Abstract
Capelin is a planktivorous key fish species in many subarctic ecosystems representing the link between lower trophic levels and apex predators. Little is known, however, of capelin in Greenland waters, but it has been suggested that size and growth increases due to difference in food availability and temperature conditions along a 1,500 km south–north gradient on the west coast. It is presently unknown how the qualitative state of capelin energy content is affected along this gradient. Based on 2007 and 2008 samples, we show that energy content increases with capelin length in both spawning and non-spawning fish and that it varies with latitude in spawning fish along West Greenland (60–71°N). Combining our results on energy content with information on capelin growth along the same latitudinal climate gradient demonstrates that less and lower-quality food is available to capelin predators in the south than in the north.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Capelin (Mallotus villosus, Osmeridae) is a planktivorous fish with a northern circumpolar distribution. Similar to other planktivorous species in the “wasp-waist species” guild (i.e., sardines, anchovy, sprat, Cury et al. 2000; Bakun 2006) capelin have a life cycle characterized by a short lifespan, high mobility, high energetic need (as a result of their numbers and high growth rates), and variable cohort strength (Frank and Carscadden 1989; Gjøsæter et al. 2002; Vilhjalmsson 2002). These characteristics can result in a fluctuating population biomass on a short time scale as demonstrated for both capelin (Gjøsæter 1998; Vilhjalmsson 2002) and sardine–anchovy systems across world oceans (Schwartzlose et al. 1999).
In Greenland, capelin is found on both the east and the west coast extending north to 73°N and 66°N, respectively (Friis-Rødel and Kanneworff 2002). On the west coast, this covers a 1,500 km unbroken latitudinal gradient making capelin in this region a perfect candidate for studying the effects of changes in environmental and climatic conditions. Capelin sensitivity to environmental changes is evident both historically and presently. In the relatively warm 1930 s, capelin distribution extended as far north as 76°N and at the same time showed reduced abundance in southern Greenland (Jensen 1939). Currently, sustained spawning is limited to the area south of 72°N. More recently, changes in life history characteristics such as growth and feeding behavior along the distributional range of capelin have been shown (Hedeholm et al. 2010, Hedeholm et al., submitted manuscript), underlining the applicability of capelin in this region as a model species for studying the effect of (future) environmental changes (Anonymous 2005) and latitudinal differences in vital life history characteristics such as fecundity, energy allocation, condition, and productivity.
Ecosystem-wide impacts of capelin, and wasp-waist species in general, have been shown theoretically (Rice 1995; Jordan et al. 2005), and the effect of a capelin-deprived diet has been shown for Canadian cod (Rose and O’Driscoll 2002) and in relation to the junk-food hypothesis in sea birds (Romano et al. 2006). In Greenland waters, the importance of capelin as prey has been demonstrated in marine mammals (Angantyr and Kapel 1990; Neve 2000), cod (Gadus morhua, Nielsen and Andersen 2001), Greenland halibut (Reinhardtius hippoglossoides, Woll and Gundersen 2004), and sea birds (Falk and Durinck 1993; Phillips et al. 1999). In all cases, capelin constituted a substantial part of the diet. Due to its lipid content, capelin has a high-energy density compared to other species (Montevecchi and Piatt 1984; Lawson et al. 1998), and thus the importance of capelin is related not only to abundance but also to tissue composition, which determines the nutritional value of this important prey species. However, the energy density and possible latitudinal trends for Greenland capelin are unknown, and satisfactory energy flow calculations including this key species cannot be developed.
In this study, we provide the first area-specific data on energetic densities, fecundity, and reproductive investment for West Greenland capelin. As stomach content and growth rates have been shown to increase with latitude in this area (Hedeholm et al. 2010, Hedeholm et al., submitted manuscript) two scenarios are possible: (1) either the observed increase in food intake is all used for growth; or (2) there is an increase in the energetic storage as well. Hence, as a null hypothesis for non-spawning capelin, we suggest that there is no increase in energy density with increasing latitude and thus no relationship between latitude and tissue energy density. In spawning fish, all accessible tissue energy will be converted to gonads as Greenland capelin are most likely semelparous (Friis-Rødel and Kanneworff 2002). Consequently, tissue energy density should not vary with latitude but be markedly reduced relative to non-spawning individuals; a non-trivial point in constructing biomass-based energetic models. Knowledge of Greenland capelin is scarce, and the present analysis will be useful in modeling (Fiksen et al. 1995). Additionally, if capelin displays a latitudinal gradient in nutritional value similar to those shown for growth, the impact of future climate changes may be more pronounced than previously thought.
Materials and methods
Sampling
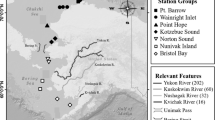
Capelin were collected during 2007 and 2008 along the west coast of Greenland (Fig. 1, Tables 1 and 2). Non-spawning capelin were collected in both years by RV “Paamiut” during yearly shrimp and fish surveys conducted by the Greenland Institute of Natural Resources. This stratified random bottom-trawl survey covered the continental shelf from the southern tip of Greenland to 72°N on the west coast. The survey was done using a bottom trawl with a vertical opening of 14 m and a towing speed of 2.5 knots. The trawl was not aimed at capelin, but these were occasionally caught, most likely during trawl haul in. All capelin caught were frozen immediately after capture. At stations where the catch exceeded 150 individuals, a random subsample of 50 individuals were taken. Spawning capelin were only sampled in 2007. The samples were collected during beach spawning events in five general areas spanning 1,300 km along the Greenland West coast: Qaqortoq (61°N), Nuuk (64°N), Sisimiut (66°N), Qeqertarsuaq (69°N), and Uummannaq (71°N, Fig. 1). Females were, however, only caught in the Nuuk area and unless specifically mentioned, all comparisons are based on male capelin. Samples of both spawning and non-spawning capelin were frozen at −20°C immediately after capture.
The southern part of Greenland with the study areas marked. The arrows mark areas where spawning capelin were sampled in 2007. From south to north, these are Qaqortoq, Nuuk, Sisimiut, Qeqertarsuaq, and Uummannaq. The circles mark the offshore areas where non-spawning capelin were sampled in 2007 and 2008
Physical measurements and fecundity
Capelin were measured to the nearest mm below (fork length) and weighed to the nearest 0.1 g. No correction was made for shrinkage due to freezing. All spawning fish were sexed based on morphological characteristics. This was not done for non-spawning fish as sexes appear similar prior to spawning. Ovaries were removed from spawning females, blotted dry, and weighed to the nearest 0.1 g. Fish that had released eggs into the body cavity were excluded from analysis as some eggs may already have been spawned.
About 20–60 eggs from the posterior part of the right ovary were weighed and counted, as the left ovary is known to be underdeveloped (Winters 1971). Based on this and total ovary wet weight, absolute fecundity was calculated assuming that no difference existed between various parts of the ovary (Huse and Gjøsæter 1997). The gonadosomatic index (GSI) was calculated as the proportional wet mass of ovaries in relation to total fish wet weight.
Energy measurements
Muscle tissue (mean ± SE = 1.09 ± 0.03 g) was removed dorsally from both sides of the fish posterior to the dorsal fin to ensure that no bones were present. All skin was subsequently removed. Muscle tissue from all fish and ovaries from spawning females were freeze dried for 24 h after which no further weight loss took place. The tissue samples were weighed (dry mass, dw), and the water content was calculated (% of wet mass, ww). Prior to any further analysis, the samples were kept in an exsiccator over a dry silica gel. The tissue samples were homogenized to a fine powder using a glass mortar and compressed into pellets of at least 25 mg. The energy density of the pellets was determined using an 1109a semimicro bomb and Parr 6725 bomb calorimeter (Parr Instruments, Moline, USA). The bomb was calibrated with benzoic acid (energy density: 26.454 kJ g−1) with regular intervals, and triplicates of randomly chosen samples demonstrated low variation between samples (<2%). Energy densities differing to a large extent from other values were reanalyzed. Energy density is reported for both wet (kJ g ww−1) and dry samples (kJ g dw−1) with the latter used in most analyses. From an ecological perspective, the wet-mass values may be more relevant (Anthony et al. 2000) but do not form the main focus of this study as energy density expressed on a dry weight basis is more appropriate for comparative purposes, as the variation caused by desiccation is minimized (Hislop et al. 1991). Additionally, wet-mass values are easily calculated by multiplying dry-mass-energy density (kJ g dw−1) by the proportion of dry mass to wet mass. Freeze-dried muscle tissue from five fish was incinerated for 24 h at 550°C to determine the ash content of the samples, and energy density was calculated based on these values (kJ g ash free dw−1). Samples from ovaries were analyzed using the same procedure.
Lastly, data on water content, energy density, and fecundity enabled calculation of individual egg energy content and, consequently, calculation of total energetic investment of the individual female. The relative reproductive investment of each female was calculated as the amount of energy in the ovaries relative to total fish energy content (ovaries excluded), under the assumption that the muscle energy density was representative of the entire fish.
Analysis
Spawning and non-spawning fish were analyzed separately. All fish were assigned to the nearest 0.5 latitudinal degree and to one of five locations: Qaqortoq (60–61°N), Nuuk (63–64°N), Sisimiut (66–67°N), Qeqertarsuaq (68.5–70°N), and Uummannaq (71–72°N, see Fig. 1) for ANOVA-based tests. However, Sisimiut was excluded from most statistical analyses concerning non-spawning fish, as few fish were caught in this area. Analyses on spawning fish only include males unless stated otherwise, as spawning females were sampled only in Nuuk. All analyses were carried out using SPSS 18.0. Standard parametric tests were preceded by tests for assumptions. When these were violated, the data were either transformed or non-parametric statistics were applied.
Results
Energy densities of 259 fish (124 spawning and 135 non-spawning) and 18 female ovaries were determined (Table 2). As no difference in energy density (kJ g dw−1) was found between 2007 and 2008 for spawning fish (ANCOVA, F(1,128) = 0.01, P = 0.94), irrespective of both size and capture location, the 2 years were pooled.
Latitudinal and length effect
In non-spawning capelin, there was a significant effect of both area (ANCOVA, F(3,122) = 3.57, P = 0.016) and length (ANCOVA, F(1,122) = 26.90, P < 0.0005) on energy density but also a significant interaction between the two (ANCOVA, F(3,122) = 4.08, P = 0.008, Fig. 2). The interaction was caused by Qeqertarsuaq and removing this area from the analysis yielded non-significant interaction and area terms (ANCOVA, F(2,98) = 2.11, P = 0.127 and F(1,98) = 34.77, P = 0.172, respectively). Using the parameter estimates (following model reduction), the overall length effect and difference between the three similar areas (Qaqortoq, Nuuk, and Uummannaq) and Qeqertarsuaq can be depicted as shown in Fig. 3. A clear positive relationship between length and energy density was seen for the pooled areas, while size effects in the Qeqertarsuaq area were positive but insignificant.
Mean energy density (kJ g dw−1) of spawning (open circles) and non-spawning (filled circles) capelin caught in 2007 and 2008 along the Greenland west coast at five locations (Nspawners, Nnon-spawners): Qaqortoq (6, 41), Nuuk (31, 39), Sisimiut (22, 5), Qeqertarsuaq (14, 26), and Uummannaq (21, 24). All areas are shown separately and combined. The combined values are unweighted averages. The lines in the combined graph represent linear regression of energy density on length for both spawning (dashed, r2 = 0.58, P = 0.03, disregarding the [110–115] category (see text)) and non-spawning (solid, r2 = 0.67, P < 0.0005). Bars represent standard error
The predicted relationship and 95% confidence intervals between energy density (kJ g dw−1) and size in non-spawning capelin from Greenland based on model parameter estimates (see text). The areas that did not differ statistically are combined (Qaqortoq, Nuuk, and Uummannaq, solid lines), while Qeqertarsuaq is depicted alone (dashed lines). Parameter estimates are (y = a*x + b): a = 0.025; b = 18.015 and a = 0.004; b = 20.571 for combined areas and Qeqertarsuaq, respectively
No length effect was seen in the individual areas in spawning capelin (linear regression, P ≥ 0.08). However, combining areas and thereby increasing the length span gave a significant effect of length (linear regression, r2 = 0.58, F1,16 = 8.24, P = 0.028) under the assumption that the single outlier in the [110–115] category can be disregarded. This was done as this point was based on a single fish from Nuuk. The data did not allow parametric statistics in area comparisons. Disregarding length, all but one of ten (Nuuk and Uummannaq, P = 0.89) intergroup comparisons were significant (Mann–Whitney, P ≤ 0.034), but the most noticeable distinction was between high values at Qeqertarsuaq and low values at Qaqortoq (medians = 21.89 and 20.83 kJ g dw−1, respectively, Fig. 4).
Spawners vs. non-spawners
Irrespective of size and location, a mean difference of 0.286 kJ g dw−1 was found in energy density (based on dry weight, kJ g dw−1) between spawners and non-spawners (ANCOVA, F(1,248) = 248.36, P < 0.0005, Fig. 2). However, when comparisons were based on wet mass (kJ g ww−1), the non-spawning fish had energy densities ranging from 4 to 4.5 kJ g ww−1 (mean ± SD = 4.32 ± 0.29 kJ g ww−1), whereas spawning fish had much lower densities (mean ± SD = 3.68 ± 0.28 kJ g ww−1, Fig. 5). The pattern was seen for all individual areas but most clearly in Uummannaq with a difference of more than 1 kJ g ww−1 in comparable weight groups. This discrepancy between dry- and wet-mass-based comparison was caused by a significant difference in water content between spawners and non-spawners, irrespective of length (mean ± SD = 82.7 ± 1.1 and 79.4 ± 1.0%, respectively, ANCOVA, F(1,199) = 452.71, P < 0.0005).
Mean energy density (kJ g ww−1) of spawning (open circles) and non-spawning (closed circles) capelin caught in 2007 and 2008 along the Greenland west coast at five locations (Nspawners, Nnon-spawners): Qaqortoq (6, 41), Nuuk (61, 42), Sisimiut (22, 5), Qeqertarsuaq (14, 26), and Uummannaq (21, 24). All areas are shown separately and combined. The combined values are unweighted averages. Bars represent standard error
Temporal effect
Because samples were taken in the summer feeding period, time-of-capture could possibly affect the results, as energetic reserves can be expected to build during summer months, thus increasing energy density. In general, for all non-spawning fish, there was no effect of time-of-capture on energy density over a period of 59 days (Spearman’s correlation, rs = −0.09, N = 135, P = 0.285). In the individual areas, the sampling time span enabled such analyses in three cases (Nuuk, Qaqortoq, and Qeqertarsuaq). In the latter two no effect of time-of-capture was seen (P ≥ 0.40), while a weak positive correlation was seen in Nuuk (Spearman’s correlation, rs = 0.900, N = 39, P = 0.037). Here, sampling took place over 14 days starting from 29 July and showed an average increase from 20.6 to 21.6 kJ g dw−1.
In spawning capelin, a temporal development in energy density was seen in the Nuuk area, which was the only area where such an analysis was possible as sampling took place over a period of 33 days. The energy density decreased from 22.37 to 21.24 kJ g dw−1 (ANCOVA, F(4,25) = 5.04, P = 0.004), while no change in size was seen during the same period (ANCOVA, F(1,25) = 1.28, P = 0.27).
Sex effect
A test of differences in energy density between sexes in spawning capelin was restricted to Nuuk, as spawning females were absent in samples from other areas. In general, energy density increased with size (kJ g dw−1, ANCOVA, F(1,57) = 19.39, P < 0.0005, Fig. 6). A difference was also found between the sexes (ANCOVA, F(1,57) = 4.89, P = 0.031), but a significant interaction (ANCOVA, F(1,57) = 5.37, P = 0.024) made comparisons across the sampled length range ambiguous. Within the overlapping size range (115–132 mm), no difference was found between sexes when pooling all individuals based on both dry (kJ g dw−1, Student’s t-test, t20.25 = −0.21, P = 0.83) and wet mass (kJ g ww−1, Student’s t-test, t30 = 0.11, P = 0.91).
Fecundity and reproductive investment
Absolute fecundity increased with gutted mass (range: 3,745–11,317 eggs, regression, r2 = 0.69, F1,16 = 35.955, P < 0.0001), and the relationship was best described as: Fecundity = 356.63 * gutted mass1.4414 (Fig. 7). The female GSI was 21.56 ± 5.98%, and on average the female ovaries contained (mean ± SD) 37.8 ± 3.3% of total female capelin energy with the mean (mean ± SD) egg energy density being 24.80 ± 0.25 kJ g dw−1. The reproductive investment was positively related to gutted mass (linear regression, r2 = 0.36, F1,16 = 8.91, P = 0.009, Fig. 7) as was GSI (linear regression, r2 = 0.35, F1,16 = 19.18, P < 0.0005, data not shown). The individual egg energy content was (mean ± SD) 2.67 ± 0.36 J, but was not related to fish gutted mass (Spearman’s correlation, rs = 0.18, N = 18, P = 0.486).
Top fecundity of female capelin from Nuuk fjord (64°N) as a function of gutted mass (N = 18). The line represents power regression (Fecundity = 356.63 *gutted mass1.4414, r2 = 0.69, P < 0.0001). Bottom the relative reproductive investment (%) measured as total ovary energy relative to total fish energy content (ovaries excluded). The line represents linear regression (Reproductive investment = 28.98 + 1.24 *gutted mass, r2 = 0.35, P = 0.01)
Discussion
To our knowledge, this study represents the first data on energy density for Greenland capelin. The densities (based on dry matter, kJ g dw−1) are lower than seen in other studies (e.g., Jangaard 1974; Lawson et al. 1998; Anthony et al. 2000). As these studies demonstrate higher energy densities than shown here, spatial differences may exist. However, most other studies convert proximate composition to energy density, thus relying on energetic conversion factors, and often use low conversion factors for protein including only assimilable energy (17.8–20.0 kJ g dw−1). Converting values given by Anthony et al. (2000) on Alaska capelin to energetic densities using an energy equivalent of 23.2 kJ g dw−1 for protein (as determined in this study using reference material), we find an energy density of 26.6 kJ g ash-free dw−1 in non-spawning capelin compared to 22.3 kJ g ash-free dw−1 in this study (the ash content of capelin was 5.72%). Similarly, Montevecchi and Piatt (1984) present results from Newfoundland, Canada, with non-spawning capelin having re-calculated energy densities of 24–29 kJ g dw−1, similar to those presented by Jangaard (1974) and Lawson et al. (1998). As calculations in the mentioned studies, and other energetic studies on fish (e.g., Van Pelt et al. 1997; Ball et al. 2007), are made from proximate fish composition, the difference may be a result of differences in methodology (i.e., lipid extraction) causing overestimation (Jezierska 1974; Craig et al. 1978). Other studies using bomb calorimetry on pelagic fishes obtain values much like those shown here (e.g., Smith et al. 1990; Kitts et al. 2004), and we believe that the values are accurate, but if they are to be used in energetic budgets of capelin predators, correction to efficiency of the metabolic pathway is needed (Ball et al. 2007). The timing of the samples collected in this study may partly explain the difference between studies, as a seasonal change in proximal composition, and thus energy density, of capelin has been reported from other waters (Jangaard 1974; Eaton et al. 1975; Montevecchi and Piatt 1984; Lawson et al. 1998 and references; Anthony et al. 2000) as well as in other fishes (e.g., Hislop et al. 1991; Schwarz and Hammer 1996; Kitts et al. 2004) with a minimum in the mid-summer months because of a bimodal production cycle in temperate regions. Hence, Jangaard (1974) observed an increase from 21.2 kJ g dw−1 in June/July to 26.7 kJ g dw−1 in August in Canadian capelin (determined from proximate fish composition). The “energy density/water” ratio changes due to reciprocal substitution of tissue and water as the tissue is metabolized. Anthony et al. (2000) used water content in capelin as a proxy for energy content explaining 97% of the variation (based on energy density in wet mass), and Jangaard (1974) found a peak in water content of 82%, to be coincident with the lowest energy density. As the mean water content in this study was 80%, a matching low-energy density may be expected. In general, we saw no effect of time-of-capture on energy density, but in Nuuk there was a 1-kJ g dw−1 increase over time in non-spawning fish, suggesting that sampling throughout the season is necessary to allow detailed comparison with other studies.
There are differences in energy density between tissues, as demonstrated here between ovaries and muscle tissue, so conclusions regarding the nutritional value of capelin to predators should ideally be based on whole fish and not assuming that muscle is representative as done here. However, non-spawning capelin do not have developed gonads, and the remaining tissues make up a small amount of total fish weight, making it unlikely that the exclusion of these tissues will influence the overall energy density significantly or change the conclusions of the present study.
The non-spawning capelin were caught as bycatch in a bottom-trawl survey and not as a part of a sampling survey directed toward capelin. This could suggest an opportunistic sampling design complicating direct hypotheses testing. However, the capelin were caught using the same standard procedure at all stations, and we have no reason to expect that the sampled capelin should be biased in a way that would alter conclusions. Furthermore, the number of capelin caught was sufficiently large to allow for a good spatial coverage and five well-defined and separate sampling areas. The data provide significant new knowledge on capelin biology in this area, sets a baseline with regard to spatial differences in capelin energy density and allocation, and will allow more specific future hypothesis to be generated along with specific and more structured sampling designs. Therefore, we consider the use of this imperfect sampling design justified.
Fecundity and reproductive investment
Fecundity estimates (mean ± SD = 6,185 ± 2,249 eggs) were much lower than estimates from the Barents Sea (approx. 11,000, e.g., Gjøsæter and Monstad 1973; Huse and Gjøsæter 1997), Pacific (approx. 20,000, Velikanov 2002), and Canada (>20,000, Nakashima 1987). However, as all studies (the present one included) show positive fecundity–size relationships (Fig. 7), the size-specific fecundity in this study is as high or higher than in other studies (converting fork length to total length using a conversion factor of 1.08).
The reproductive investment was high (38%), which was as expected for a—to a varying degree—semelparous species (Karamushko and Christiansen 2002) that undertakes at least some migration associated with spawning (Behrens et al. 2006) and corresponds well with the semelparous/iteroparous limit of 60% (migration and spawning) suggested by Glebe and Leggett (1981). Hence, values of 10–30% have been demonstrated for iteroparous species such as Arctic char (Jonsson and Jonsson 1997; Finstad et al. 2002) and Atlantic cod in Greenland (Hedeholm et al., unpublished manuscript).
Reproductive investment increased with mass, but individual egg energy content (mean ± SD = 2.67 ± 0.36 J) did not. This has several possible explanations. The increase in investment could simply be a result of body cavity volume increasing more with a given mass increase (i.e., higher fecundity) than somatic energy content, or that smaller fish have a higher proportion of essential tissue that is not convertible to ovaries. However, it could also reflect a need to conserve more energy in smaller capelin as a response to an iteroparous life history strategy, which has been suggested for female capelin (Sørensen 1985; Vilhjálmsson 1994; Huse 1998; Friis-Rødel and Kanneworff 2002). In iteroparous species, it might be expected that first-time spawners produce lower-quality eggs and/or invest less in reproduction than more experienced individuals (Trippel 1998; Carr and Kaufman 2009). This study supports the latter of these notions and encourages further research on both male and female capelin.
Latitudinal and length effects
There was no evidence of a clear latitude-related trend in energetic density in non-spawning capelin. As growth increases along the same latitudinal gradient (Hedeholm et al. 2010), this indicates that the increase in energy intake with latitude is all converted to growth and not stored as energy. However, there was a significant difference between Qaqortoq and Qeqertarsuaq in spawning fish (20.86 and 21.87 kJ g dw−1, respectively), which are also the two areas showing the largest growth differences, indicating that these areas represent the southern distributional limit where environmental constraints limit capelin growth (Qaqortoq) and the optimal living conditions (Qeqertarsuaq), respectively. Hence, the decline in energy density in Uummannaq north of Qeqertarsuaq may represent the early signs of a northern distributional limit, and the decrease is again similar to that observed in capelin growth. Indeed, the latitudinal gradient in growth may be viewed as energy storage in terms of increased somatic mass, which serves as a positive fitness parameter in that the reproductive investment was found to be positively related to length (Fig. 7). However, the possibility of a temporal artifact needs to be considered, as energy density in Nuuk was shown to decline with almost 1 kJ g dw−1 over a 1-month period. This needs to be confirmed with sampling on a finer temporal scale along the latitudinal gradient.
There was a clear general increase in energy density (kJ g dw−1) with size in non-spawning capelin in three (Qaqortoq, Nuuk, and Uummannaq) of the four analyzed areas (Sisimiut excluded, Fig. 2). The reason for the inconsistency in the Qeqertarsuaq area, where only a slight increase in energy density with length was seen, is unknown. However, the analyzed size spectrum is smaller in this area, and the inclusion of smaller individuals may have resulted in an increase similar to that seen in other areas.
The positive relationship between size and energy density is similar to that shown in Alaska capelin (Anthony et al. 2000) as well as other pelagic species (Hislop et al. 1991). This length-related difference in nutritional value in interaction with similar latitudinal growth variation may have an important impact on higher trophic levels depending on capelin (Lavigne et al. 1985; Falk and Durinck 1993; Nielsen and Andersen 2001). As the average length-at-age increases by more than 50% across the latitudinal gradient, changes in capelin growth conditions may have a large effect on the ecosystem (Carscadden et al. 2001) as energetic densities can be expected to change accordingly—further emphasized by the fact that a reduction in size reduces not only energy density but also energy intake, as capelin weight can be expected to decrease by 87% with a 50% length reduction (Hedeholm et al. 2010). Faced with climate change (Holland and Bitz 2003) and a partly temperature-controlled growth pattern (Gjøsæter and Loeng 1987; Hedeholm et al. 2010), such changes are likely to occur in Greenland waters (Huse and Ellingsen 2008) and they will affect energy flow (Carscadden et al. 2001) and probably species distribution (Drinkwater 2005).
Although less clear, there was also a length effect on energy density in male spawning capelin. Capelin spawning takes place in shallow waters (Friis-Rødel and Kanneworff 2002) forcing capelin into large easily accessible shoals during this period, and it is during this period that they are preyed heavily upon by cod, seabirds, whales, and seals. Hence, the latitudinal growth (Hedeholm et al. 2010) and energy density (this study) gradients present along the Greenland west coast indicate that increasing temperatures will increase both capelin size and energy density, providing better feeding conditions for many predators. This is, however, dependent on temperatures staying within the optimal range and food being sufficient to meet metabolic demand.
The energy density was similar to that of non-spawning capelin based on dry mass (mean ± SD = 21.4 ± 0.5 kJ g dw−1), but differed significantly based on wet mass (mean ± SD = 3.44 ± 0.97 kJ g ww−1) because of a significant difference in water content (Figs. 2 and 5). This leads to the conclusion that as spawning approaches, feeding is reduced (O’Driscoll et al. 2001) and tissue is broken down and progressively replaced by water, while remaining tissue maintains the same composition until metabolized. The decline in energy content with time in male spawning capelin is consistent with knowledge on capelin spawning behavior. Males stay on the spawning grounds for longer periods awaiting small schools of females (Vilhjálmsson 1994) and to support metabolism in this period of limited food intake (Templeman 1948; R. Hedeholm, pers. obs.), tissue must be metabolized.
In addition to the latitudinal and length-related patterns demonstrated here, a possible genetic component to variations in capelin life history traits may also be relevant. Genetic differences along the latitudinal gradient examined here have been suggested (Sørensen and Simonsen 1988) and clearly demonstrated throughout capelin-inhabited waters (Præbel et al. 2008). Such differences can affect capelin life history traits (Christiansen et al. 2008) as also shown for similar species such as Atlantic silverside (Menidia menidia), and a genetic component to the patterns demonstrated here cannot be ruled out. However, present knowledge does not allow for an evaluation of such an effect, and further research into not only this aspect but also the overall genetic structure of Greenland capelin is encouraged as it would benefit this and other studies (Hedeholm et al. 2010).
In response to expected temperature changes in Greenland waters (Holland and Bitz 2003), the future distribution of capelin might well change (Huse and Ellingsen 2008) causing this key species to inhabit new areas. Capelin is found further north in other waters (i.e., The Barents Sea, Præbel et al. 2008) suggesting that it is not the potential length of the growing season that prevents capelin from extending its Greenland distribution further north. More likely, capelin distribution is limited by extensive sea ice cover. A late sea ice break up can prevent capelin from reaching spawning grounds in a given year, which could prove detrimental to a species with a large degree of semelparity (Friis-Rødel and Kanneworff 2002). Furthermore, as primary production in ice-covered regions is initiated by the increasing spring irradiance as well as the disappearance of sea ice (Madsen et al. 2008), a sea ice cover extending for much of the year reduces the growing season beyond that caused solely by a lack of sunlight. This could limit capelin distribution, as physiological limits of capelin feeding and assimilation may restrict them from obtaining sufficient energy in the short time period available. Feeding migrations as in other capelin populations (e.g., Vilhjalmsson 2002) could compensate for this, but Greenland capelin tends to be fairly non-migratiory (Friis-Rødel and Kanneworff 2002). Hence, if increasing ocean temperatures limits the extent (spatial and temporal) of sea ice, the distribution of capelin will most likely be extended further north, which is also supported by the fact that capelin in Greenland show positive latitudinal responses in both growth (Hedeholm et al. 2010) and prey availability (Hedeholm et al., submitted manuscript).
References
Angantyr LA, Kapel FO (1990). Harp seal feeding habits in Greenland—our present knowledge, April 1990. Technical Report of the Greenland Fisheries Research Institute, p 72
Anonymous (2005) Arctic climate impact assessment. Cambridge University Press, Cambridge
Anthony JA, Roby DD, Turco KR (2000) Lipid content and energy density of forage fishes from the northern Gulf of Alaska. J Exp Mar Biol Ecol 248:53–78
Bakun A (2006) Wasp-waist populations and marine ecosystem dynamics: navigating the “predator pit” topographies. Prog Oceanogr 68:271–288
Ball JR, Esler D, Schmutz JA (2007) Proximate composition, energetic value, and relative abundance of prey fish from the inshore eastern Bering Sea: implications for piscivorous predators. Pol Bio 30:699–708
Behrens JW, Præbel K, Steffensen JF (2006) Swimming energetics of the Barents Sea capelin (Mallotus villosus) during the spawning migration period. J Exp Mar Biol Ecol 331:208–216
Carr JP, Kaufman L (2009) Estimating the importance of maternal age, size, and spawning experience to recruitment of Atlantic cod (Gadus morhua). Bio Cons 142:477–487
Carscadden JE, Frank KT, Leggett WC (2001) Ecosystem changes and the effects on capelin (Mallotus villosus), a major forage species. Can J Fish Aquat Sci 58:73–85
Christiansen JS, Præbel K, Siikavuopio SI, Carscadden JE (2008) Facultative semelparity in capelin Mallotus villosus (Osmeridae)—an experimental test of a life history phenomenon in a sub-arctic fish. J Exp Mar Biol Ecol 360:47–55
Craig JF, Kenley MJ, Talling JF (1978) Comparative estimations of the energy content of fish tissue from bomb calorimetry, wet oxidation and proximate analysis. Fresh Bio 8:585–590
Cury P, Bakun A, Crawford RJM, Jarre A, Quinones RA, Shannon LJ, Verheye HM (2000) Small pelagics in upwelling systems: patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J Mar Sci 57:603–618
Drinkwater KF (2005) The response of Atlantic cod (Gadus morhua) to future climate change. ICES J Mar Sci 62:1327–1337
Eaton CA, Ackman RG, Tocher CS, Spencer KD (1975) Canadian Capelin 1972–1973—fat and moisture composition, and fatty-acids of some oils and lipid extract triglycerides. J Fish Res Board Can 32:507–513
Falk K, Durinck J (1993) The winter diet of thick-billed murres, Uria Lomvia, in Western Greenland, 1988–1989. Can J Zool 71:264–272
Fiksen O, Giske J, Slagstad D (1995) A spatially explicit fitness based model of capelin migrations, the Barents Sea. Fish Oceanogr 4:193–208
Finstad AG, Berg OK, Langeland A, Lohrmann A (2002) Reproductive investment and energy allocation in an alpine Arctic charr, Salvelinus alpinus, population. Environ Biol Fishes 65:63–70
Frank KT, Carscadden JE (1989) Factors Affecting Recruitment Variability of Capelin (Mallotus villosus) in the Northwest Atlantic. ICES J Mar Sci 45:146–164
Friis-Rødel E, Kanneworff P (2002) A review of capelin (Mallotus villosus) in Greenland waters. ICES J Mar Sci 59:890–896
Gjøsæter H (1998) The population biology and exploitation of capelin (Mallotus villosus) in the Barents Sea. Sarsia 83:453–496
Gjøsæter H, Loeng H (1987) Growth of the Barents Sea Capelin, Mallotus Villosus, in Relation to Climate. Environ Biol Fishes 20:293–300
Gjøsæter J, Monstad T (1973) Fecundity and egg size of spring spawning Barents sea capelin. FiskDir Skr (Ser Havunders) 16:98–104
Gjøsæter H, Dalpadado P, Hassel A (2002) Growth of Barents Sea capelin (Mallotus villosus) in relation to zooplankton abundance. ICES J Mar Sci 59:959–967
Glebe BD, Leggett WC (1981) Temporal, intra-population differences in energy allocation and use by American Shad (Alosa sapidissima) during the spawning migration. Can J Fish Aquat Sci 38:795–805
Hedeholm R, Grønkjær P, Rysgaard S (submitted) Feeding ecology of capelin (Mallotus villous Müller) in West Greenlandic waters. Pol Bio
Hedeholm R, Grønkjær P, Rysgaard S (2010) Differentiated growth of Greenlandic capelin (Mallotus villosus Müller) along a latitudinal gradient. ICES J Mar Sci 67:1128–1137
Hislop JRG, Harris MP, Smith JGM (1991) Variation in the calorific value and total energy content of the lesser Sandeel (Ammodytes marinus) and other fish preyed on by seabirds. J Zool 224:501–517
Holland MM, Bitz CM (2003) Polar amplification of climate change in coupled models. Clim Dyn 21:221–232
Huse G (1998) Sex-specific life history strategies in capelin (Mallotus villosus)? Can J Fish Aquat Sci 55:631–638
Huse G, Ellingsen I (2008) Capelin migrations and climate change—a modelling analysis. Clim Change 87:177–197
Huse G, Gjøsæter H (1997) Fecundity of the Barents Sea capelin (Mallotus villosus). Mar Biol 130:309–313
Jangaard PM (1974) The capelin (Mallotus villosus). Biology, distribution, exploitation, utilization and composition. Fish Res Bd Can Bull 186:1–70
Jensen AS (1939) Concerning a change of climate during recent decades in the Arctic and Subarctic regions, from Greenland in the west to Eurasia in the east, and contemporary biological and physical changes. Det. Kgl. Danske Videnskabernes Selskab Biologiske Medd 14:1–77
Jezierska B (1974) The effect of various type of food on the growth and chemical composition of the body of perch (Perca fluviatilis L.) in laboratory conditions. Pol Arch Hydrobiol 21:467–479
Jonsson N, Jonsson B (1997) Energy allocation in polymorphic brown trout. Func Ecol 11:310–317
Jordan F, Liu WC, Wyatt T (2005) Topological constraints on the dynamics of wasp-waist ecosystems. J Mar Syst 57:250–263
Karamushko LI, Christiansen JS (2002) Aerobic scaling and resting metabolism in oviferous and post-spawning Barents Sea capelin Mallotus villosus villosus (Müller, 1776). J Exp Mar Biol Ecol 269:1–8
Kitts DD, Huynh MD, Hu C, Trites AW (2004) Season variation in nutrient composition of Alaskan walleye pollock. Can J Zool/Rev Can Zool 82:1408–1415
Lavigne DM, Innes S, Stewart REA, Worthy GAJ (1985) An annual energy budget for north-west Atlantic harp seals. In: Marine mammals and fisheries, 319–335
Lawson JW, Magalhaes AM, Miller EH (1998) Important prey species of marine vertebrate predators in the northwest Atlantic: proximate composition and energy density. Mar Ecol Prog Ser 164:13–20
Madsen SD, Nielsen TG, Hansen BW (2008) Annual population development and production by small copepods in Disko Bay, western Greenland. Mar Biol 155:63–77
Montevecchi WA, Piatt J (1984) Composition and energy contents of mature inshore Spawning Capelin (Mallotus villosus)—implications for seabird predators. Comp Biochem Physiol A: Mol Integr Physiol 78:15–20
Nakashima BS (1987) Regional and temporal variations in fecundity of capelin in Newfoundland waters. T Am Fish Soc 116:864–873
Neve PB (2000) The diet of the minke whale in Greenland—a short review. Minke Whales, Harp and Hooded Seals: Major Predators in the North Atlantic Ecosystem. NAMMCO Scientific Publications 2:92–96
Nielsen JR, Andersen M (2001) Feeding habits and density patterns of Greenland cod (Gadus ogac) (Richardson 1836), at West Greenland compared to those of the coexisting Atlantic cod, Gadus morhua L. J Northwest Atl Fish Sci 29:1–22
O’Driscoll RL, Parsons MJD, Rose GA (2001) Feeding of capelin (Mallotus villosus) in Newfoundland waters. Sarsia 86:165–176
Phillips RA, Petersen MK, Lilliendahl K, Solmundsson J, Hamer KC, Camphuysen CJ, Zonfrillo B (1999) Diet of the northern fulmar Fulmarus glacialis: reliance on commercial fisheries? Mar Biol 135:159–170
Præbel K, Westgaard JI, Fevolden SE, Christiansen JS (2008) Circumpolar genetic population structure of capelin Mallotus villosus. Mar Ecol Prog Ser 260:189–199
Rice J (1995) Food web theory, marine food webs, and what climate change may do to northern marine fish populations. In: Beamish RJ (ed) Climate change & Northern fish populations. Canadian special Publications of Fisheries and Aquatic Sciences, vol 121, pp 561–568
Romano MD, Piatt JF, Roby DD (2006) Testing the junk-food hypothesis on marine birds: effects of prey type on growth and development. Waterbirds 29(4):407–414
Rose GA, O’Driscoll RL (2002) Capelin are good for cod: can the northern stock rebuild without them? ICES J Mar Sci 59:1018–1026
Schwartzlose RA et al (1999) Worldwide large-scale fluctuations of sardine and anchovy populations. S Afr J Mar Sci-S-Afr Tydskr Seewe 21:289–347
Schwarz G, Hammer C (1996) Size and seasonal effects on the body calorific content of North Sea whiting (Merlangius merlangus L, Gadidae). Arch Fish Mar Res 44:33–46
Smith RL, Paul AJ, Paul JM (1990) Seasonal-changes in energy and the energy-cost of spawning in Gulf of Alaska Pacific Cod. J Fish Biol 36:307–316
Sørensen EF (1985) Ammassat ved Vestgrønland [Capelin at West Greenland], Fiskeri- og Miljøundersøgelser i Grønland
Sørensen EF, Simonsen V (1988) Genetic differentiation among populations of Capelin Mallotus villosus from the West-Coast of Greenland. J Appl Ichthyol/Z Angew Ichtyol 4:23–28
Templeman W (1948) The life history of the capelin (Mallotus villosus O. F. Müller) in Newfoundland waters. Research Bulletin of the Newfoundland Government Laboratory 17:151
Trippel EA (1998) Egg size and viability and seasonal offspring production of young Atlantic cod. T Am Fish Soc 127:339–359
Van Pelt TI, Piatt JF, Lance BK, Roby DD (1997) Proximate composition and energy density of some North Pacific forage fishes. Comp Biochem Physiol A: Mol Integr Physiol 118:1393–1398
Velikanov AY (2002) Spatial differences in reproduction of capelin (Mallotus villosus socialis) in the coastal waters of Sakhalin. ICES J Mar Sci 59:1011–1017
Vilhjalmsson H (2002) Capelin (Mallotus villosus) in the Iceland-East Greenland-Jan Mayen ecosystem. ICES J Mar Sci 59:870–883
Vilhjálmsson H (1994) The Icelandic Capelin Stock. J Marine Res Inst (281 pp)
Winters GH (1971) Fecundity of left and right ovaries of Grand Bank Capelin (Mallotus villosus). J Fish Res Board Can 28:1029–1033
Woll AK, Gundersen AC (2004) Diet composition and intra-specific competition of young Greenland halibut around southern Greenland. J Sea Reas 51:243–249
Acknowledgments
We thank the staff at the Greenland Institute of Natural Resources for help with sampling. Additionally, we thank two anonymous reviewers for helpful comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Harrod.
Rights and permissions
About this article
Cite this article
Hedeholm, R., Grønkjær, P. & Rysgaard, S. Energy content and fecundity of capelin (Mallotus villosus) along a 1,500-km latitudinal gradient. Mar Biol 158, 1319–1330 (2011). https://doi.org/10.1007/s00227-011-1651-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1651-5