Abstract
The quahog Mercenaria mercenaria has been introduced repeatedly to the Pacific coast of North America, but only one population is known to have become established. In the 1970s, the population of M. mercenaria at Colorado Lagoon, in Los Angeles County, California (33o46′16″N, 118o08′05″W), was estimated at more than 300,000 individuals. To determine the current status of this non-indigenous species (NIS), in 2009, we sampled 57 intertidal and 20 shallow subtidal plots, identifying and quantifying collected bivalves. No quahogs were found among the 2,490 living bivalves in our plots, though two were found intertidally outside of our plots. The M. mercenaria population has thus collapsed since 1980, but the native community has not recovered. Six of the fourteen living bivalve species we encountered were NIS; three are new records for the location, including the clam Venerupis philippinarum, which made up 87.6% of collected individuals. Though M. mercenaria is likely on its way to extinction on the US Pacific coast, the bivalve assemblage at this location remains heavily dominated by NIS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human-mediated introductions of marine and estuarine species to habitats outside of their native ranges have often led to the establishment and spread of these non-indigenous species (NIS), sometimes with dramatic ecological and economic consequences (e.g., Williams and Grosholz 2008). Predicting the trajectory of population growth and spread of NIS is challenging because it can be influenced by multiple factors, including biological traits of the NIS, biological traits of the recipient community, and physical traits of the habitat (Davis 2009). However, it is clear that gaining an understanding of the population biology of NIS is important, as such information may be very useful in their management (Sakai et al. 2001).
One aspect of the population dynamics of NIS that is of special interest in this regard is that of “population collapse”. Simberloff and Gibbons (2004) defined these events as drops in population size or density by at least 90% in 30 or fewer years, in the absence of management action. Although such declines are of obvious interest to managers of NIS populations, relatively little is known of the phenomenon at this point (Simberloff and Gibbons 2004). Gaining a better understanding of the frequency and causes of such dramatic declines may help us increase the effectiveness of management and eradication strategies for other NIS populations.
Here, we document population collapse in a marine NIS, the bivalve mollusk Mercenaria mercenaria. Also known as the hard clam or northern quahog, M. mercenaria is native to the Northwest Atlantic (Mikkelsen and Bieler 2008). This species is commercially important: from 2006 to 2008, more than 26,000 tonnes of M. mercenaria were harvested globally (FAO 2010). In that same period, more than 81,000 tonnes of M. mercenaria were produced globally via aquaculture (FAO 2011). Because of their economic importance, quahogs have been planted in marine habitats across the globe (Chew 2001). On the North American Pacific coast, intentional plantings of this species have been carried out at multiple sites since 1935 (Carlton 1979). Although the quahog has been reported as present in Boundary Bay, British Columbia (Forsyth 1997; Turgeon et al. 1998; Gillespie et al. 1999; Coan et al. 2000), all of these reports are based on the discovery of a single M. mercenaria individual found in the bay in 1996 (Forsyth and Forsyth 2001). Other than that single observation of a single individual, there are no published records of Mercenaria mercenaria in Boundary Bay, and recent reviews have concluded that there was no established population in British Columbia (Gillespie 2007). Therefore, only one population of the quahog is known to have become established on the North American Pacific coast, at Colorado Lagoon, in Long Beach, California (Carlton 1992, 1999). The timing and vector of the introduction of M. mercenaria into the lagoon are not known, but previous researchers have suggested that the population became established sometime after 1950, either as a result of migration from a population of adult clams intentionally introduced into a nearby beach in contiguous Alamitos Bay or as the result of an introduction directly into the lagoon (Crane et al. 1975). The earliest record of M. mercenaria in Colorado Lagoon is from 1967, and by 1969 intertidal densities of 8.6 quahogs m−2 were recorded, with clams at the site ranging in shell length from 8 to 101 mm (Table 1; Salchak and Haas 1971).
From the late 1960s through 1980, Mercenaria mercenaria were extremely abundant at Colorado Lagoon. In the first major study of quahogs at the site, Crane et al. (1975) reported on intertidal and subtidal bivalve assemblages based on surveys conducted from 1970 to 1972. Densities of M. mercenaria in the intertidal zone varied spatially, from 0 to 1 quahogs m−2 in “low density” areas, to a maximum of 18 quahogs m−2 in “intermediate density” areas. Crane et al. (1975) found extremely high densities of M. mercenaria in the subtidal zone, exceeding 500 quahogs m−2 in one area. The authors made a “conservative estimate” of the total population size of M. mercenaria at the site of 300,000–500,000 clams. Based on the presence of reproductively mature clams and the apparent presence of larvae of M. mercenaria in the plankton, they concluded the population was established and breeding (Crane et al. 1975). Subsequent surveys conducted from 1978 to 1980 by Murphy (1983, 1985) revealed that the quahog was still very abundant and a dominant component of the bivalve assemblage at the lagoon. Murphy recorded an average subtidal density of 78.61 quahogs m−2, with a maximum observed density of 400 quahogs m−2; M. mercenaria was the single most abundant of the 11 species of bivalves recovered from the site, with an average density more than 2.5× higher than any other species (Murphy 1983). Murphy estimated that the total quahog population at Colorado Lagoon included more than 4 million individuals and concluded that the population must be breeding, based on the presence of juveniles as small as 5 mm in length, as well as large, presumably reproductive adults (up to 95 mm in length: Murphy 1983). These studies indicate that Colorado Lagoon was the location of a large, established, breeding population of the introduced quahog from the late 1960s through 1980. Because there are no known records of M. mercenaria occurring in other areas of Alamitos Bay after 1954 (Crane et al. 1975; Murphy 1983), it appears that the lagoon population was self-sustaining.
To the best of our knowledge, Murphy’s work was the last major study of this introduced population of quahogs; since then, the population has been largely unmonitored. Our study was inspired by the fact that in numerous visits to Colorado Lagoon from 2004 to 2008, one of us (BP) did not observe a single M. mercenaria, living or dead. The apparent absence of quahogs was unexpected given their previous abundance at the site. Thus, in 2009, we carried out a study to determine the current status of the population of quahogs at Colorado Lagoon. Our data demonstrate not only a population collapse in this species, but also an important shift in the composition of the assemblage of benthic bivalves, with a previously unreported invasive bivalve replacing M. mercenaria as the dominant species at this location.
Methods
Site description
Colorado Lagoon (33°46′16″N, 118°08′05″W) is a small urban body of water with a low-tide perimeter of approximately 1,700 m and a high tide maximum water depth of approximately 5.5 m. The lagoon, which is bordered to the north by a golf course, is connected to the rest of Alamitos Bay by a culvert, which has dramatic effects on the lagoon’s tidal cycle. Tidal fluctuations in the lagoon are muted and offset in time relative to the tidal cycle in Alamitos Bay; although high tide level in Colorado Lagoon is approximately the same height as in Alamitos Bay, low tides in the lagoon are ~0.6 m higher and 3 h later than those in the rest of the bay (Moffatt and Nichol 2004). Eleven storm drains channel run-off from nearby surface streets into the lagoon (Moffatt and Nichol 2010).
Because of its small size, restricted connection to the rest of Alamitos Bay, and drainage from surrounding surface streets, physical conditions in the lagoon are more variable than those of nearshore coastal waters. Water temperatures reach 12°C in winter and 20–26°C in summer; surface salinities, lowest after heavy rainfalls, range from 2 to 35 ppt, and dissolved oxygen ranges from 3.8 to 10.8 ppm (Crane et al. 1975; Chambers Group 2004; Murphy 1983; Murphy and Kremer 1985). The lagoon is also periodically affected by major human-related environmental events. For example, in 1972, a failure of a nearby pumping station led to the sudden influx of 3,407,000 l of raw sewage into the lagoon, followed by 1,890,000 l of freshwater and 318 kg of sodium hypochlorite used to flush the surrounding streets (Crane et al. 1975). More recently, in July 2010, the culvert connecting the lagoon to the rest of Alamitos Bay was sealed off during an extremely low tide to permit maintenance work on the culvert. Most of the lagoon’s intertidal zone was emersed for ~5 days, during a period of warm summer weather. Several weeks later, hundreds of thousands of dead bivalves were observed in the intertidal zone (B. Pernet and C. Whitcraft, personal observation). Though the history of such major human-related events in Colorado Lagoon is not well documented, they have undoubtedly occurred repeatedly over the past few decades.
Intertidal and subtidal sampling
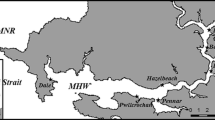
Because our primary goal was to determine whether a population of Mercenaria mercenaria was still present at the site, we focused most of our sampling effort on areas identified by Crane et al. (1975) as containing high densities of M. mercenaria. Intertidal samples were collected between March 2009 and February 2010. The majority of intertidal plots were placed in the two regions of the lagoon with the highest 1970–1972 intertidal densities of M. mercenaria (2–18 quahogs m−2: Crane et al. 1975). These two regions were the southwest side of the west arm (our “West Arm Intensive Intertidal Sampling Area”), and the west side of the north arm (our “North Arm Intensive Intertidal Sampling Area”) (Fig. 1; see Fig. 4 in Crane et al. 1975 for comparison). In the North Arm Intensive Intertidal Sampling Area, we sampled 20 randomly located plots along a 270-m stretch of intertidal zone. In the West Arm Intensive Intertidal Sampling Area, we sampled 26 randomly placed plots along a 150-m stretch of intertidal. In addition to these intensive sampling areas, we sampled 11 intertidal plots at approximately 100-m intervals around the remaining perimeter of the lagoon, except for the northeast side of the north arm, where the intertidal mud was too soft to permit sampling (Fig. 1). Our plots were placed throughout the intertidal zone, which has a tidal range of approximately 1.5 m (Moffatt and Nichol 2004).
Sample locations at Colorado Lagoon, Long Beach, CA. Intensive intertidal sampling (in the North and West Arms) was conducted in areas of the lagoon with the highest intertidal densities of Mercenaria mercenaria (2–18 clams m−2) recorded by Crane et al. (1975). The remainder of the intertidal zone around the lagoon was identified by Crane et al. (1975) as “low M. mercenaria density” (0–1 clams m−2); in these areas, we collected intertidal samples (I) at ~100-m intervals. Subtidal samples (S) were collected at ~100-m intervals around most of the lagoon, with additional effort along the south side of the west arm, because this area was identified as an area of high subtidal M. mercenaria density (18–556 clams m−2) by Crane et al. (1975). Stars indicate intertidal locations where two living individuals of M. mercenaria were discovered
In each intertidal plot, we used trowels to remove sediment from a 0.25-m2 area to a depth of 20 cm. All sediment was immediately sieved through 6 × 6 mm mesh. Any material remaining in the mesh was transported to the laboratory and frozen for later sorting and identification.
Subtidal samples were collected between May 2009 and February 2010. We located 20 shallow subtidal samples (each sample = a single replicate approximately 1 × 0.5 × 0.15 m) at approximately 100-m intervals around the lagoon, with two exceptions (Fig. 1). We increased our sampling effort on the south side of the west arm relative to other regions of the lagoon, because this area was determined by Crane et al. (1975) to be the area of the highest subtidal densities of M. mercenaria. The other exception to our 100-m subtidal sampling interval was the northwest tip of the north arm, where the sediment was too soft to permit sampling.
For subtidal sampling, we waded into the water to a depth of ~0.75 m. From this point, we used a clam rake (Model C002, Clamout Equipment Co., PA) to collect clams from the upper 10–15 cm of sediment. The rake head was 28 cm wide, with tines spaced 2.5 cm apart, and a handle 2 m long. We extended the rake into deeper water and combed a sample area approximately 1 × 0.5 m; this required several consecutive pulls of the rake. After each pull, material collected by the rake was pooled into a single bucket. We then sieved all collected material through 6 × 6 mm mesh. All material retained by the mesh (including algae or plant material) was returned to the laboratory and frozen for later sorting and identification. Our deepest subtidal samples were taken approximately 0.9 m below the lowest low tide of the year at the lagoon.
Sample processing and species identification
In the laboratory, we sorted all collected material and separated bivalves that were collected alive (whose valves contained tissue; hereafter, we refer to these as “living” bivalves) from those that were collected only as shells (“dead” bivalves). For each plot, we kept separate species richness tallies for living and dead bivalves. We used Coan et al. (2000) and Coan and Valentich-Scott (2007) as our primary sources for identification and taxonomy. Identifications of a subset of our samples (at least one individual of each species encountered) were confirmed by Dr. Paul Valentich-Scott of the Santa Barbara Museum of Natural History (SBMNH). Voucher specimens were deposited at SBMNH (catalog numbers 149505, 149506, and 211774–211784).
We collected and identified 12 species of living bivalves at the lagoon. In addition, we encountered occasional small individuals that could only be identified conclusively to genus. These specimens of undetermined species belonged to three genera: Chione, Leukoma, and Tagelus. All three of these genera were also represented by living individuals that we conclusively identified to species (C. fluctifraga, L. staminea, T. affinis, and T. californianus). To be conservative in our conclusions, we counted undetermined members of a genus separately from conclusively identified species for estimates of abundance in plots. For estimates of species richness in plots, we counted undetermined individuals as a species only if there were no conclusively identified individuals of that genus in the plot, because we considered it likely that these small undetermined individuals were members of common species. For example, if a plot contained an individual of C. fluctifraga and an individual of an undetermined Chione sp., we counted that plot as containing only a single species of Chione so as not to overestimate richness. However, if a plot contained an individual of an undetermined Chione sp. and no individuals of C. fluctifraga, we counted the undetermined Chione sp. as one species in richness calculations.
For living individuals of the two most abundant species in our samples (Venerupis philippinarum and C. fluctifraga), we measured shell length to the nearest 0.1 mm using digital calipers. Within these species, individuals were grouped into 2 mm size classes for presentation of size distributions.
Data analysis
To determine whether there was evidence of a negative relationship between the abundance of dominant bivalve species and bivalve species richness in our sample plots, we used Kendall rank correlation analysis (because data could not be transformed to meet assumptions for standard correlation analysis). Four of our sampled intertidal plots and seven of our sampled subtidal plots had no living bivalves: these plots were not included in the correlation analysis. Following Zar (2010), we evaluated linear regression models to characterize the relationship between the abundance of dominant species and the abundance of other bivalves and determined that the relationship was best characterized by polynomial (quadratic) regression. Data analysis was performed in JMP version 8.0 (SAS, Inc.).
Results
We found a total of 2,490 living bivalves in our 77 sample plots. None of these were individuals of Mercenaria mercenaria. However, the quahog may not be extinct at Colorado Lagoon—two living individuals were found by chance in the intertidal zone outside of our plots (Fig. 1, stars). If we calculate a maximum density of M. mercenaria using these two living individuals (encountered outside of our sampling plots) and the intertidal area we sampled in our plots, we obtain an estimate of current intertidal quahog abundance at the lagoon of 0.14 quahogs m−2. In addition to these two living quahogs, we found shells or shell fragments of dead M. mercenaria in eight intertidal plots and one subtidal plot.
We observed living representatives of a total of 12 species of bivalves at Colorado Lagoon either in our sample plots or directly outside of them (M. mercenaria, discussed above, and the oyster Crassostrea gigas, observed on an intertidal rock). In addition, living members of two species that we found only as shells were subsequently collected at the lagoon in the summer of 2010 (Chione californiensis and Geukensia demissa; C. Whitcraft, personal communication), bringing the total number of species with living representatives to 14 species (Table 2). Six of these 14 species were NIS: C. gigas, G. demissa, M. mercenaria, Musculista senhousia, Mytilus galloprovincialis, and Venerupis philippinarum. In addition to the species listed in Table 2, we encountered a shell of a tellinid clam, which was too small to identify to the genus level. We also encountered nine species represented only by dead individuals (shells) in our samples (Table 2). In total, pooling living and dead individuals, we found evidence of 23 species of bivalves (from 11 families) at the lagoon.
Living representatives of all of the living species encountered in this study were found in the intertidal zone. The species richness of living bivalves in the intertidal zone ranged from 0 to 5 species 0.25 m−2 plot, with an average of 2.1 species (±1.19 SD, N = 57 plots). Subtidal richness was lower, ranging from 0 to 3 species 0.5 m−2 sample, with an average of 1.05 species (±1.0 SD, N = 20 samples). We found living representatives of a total of 4 species in the subtidal zone; all of these species were also found in intertidal plots.
The spatial distribution of living bivalves in the lagoon was extremely patchy. Total bivalve density in intertidal plots ranged from 0 to 353 individuals 0.25 m−2 plot. Subtidal densities were much lower, ranging from 0 to 20 individuals 0.5 m−2 sample. Intertidal bivalve abundance in the west side of the north arm of the lagoon was dramatically higher than in the south side of the west arm. Average living bivalve abundance in the North Arm Intensive Intertidal Sampling Area was 79.15 individuals per plot (±82.76 SD, N = 20 plots) compared to an average of only 2.42 individuals per plot (±2.37 SD, N = 26 plots) in the West Arm Intensive Intertidal Sampling Area. These differences in abundance between arms of the lagoon were primarily the result of differences in abundance of the non-native clam Venerupis philippinarum (Fig. 2).
Spatial distribution of Venerupis philippinarum in intertidal sample plots at Colorado Lagoon. The abundance of V. philippinarum in plots in the North and West arm intensive intertidal sampling areas is shown graphically, while abundance in extra intertidal plots is represented numerically. Although plots were spaced at random intervals along the West and North arms of the lagoon, they are placed on the y axis at regular intervals for ease of presentation. Note differences in x-axis scales
Non-indigenous species made up 89% of the living bivalves collected in our sample plots (Table 3). The non-native clam V. philippinarum was the most abundant single species, making up 87.6% of the living individuals in our samples. The second most abundant species, the native clam C. fluctifraga, made up only 7.8% of collected individuals. Individuals of another NIS, M. senhousia, made up 1.7% of the total living bivalves in our sample and thus were the third most abundant species.
Venerupis philippinarum, the most abundant species in our surveys, made up on average 66.9% of the living individuals in intertidal plots (±33.09% SD, N = 57 plots) and 57.8% of individuals in subtidal plots (±43.16% SD, N = 20 plots). In the intertidal zone, the abundance of living V. philippinarum was positively correlated with bivalve species richness (Kendall rank correlation, τ = 0.64, p < 0.0001; Fig. 3a). This relationship was not present in the subtidal zone, where overall abundance and species richness were lower (Kendall rank correlation, τ = −0.02, p = 0.95; Fig. 3b). In the intertidal zone, plots with intermediate abundances of living V. philippinarum (65–215 individuals) had the highest abundance of other living bivalves (polynomial [quadratic] fit for relationship between abundance of other bivalves and abundance of V. philippinarum, R2 = 0.62, p < 0.0001; polynomial [quadratic] fit for relationship between abundance of C. fluctifraga and abundance of V. philippinarum, R2 = 0.61, p < 0.0001; Fig. 4).
Relationship between the abundance of Venerupis philippinarum and total bivalve species richness in sampled plots. a Intertidal sample plots (n = 53 plots with living bivalves). b Subtidal sample plots (total n = 13 plots with living bivalves). Four intertidal plots and seven subtidal plots had no living bivalves: These plots are not included in the figure or used in analysis. The abundance of V. philippinarum was positively correlated with species richness in the intertidal zone (Kendall rank correlation τ = 0.64, p < 0.0001) but not in the subtidal zone (τ = −0.02, p = 0.95)
The two most abundant species in our surveys both showed a wide range of individual sizes at Colorado Lagoon. Individuals of V. philippinarum in the intertidal retained in our 6 × 6 mm mesh size sieves ranged in length from 6.1 to 71.9 mm (Fig. 5a). Individuals of V. philippinarum collected in the subtidal zone were smaller, on average, than those collected in the intertidal (subtidal length range 7.8–52.1 mm; Fig. 5a). Subtidal individuals of V. philippinarum smaller than the 25-mm spacing between the tines of the rake were retained in masses of mud or algae captured by the rake. Intertidal specimens of C. fluctifraga retained in our 6 × 6 mm mesh size sieves ranged in length from 6.2 to 49.1 mm (Fig. 5b).
Size distributions of the two most abundant bivalves at Colorado Lagoon. For both species, all living individuals with unbroken shells (including both intertidal and subtidal specimens) were measured. Lengths are presented in 2 mm size classes. a Venerupis philippinarum, n = 2,139 individuals in 57 intertidal and 20 subtidal plots. b Chione fluctifraga, n = 191 individuals in 57 intertidal plots. No unbroken individuals of C. fluctifraga were found in subtidal plots. Note differences in the y-axis scales
Discussion
We found evidence of 23 species of bivalves at Colorado Lagoon, including 14 species with living representatives. Our results are roughly similar to those of the two previous surveys of Colorado Lagoon bivalves, those of Crane et al. (1975) and Murphy (1983, 1985) (see Table 2). Crane et al. (1975) identified living members of nine species at the lagoon, and Murphy (1983) recorded 11 species. Our samples included living members of five species that had not been recorded in either of those publications: Adula diegensis, Cryptomya californica, Musculista senhousia, Tagelus affinis, and Venerupis philippinarum. We also found shell material (dead individuals) of seven species that had not previously been described at the lagoon (Table 2). We have information on the history of only two of these species at the lagoon. Although Donax gouldii, present in our samples only as shell material, was not mentioned in either Crane et al. (1975) or Murphy (1983), living individuals of an unidentified species in the genus were found at the lagoon in 1977 (J. T. Carlton, personal communication). Similarly, shells of a species of Argopecten were found at the site in August 2000 (J. T. Carlton, personal communication), indicating that members of this genus were present in the lagoon before that time.
Conversely, seven species of bivalves previously recorded at the lagoon were not represented by living specimens in our samples. We found shell material, but no living specimens, of Chione undatella, Laevicardium substriatum, Lyonsia californica, and Leukoma laciniata. Three additional species recorded in earlier studies were entirely absent from our samples. No specimens, living or dead, of Laevicardium elatum (recorded by Crane et al. 1975), Psammotreta obesa (Crane et al. 1975; Murphy 1983) or Tellina bodegensis (Murphy 1983) were found in our sample plots. It is possible that these species are now locally extinct in the lagoon; alternatively, they may be present in the lagoon at abundances too low to be captured by our sampling methods.
In total, we found evidence of six NIS currently living in Colorado Lagoon. Three of these had been recorded at the lagoon in previous studies. Mercenaria mercenaria and Geukensia demissa were both reported as present at the lagoon by Crane et al. (1975) and Murphy (1983); G. demissa was also reported by Cohen et al. (2002). The mussel Mytilus galloprovincialis was recorded as present at the site by Cohen et al. (2002) but had not been recorded in the earlier surveys of Crane et al. (1975) or Murphy (1983, 1985). However, the absence of this mussel from species lists in these older papers is most likely a result of nomenclature changes and not evidence of a new invasion. Crane et al. (1975) did report the presence of Mytilus edulis, which was simply the name being used at the time for bay-dwelling Mytilus; southern California bay-dwelling Mytilus were subsequently shown to be Mytilus galloprovincialis (Geller 1999).
Three of the NIS we observed were new records for Colorado Lagoon: C. gigas, M. senhousia, and V. philippinarum. Crassostrea gigas, the Japanese oyster, has been repeatedly introduced to the west coast of North America for commercial purposes (Carlton 1992). The earliest reported introduction in southern California was in 1932 in Newport Bay (Carlton 1979), and the species is now present at numerous southern California coastal sites (J. Burnaford, personal observation). Since the oyster is primarily found on hard substrata, the absence of records for this species from earlier studies of Colorado Lagoon may simply be due to the fact that those previous researchers focused on infaunal bivalves. Cohen et al. (2002) recorded the fauna on floats and bridge supports in the lagoon but did not find C. gigas. Musculista senhousia, native to the NW Pacific, was recorded in southern California in 1964 (Mission Bay; MacDonald 1969) but was first recorded in the Los Angeles/Orange County region around 1977 (Newport Bay; Carlton 1979, 1992). It is possible that M. senhousia did not arrive in Colorado Lagoon until after Murphy’s 1978–1980 surveys. Venerupis philippinarum, an extremely abundant NIS at Colorado Lagoon, is discussed further below.
The decline of Mercenaria and the rise of Venerupis
Two of the species of NIS we observed have undergone profound changes in population size since 1980, causing a major shift in the bivalve assemblage at Colorado Lagoon. First, in the earlier surveys of Crane et al. (1975) and Murphy (1983, 1985), M. mercenaria was found in extraordinary abundance at the site, with an estimated population size of up to four million individuals (Murphy 1983). We did not find any living individuals of M. mercenaria in our 57 intertidal sample plots, many of which were placed in regions which contained densities up to 18 quahogs m−2 in the 1970s. Quahogs typically bury <10 cm below the surface of the sediment (Doering 1976; Roberts et al. 1989), and our plots were excavated to a depth of 20 cm; this should have been more than adequate to recover individuals of all sizes, were they present. Both living individuals of M. mercenaria discovered outside of our sample plots were protruding from the surface of the sediment, clearly visible to persons walking along the shore (T. Parker and B. Pernet, personal communication). Given that these quahogs were apparent to pedestrians, the fact that we did not observe any other specimens of M. mercenaria in the course of our sampling or in repeated visits to the site from 2004 to 2010 indicates that the clam is no longer a major component of the intertidal bivalve assemblage at Colorado Lagoon. Our data also show that the quahog has declined in abundance in the subtidal zone. Murphy (1983) reported that quahogs comprised approximately 50% of individuals in the subtidal zone, yet we did not find any living individuals of M. mercenaria in our shallow subtidal samples, even those taken in an area reported by Crane et al. (1975) as having densities of M. mercenaria of 556 m−2. We did not sample the deepest subtidal areas, but there is no evidence to suggest that they contain high abundances of the quahog. In 2004, a small-scale study at the site included nine samples (cores 10 cm in diameter, 10 cm depth) from deep subtidal sediments; no specimens of M. mercenaria were collected in these cores (Chambers Group 2004). Further observations suggest that the quahog had declined in abundance by 2000, as searches of shell debris around the lagoon perimeter in that year did not lead to the discovery of any shells of M. mercenaria, in contrast to similar observations in the late 1970s when quahog shells were frequently found washed up on the shore (J.T. Carlton, personal communication).
The smaller of the two living individuals of M. mercenaria that we recovered at Colorado Lagoon measured 7.83 cm in shell length. Using shell growth increment analysis, and taking into account estimated water temperature and exposure times for the intertidal location at which this clam was recovered, it was determined to be 9 years old at capture (J. Harding, personal communication; SBMNH 149505). The other living quahog measured 8.9 cm. Although we were unable to obtain an age estimate for this second individual, it seems reasonable based on its size to conclude that it was at least 9 years old. Of the few shells of M. mercenaria that we recovered in our plots, none retained any tissue remnants, but one articulated shell with a partially intact hinge ligament was discovered in an intertidal plot. This individual was determined to be 4 years old when it died (J. Harding, personal communication; SBMNH 149506). Ligaments can persist in shells up to 5 years postmortem (J. Harding, personal communication). If the two living specimens we collected were descendants of the large quahog population present in the lagoon in 1970, these limited data suggest that the last successful recruitment event for M. mercenaria at Colorado Lagoon may have occurred somewhere around the year 2000. Given the 1978–1980 surveys in which M. mercenaria was reported at high densities in the lagoon (Murphy 1985), the above inference on recruitment, and the fact that Cohen et al. (2002) and J.T. Carlton (personal communication) did not observe quahogs or their shells in the intertidal zone during a rapid survey for invasive species in 2000, we conclude that a major decline in quahog abundance at the lagoon occurred between 1980 and 2000. This decline—from estimated densities of >500 quahogs m−2 in the early 1970s (Crane et al. 1975) to our conservative estimate of 0.14 quahogs m−2 in 2009–2010—clearly qualifies as a “population collapse” in the sense of Simberloff and Gibbons (2004): a decline of 90% or more in abundance or density within a period of 30 years.
It is also possible that instead of simply experiencing a population collapse, the quahog population actually went extinct between 1980 and 2000, and the two living specimens we observed were introduced to the lagoon more recently. Isolated specimens of M. mercenaria have been occasionally found in southern California (e.g., Hertz and Hertz 1992), presumably the result of discarding or intentional introduction. In a cursory survey in February 2011, we identified six large grocery stores selling living M. mercenaria (as “eastern littlenecks”) within a 5 km radius of the lagoon (unpublished data). Therefore, living M. mercenaria are widely available in the area, and specimens could have been released in the lagoon either purposefully or accidentally.
In Colorado Lagoon, the once dominant NIS M. mercenaria has been replaced by a new NIS: the Japanese littleneck V. philippinarum. Venerupis philippinarum is by far the most abundant bivalve in soft sediments in the lagoon, making up 87.6% of the total bivalve individuals we collected in this study. In some areas of the lagoon, especially in the north arm (Fig. 2), it occurs at densities of up to 220 clams 0.25 m−2, far higher than that of any other species we observed in this study.
This represents a rather rapid transition, as V. philippinarum was not recorded at this site—or indeed, in southern California at all—until quite recently. V. philippinarum is thought to have been introduced to the northern Pacific coast of North America in the mid 1930s as a byproduct of the introduction of Japanese oysters (Carlton 1992). The range of the clam extended south along the Pacific coast in the years following the introduction, most likely through a combination of natural dispersal and intentional plantings (Carlton 1992). In 1953, two thousand individuals of V. philippinarum were intentionally planted in Newport Bay (J. T. Carlton, personal communication), but the planting did not appear to be successful in generating a self-sustaining population. After a gap of many years, it appears that the clam again reached southern California in the mid 1990s; the earliest record we could uncover for V. philippinarum in southern California is that of a single individual collected in Newport Bay, Orange County, in 1994 (Le Page 1996). At Colorado Lagoon, no living individuals or shells of V. philippinarum were noticed by researchers who examined the sediment surface in 2000, but by March 2003, shells of V. philippinarum were abundant on the surface of the sediment and live individuals were observed being consumed by gulls at the site (J. T. Carlton, personal communication). Therefore, from our limited data, it appears that the arrival and increase in abundance of V. philippinarum at Colorado Lagoon occurred between 2000 and 2003.
What were the causes of this transition in bivalve assemblages?
The major differences between the bivalve assemblages of Colorado Lagoon in 1970–1980 and 2009–2010 are the absence of M. mercenaria, and the appearance and explosion in abundance of V. philippinarum. What might have caused this major transition? Though we cannot determine cause from our observational study, we can make inferences about several factors that might have contributed to these changes.
An obvious potential explanation is competition between the two species. However, even given the coarse temporal resolution of the historical record of clam abundances at the site, this appears unlikely. Studies of other sites with high densities of the Manila clam have failed to find negative effects of V. philippinarum on coexisting bivalve species (Byers 2005; Whiteley and Bendell-Young 2007). Similarly, our data from Colorado Lagoon do not suggest strong negative effects of V. philippinarum on the species richness or abundance of other bivalves (Figs. 3, 4). As described above, we infer that the major decline in the population of M. mercenaria occurred sometime between 1980 and 2000, while the major expansion of the population of V. philippinarum occurred sometime between 2000 and 2003. It is possible that the decline in M. mercenaria in Colorado Lagoon permitted V. philippinarum to invade this suddenly “open” habitat. Overall, it seems likely that V. philippinarum arrived at the lagoon and increased in abundance as the population of M. mercenaria was already in decline.
A variety of factors other than competition with V. philippinarum might have led to the decline in the population of M. mercenaria at Colorado Lagoon after 1980. However, none of these potential causes stand out as likely causes of the decline. Harvesting by humans has been implicated in the decline of M. mercenaria populations in locations on the US Atlantic Coast (Peterson 2002). Humans do remove clams from the intertidal zone at Colorado Lagoon: Crane et al. (1975) reported up to 4,000 clams removed per hour in the intertidal zone in the winter of 1970–1971. However, while harvesting might have played a role in reducing the abundance of intertidal M. mercenaria through the 1980s or 1990s, it does not seem a likely explanation for the massive decline in abundance in the subtidal zone at the site.
Non-human predators might also have contributed to the quahog’s decline. The only published comprehensive studies of fishes at Colorado Lagoon are those of Allen and Horn (1975) and Allen (1976). Of the 33 fish species recorded in these studies, three were potential lethal predators of juvenile or adult M. mercenaria: shovelnose guitarfish, bat rays, and spotted sand bass (C. Lowe, personal communication). Six more species might be non-lethal predators (siphon nippers: round stingrays, staghorn sculpins, spotfin croaker, pile surfperch, California corbina, and diamond turbot), which could have negative effects on clam growth and reproduction. These studies were conducted in 1973, when M. mercenaria was very abundant in the lagoon. Surveys conducted in 2009 and 2010 (C. Whitcraft, unpublished data) suggest that the existing fish assemblage in the lagoon is very similar in species composition to the assemblage reported in those earlier studies. It seems unlikely that any of these species increased in abundance in the intervening time period enough to cause the collapse of the quahog population.
Heavy cover of benthic seaweeds (Gracilaria and Ulva/Enteromorpha) has been linked to low densities of M. mercenaria in East Coast waterways (Tyler 2007), presumably because dense mats of the algae restrict bivalve access to the overlying water. Seaweeds in both of these genera were present at Colorado Lagoon during our study, and our observations suggest that their cover in the intertidal and subtidal zone of the lagoon varies over space and time. However, both Crane et al. (1975) and Murphy (1983) mentioned similar variation in the abundance of these seaweeds at the site during time periods when quahogs were extremely abundant.
Finally, it is possible that fluctuations in habitat quality in the lagoon might have led to the decimation of the quahog population. Water column salinities lower than 15 ppt or higher than 32 ppt and dissolved oxygen levels below 5 mg l−1 or above 100% saturation have been shown to have substantial negative effects on quahogs (Grizzle et al. 2001). These parameters have certainly varied outside of these ranges in Colorado Lagoon (Crane et al. 1975; Chambers Group 2004). Previous studies have shown that anoxic sediments support only low densities of M. mercenaria (Mann et al. 2005). We qualitatively noted anoxic conditions just below the sediment surface in many of our intertidal and subtidal study plots; these observations were supported by quantitative intertidal measurements in the north arm of the lagoon showing strongly negative redox potential (C. Whitcraft, unpublished data). Mercenaria mercenaria at this site may have been affected by these water or sediment quality parameters or by major human-related environmental events like sewage spills (e.g., Crane et al. 1975).
Given the 30-year gap between comprehensive studies at Colorado Lagoon, we cannot determine what factors are responsible for the decline in M. mercenaria; all or none of them may have contributed to the population collapse we document here. Whether the decline in the quahog was gradual or catastrophic (e.g., due to a mass mortality event), it seems unlikely that the population will be able to recover. All evidence suggests that the original Alamitos Bay population of M. mercenaria was extinct by the mid 1980s, if not earlier (Crane et al. 1975; Murphy 1985). There are no other known populations of M. mercenaria on the North American Pacific coast (Carlton 1992, 1999; Chew 2001). Therefore, there are no known sources that could supply larvae for re-colonization of Colorado Lagoon. Allee effects are known to reduce fertilization success in low-density populations of free-spawning marine invertebrates like M. mercenaria (Levitan 1995), which suggests that any remaining individuals in the lagoon are unlikely to have high enough reproductive success to allow an increase in population abundance.
Conclusions
The population of M. mercenaria at Colorado Lagoon thus joins the ranks of NIS populations that have collapsed from undetermined causes, which make up 58% of the examples cited by Simberloff and Gibbons (2004). Developing a better understanding of the causes of these major population changes will require both the accumulation of long-term data describing the patterns of change in population and community structure over time and focused experimental manipulations to clarify their underlying mechanisms.
Though the population of M. mercenaria in Colorado Lagoon has collapsed, the site remains heavily dominated by NIS, and the native bivalve community has not recovered. Native species made up less than 11% of the individual bivalves we collected at the site; instead, the NIS V. philippinarum and M. senhousia have replaced quahogs as the most abundant bivalves in the system. Though these two “new” residents of the lagoon may experience many of the same environmental and biological factors that presumably contributed to the collapse of the population of M. mercenaria, neither seems to be at much risk of long-term population collapse at the site. Both occur in large populations in other parts of Alamitos Bay and many other bays in southern California (Ranasinghe et al. 2005; J. Burnaford, S. Henderson, and B. Pernet, personal observation), and both have planktonic, dispersing larval stages that would likely rapidly re-colonize Colorado Lagoon should local populations decline.
References
Allen LG (1976) Additions to the list of fish species known from Alamitos Bay, California, based on studies in Colorado Lagoon. Calif Fish and Game 62:310–314
Allen LG, Horn MH (1975) Abundance, diversity and seasonality of fishes in Colorado Lagoon, Alamitos Bay, California. Estuar Coast Mar Sci 3:371–380
Byers JE (2005) Marine reserves enhance abundance but not competitive impacts of a harvested nonindigenous species. Ecology 86:487–500
Carlton JT (1979) History, biogeography, and ecology of the introduced marine and estuarine invertebrates of the Pacific coast of North America. Ph.D. dissertation, University of California, Davis
Carlton JT (1992) Introduced marine and estuarine mollusks of North America: an end-of-the-20th-century perspective. J Shellf Res 11:489–505
Carlton JT (1999) Molluscan invasions in marine and estuarine communities. Malacologia 41:439–454
Chambers Group (2004) Habitat assessment for the Colorado Lagoon restoration feasibility study for the City of Long Beach. Prepared for Moffatt and Nichol. p 25. Downloaded from http://www.longbeach.gov/news/displaynews.asp?NewsID=2179
Chew KK (2001) Introduction of the hard clam (Mercenaria mercenaria) to the Pacific Coast of North America with notes on its introduction to Puerto Rico, England, and France. In: Kraueter JN, Castagna M (eds) Biology of the Hard Clam. Elsevier Science, New York, pp 701–709
Coan EV, Valentich-Scott P (2007) Bivalvia. In: Carlton JT (ed) The Light and Smith Manual, 4th edn. UC Press, Berkeley, pp 807–859
Coan EV, Valentich-Scott P, Bernard FR (2000) Bivalve Seashells of Western North America. Santa Barbara Museum of Natural History, Santa Barbara
Cohen AN, Harris LH, Bingham BL, Carlton JT, Chapman JW, Lambert CC, Lambert G, Ljubenkov JC, Murray SN, Rao LC, Reardon K, Schwindt E (2002) Project report for the Southern California exotics expedition 2000, a rapid assessment survey of exotic species in sheltered coastal waters. Report prepared for CA department of fish and game, state water resources control board, and natl fish and wildlife foundation, p 23
Crane JM, Allen LG, Eisemann C (1975) Growth rate, distribution, and population density of the northern quahog Mercenaria mercenaria in Long Beach, California. Calif Fish and Game 61:68–81 (errata 61:261)
Davis MA (2009) Invasion biology. Oxford University Press Inc., New York, p 244
Doering PH (1976) A burrowing response of Mercenaria mercenaria (Linnaeus, 1758) elicited by Asterias forbesi (Desor, 1848). The Veliger 19:167–175
FAO (2010). Fishery and Aquaculture Statistics Capture Production 2008 Yearbook. 590 pp. Downloaded from ftp://ftp.fao.org/FI/CDrom/CD_yearbook_2008/navigation/index_content_capture_e.htm
FAO (2011) Cultured aquatic species information programme. Mercenaria mercenaria. Cultured aquatic species information programme. Rome. FAO© 2004–2011. Downloaded 24 March 2011 from http://www.fao.org/fishery/culturedspecies/Mercenaria_mercenaria/en
Forsyth R (1997) An introduction to introduced marine molluscs in the Pacific Northwest. Of Sea and Shore 19:188–190, 202
Forsyth RG, Forsyth TJ (2001) A note on Mercenaria mercenaria in British Columbia, Canada. Festivus 33:85
Geller JB (1999) Decline of a native mussel masked by sibling species invasion. Conserv Biol 13:661–664
Gillespie GE (2007) Distribution of non-indigenous intertidal species on the Pacific Coast of Canada. Nippon Suisan Gakkaishi 73:1133–1137
Gillespie GE, Parker M, Merilees W (1999) Distribution, abundance, biology and fisheries potential of the exotic varnish clam (Nuttallia obscurata) in British Columbia. Canadian Stock Assessment Secretariat Research Document 99/193
Grizzle RE, Bricelj VM, Shumway SE (2001) Physiological ecology of Mercenaria mercenaria. In: Kraueter JN, Castagna M (eds) Biology of the hard clam. Elsevier Science, New York, pp 305–382
Hertz J, Hertz CM (1992) Unusual finds at Mission Bay, San Diego. Festivus 24:61–62
Le Page SD (1996) Spatial distribution and temporal fluctuations of bivalve assemblages in Newport Bay, California. M.S. thesis, California State University, Fullerton
Levitan DR (1995) The ecology of fertilization in free-spawning invertebrates. In: McEdward LR (ed) Ecology of marine invertebrate larvae. CRC Press, Boca Raton, pp 123–156
MacDonald KB (1969) Quantitative studies of salt marsh mollusc faunas from the North American Pacific coast. Ecol Mon 39:33–60
Mann R, Harding JM, Southworth MJ, Wesson JA (2005) Northern quahog (hard clam) Mercenaria mercenaria abundance and habitat use in Chesapeake Bay. J Shellf Res 24:509–516
Mikkelsen PM, Bieler R (2008) Seashells of southern Florida: living marine mollusks of the Florida Keys and adjacent regions: bivalves. Princeton University Press, Princeton, p 503
Moffatt and Nichol (2004) Tidal and flood hydraulics study. Prepared for the City of Long Beach, p 25. Downloaded from http://www.longbeach.gov/news/displaynews.asp?NewsID=2179
Moffatt and Nichol (2010) Alternatives analysis report, Phase 2 study, Colorado Lagoon Restoration Project. Prepared in association with WRA and Blue Line Consulting for the City of Long Beach and Port of Long Beach, p 88. Downloaded from http://www.longbeach.gov/cd/property_services/project_development/colorado.asp
Murphy RC (1983) The introduced bivalve, Mercenaria mercenaria, in a shallow coastal ecosystem: 1) factors affecting its distribution, and 2) contribution to benthic community metabolism. Ph.D. dissertation, University of Southern California
Murphy RC (1985) Factors affecting the distribution of the introduced bivalve, Mercenaria mercenaria, in a California lagoon—the importance of bioturbation. J Mar Res 43:673–692
Murphy RC, Kremer JN (1985) Bivalve contribution to benthic metabolism in a California lagoon. Estuaries 8:330–341
Peterson CH (2002) Recruitment overfishing in a bivalve mollusc fishery: hard clams (Mercenaria mercenaria) in North Carolina. Can J Fish Aquat Sci 59:96–104
Ranasinghe JA, Mikel TK, Velarde RG, Weisberg SB, Montagne DE, Cadien DB, Dalkey A (2005) The prevalence of non-indigenous species in southern California embayments and their effects on benthic macroinvertebrate communities. Biol Inv 7:679–686
Roberts D, Rittschof D, Gerhart DJ, Schmidt AR, Hill LG (1989) Vertical migration of the clam Mercenaria mercenaria (L.) (Mollusca:Bivalvia): environmental correlates and ecological significance. J Exp Mar Biol Ecol 126:271–280
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Ann Rev Ecol Syst 32:305–332
Salchak A, Haas J (1971) Occurrence of the northern quahog, Mercenaria mercenaria, in Colorado Lagoon, Long Beach, California. Calif Fish and Game 57:126–128
Simberloff D, Gibbons L (2004) Now you see them, now you don’t!—population crashes of established introduced species. Biol Inv 6:161–172
Turgeon DD, Quinn JF, Bogan AE, Coan EV, Hochberg FG, Lyons WG, Mikkelsen PM, Neves RJ, Roper CFE, Rosenberg G, Roth B, Scheltema A, Thompson FG, Vecchione M, Williams JD (1998) Common and scientific names of aquatic invertebrates from the United States and Canada: Mollusks. Second edition. American Fisheries Society special publication 26. American Malacological Union, Bethesda, p 526
Tyler RM (2007) Effects of coverage by benthic seaweed mats on (northern quahog = hard clam) Mercenaria mercenaria in a eutrophic estuary. J Shellf Res 26:1021–1028
Whiteley J, Bendell-Young L (2007) Ecological implications of intertidal mariculture: observed differences in bivalve community structure between farm and reference sites. J Appl Ecol 44:495–505
Williams SL, Grosholz ED (2008) The invasive species challenge in estuarine and coastal environments: marrying management and science. Estuaries Coasts: J CERF 31:3–20
Zar JH (2010) Biostatistical analysis, 5th edn. Pearson Prentice Hall, New Jersey, p 944
Acknowledgments
We thank T. Parker and E. Zahn for facilitating our work at Colorado Lagoon and for answering many questions about the history and management of the lagoon. Intertidal surveys would not have been possible without the help of numerous volunteers from CSU Fullerton, CSU Long Beach, and the residential neighborhood surrounding Colorado Lagoon. We are grateful to Dr. P. Valentich-Scott for verifying identifications, Dr. J. Harding for estimating the ages of two specimens of Mercenaria mercenaria, and T. Vu and L. Cano for measuring many clams. Drs. J. Carlton, C. Lowe, D. Reish, and C. Whitcraft provided much appreciated information about the site and its inhabitants. Drs. J. Carlton, F. Bulleri, C. Whitcraft, and an anonymous reviewer made very helpful comments on an earlier draft of the paper. This work was partially supported by the Departments of Biological Science of both CSU Fullerton and CSU Long Beach.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bulleri.
Rights and permissions
About this article
Cite this article
Burnaford, J.L., Henderson, S.Y. & Pernet, B. Assemblage shift following population collapse of a non-indigenous bivalve in an urban lagoon. Mar Biol 158, 1915–1927 (2011). https://doi.org/10.1007/s00227-011-1703-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1703-x