer DW, Lemeshow S (2000) Applied logistic regression, 2nd edn. Wiley, New York
Hyman LH (1955) Echinodemata. The Invertebrates. IV. McGraw-Hill, New York
Kamel SJ, Grosberg RK, Marshall DJ (2010) Family conflicts in the sea. Trends Ecol Evol 5:442–449. doi:10.1016/j.tree.2010.05.008
Koehler R (1906) Echinodermes. Exped Ant Française (1903–1905), 41 p
Komatsu M, Kano YT, Yoshizawa H, Akabane S, Oguro C (1979) Reproduction and development of the hermaphroditic sea-star, Asterina minor Hayashi. Biol Bull 157:258–274
Komatsu M, O’Loughlin PM, Bruce B, Yoshizawa H, Tanaka K, Murakami C (2006) A gastric-brooding asteroid, Smilasterias multipara. Zool Sci 23:699–705
Lane DJW, Beaumont AR, Hunter JR (1985) Byssus drifting and the drifting threads of the young post-larval mussel Mytilus edulis. Mar Biol 84:301–308. doi:10.1007/BF00392500
Lawrence JM, Herrera J (2000) Stress and deviant reproduction in echinoderms. Zool Stud 39:151–171
Lawrence JM, McClintock JB, Guille A (1984) Organic level and caloric caloric content of eggs of brooding asteroids and an echinoid (Echinodermata) from Kerguelen (South Indian Ocean). Int J Invert Reprod Dev 7:249–257
Lieberkind I (1920) On a starfish (Asterias groenlandica) which hatches its young in its stomach. Vidensk Medd Dan Naturhist Foren 72:121–126
Lindegarth M, Gamfeldt L (2005) Comparing categorical and continuous ecological analyses: effects of “wave exposure” on rocky shores. Ecology 86:1346–1357
Litulo C (2005) Fecundity and size at sexual maturity of the fiddler crab Uca vocans (Linnaeus, 1758) (Brachyura: Ocypodidae). Thalassas 21:59–65
Ludwig H (1903) Seesterne. Résultats du voyage du S.Y. Belgica en 1897-1898-1899. Rapp Sci, Zool R20:1–72
Macbride EW, Simpson JC (1908) Echinoderma II. Echinoderm larvae. Nat Ant Exp (1901–1904) 4 (Zool):1–9
McClary DJ, Mladenov PV (1990) Brooding biology of the sea star Pteraster militaris (O.F. Müller): energetic and histological evidence for nutrient translocation to brooded juveniles. J Exp Mar Biol Ecol 142:183–199. doi:10.1016/0022-0981(90)90090-Y
McClintock JB, Pearse JS (1986) Organic and energetic content of eggs and juveniles of antarctic echinoids and asterids with lecithotrophic development. Comp Bioch Physiol A 85:341–345
McEdward LR, Janies DA (1993) Life cycle evolution in asteroids: What is a larva? Biol Bull 184:255–268
McEdward LR, Janies DA (1997) Relationships among development, ecology, morphology in the evolution of echinoderm larvae and life cycles. Biol J Linn Soc 60:381–400
McEdward LR, Miner BG (2001) Larval and life-cycle patterns in echinoderms. Can J Zool 79:1125–1170. doi:10.1139/cjz-79-7-1125
Menge BA (1974) Effect of wave action and competition on brooding and reproductive effort in the seastar, Leptasterias hexactis. Ecology 55:84–93
Menge BA (1976) Organization of the New England rocky intertidal community: role of predation, competition, and environmental heterogeneity. Ecol Monogr 46:355–393
Mercier A, Hamel JF (2008) Depth-related shift in life history strategies of a brooding and broadcasting deep-sea asteroid. Mar Biol 156:205–223. doi:10.1007/s00227-008-1077-x
Metaxas A, Scheibling RE (1993) Community structure and organization of tidepools. Mar Ecol Prog Ser 98:187–198
Meyer E, Manahan DT (2009) Nutrient uptake by marine invertebrates: cloning and functional analysis of amino acid transporter genes in developing sea urchins (Strongylocentrotus purpuratus). Biol Bull 217:6–24
Mortensen T (1920) Studies in the development of crinoids. Pap Department of Marine Biology, Carnegie Institution of Washington, vol 16, pp 1–94
Naughton KM, O’Hara TD (2009) A new brooding species of the biscuit star Tosia (Echinodermata: Asteroidea: Goniasteridae), distinguished by molecular, morphological and larval characters. Invertebr Syst 23:348–366
Niesen TM (1973) Population and reproductive biology of the six-rayed sea star Leptasterias hexactis on the protected outer coast. PhD Thesis, University of Oregon, USA
O’Brien FX (1976) Some adaptations of the seastar, Leptasterias littoralis (Stimpson) to life in the intertidal zone. Thalassia Jugoslavica 12:237–243
O′Loughlin PM, O′Hara DT (1990) A review of the genus Smilasterias (Echinodermata, Asteroidea) with descriptions of two new species from south-eastern Australia, one a gastric brooder, and a new species from Maqcuarie Island. Mem mus Victoria 50:307–329
Osterud HL (1918) Preliminary observations on the development of Leptasterias hexactis. Publ Puget Sound St 2:1–15
Pearse JS, Bosch I (1994) Brooding in the Antarctic: Östergren had it nearly right. In: David B, Guille A, Féral JP, Roux M (eds) Echinoderms through time. Balkema, Rotterdam, pp 111–120
Pearse JS, Mooi R, Lockhart SJ, Brandt A (2009) Brooding and species diversity in the Southern Ocean: selection for brooders or speciation within brooding clades? In: Krupnik I, Lang MA, Miller SE (eds) Smithsonian at the poles: contributions to international polar year science. Smithsonian Institution, Washington, pp 181–196
Perrier E (1891) Echinodermes de la mission scientifique du Cap Horn. Stellérides. Miss Sci Cap Horn Zool 6:1–168
Philippi RA (1870) Neue Seesterne aus Chile. Arch Naturges 36:268–275
Prowse TAA, Pile AJ (2005) Phenotypic homogeneity of two intertidal snails across a wave exposure gradient in South Australia. Mar Biol Res 1:176–185
Prowse TAA, Sewell MA, Byrne M (2008) Fuels for development: evolution of maternal provisioning in asterinid sea stars. Mar Biol 153:337–349. doi:10.1007/s00227-007-0809-7
Raff RA, Byrne M (2006) The active evolutionary lives of echinoderm larvae. Heredity 97:244–252
Raymond JF, Himmelman JH, Guderley HE (2004) Sex differences in biochemical composition, energy content and allocation to reproductive effort in the brooding sea star Leptasterias polaris. Mar Ecol Prog Ser 283:179–190
Salvat MB (1985) Biología de la reproducción de Anasterias minuta Perrier (Echinodermata, Asteroidea), especie incubadora de las costas patagonicas. PhD thesis. Universidad de Buenos Aires, Argentina
Sewell MA (1996) Mortality of pentactulae during intraovarian brooding in the apodid sea cucumber Leptosynapta clarki. Biol Bull 190:188–194
Simpson RD (1982) The reproduction of some echinoderms from Macquarie Island. Austr Mus Mem 16:39–52
Smith EA (1876) Descriptions of species of Asteriidae and Ophiuridae from Kerguelen′s Island. Ann and Mag Nat Hist ser 4(17):105–113
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practice of statistics in biological research, 3rd edn. WH Freeman and Company, New York
Soliman ES, Nojima S (1984) Some observations on dispersal behavior of the early juvenile of the sea-star, Asterina minor. Publ Anakrrsa Mar Biol Lab 7:81–93
Strathmann RR (1975) Larval feeding in echinoderms. Am Zool 15:717–730
Strathmann RR, Strathmann MF (1982) The relationship between adult size and brooding in marine invertebrates. Am Nat 119:91–101
Strathmann RR, Strathmann MF (1995) Oxygen supply and limits on aggregation of embryos. J Mar Biol Assoc UK 75:413–428. doi:10.1017/S0025315400018270
Strathmann RR, Strathmann MF, Emson RH (1984) Does limited brood capacity link adult size, brooding and simultaneous hermaphroditism? A test with the starfish Asterina phylactica. Am Nat 123:796–818
Turner RL, Dearborn JH (1979) Organic and inorganic composition of post-metamorphic growth stages of Ophionotus hexactis (E.A. Smith) (Echinodermata: Ophiuroidea) during intraovarian incubation. J Exp Mar Biol Ecol 36:41–51
Turner RL, Lawrence JM (1979) Volume and composition of echinoderm eggs: implications for use of egg size in life-history models. In: Stancky SE (ed) Reproductive ecology of marine invertebrates. Univ South Carolina Press, Columbia, pp 25–40
Vadas RL, Wright WA, Mille SL (1990) Recruitment of Ascophyllum nodosum: wave action as a source of mortality. Mar Ecol Prog Ser 61:263–272
Vance RR (1973) On reproductive strategies in marine benthic invertebrates. Am Nat 107:339–352
Verrill AE (1914) Monograph of the shallow-water starfishes of the North Pacific coast from the Arctic Ocean to California. Harriman Alaska Ser US Nat Mus 14:1–408
Villinski JT, Villinski JC, Byrne M, Raff RA (2002) Convergent maternal provisioning and life-history evolution in echinoderms. Evolution 56:1764–1775. doi:10.1111/j.0014-3820.2002.tb00190.x
Worley EK, Franz DR, Hendler G (1977) Seasonal patterns of gametogenesis in a North Atlantic brooding asteroid, Leptasterias tenera. Biol Bull 153:237–253
Zaixso HE (1975) Distribución vertical de los moluscos marinos de la ria Deseado (Santa Cruz, Argentina): sustratos con fracción limosa. Physis 34:229–243
Zaixso HE, Pastor CT (1977) Observaciones sobre la ecología de los mitílidos de la ría Deseado. I. Distribución y análisis biocenótico. Ecosur 4:1–46
Acknowledgments
The authors would like to thank Silvina Rosales and Maria Belén Reartes for their support during field surveys and Bárbara Kotoucek (UNPSJB) for assistance with German translation. This manuscript was further improved by comments of Howard Feder and two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Byrne.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gil, D.G., Escudero, G. & Zaixso, H.E. Brooding and development of Anasterias minuta (Asteroidea: Forcipulata) in Patagonia, Argentina. Mar Biol 158, 2589–2602 (2011). https://doi.org/10.1007/s00227-011-1760-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1760-1