Abstract
Capelin occupy a key trophic role and have a broad latitudinal distribution in the northeastern Pacific and Arctic Oceans. Understanding their adaptation to a range of conditions is important to predicting how they will respond to climate change. To quantify the variation in body condition in different physical environments, we measured energy density, RNA/DNA ratios, carbon and nitrogen stable isotope ratios in 62 juvenile capelin along the Western Alaskan coast from Bristol Bay to Point Barrow ranging across approximately 14° of latitude. Energy density correlated positively with latitude, whereas RNA/DNA (instantaneous growth index) was strongly correlated with sea surface temperature, indicating that optimal growth of capelin was achieved at ~9 °C, followed by rapid decreases in RNA/DNA ratios at higher temperatures. δ13C and δ15N had strong, inverse nonlinear relationships with latitude. Depletion of δ13C seen in capelin North of Bristol Bay may be related to the incorporation of allochthonous basal resources into the diets of juvenile capelin from nearby riverine inputs. Observed enrichment of δ15N North of Bristol Bay is likely to be related to incorporation of higher trophic level prey items. Given inverse relationship between δ13C and δ15N, these prey items are likely available due to the increased diversity of basal resources from increased inputs of riverine organic material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Latitudinal variation exists across seascapes and some species found across a wide range of climatic conditions have different feeding and life-history strategies according to the conditions they face (Conover 1988; Post and Parkinson 2001; Shoji et al. 2011; Rypel 2012). Life-history strategy is defined as the allocation of energy throughout a lifetime to optimize growth, survival, and reproduction (Noordwijk and Jong 2014). Energy allocation in juvenile fish is particularly important, when there are concurrent energetic demands to grow (predator avoidance, increase accessibility to prey) and store energy to survive their first winter (Mogensen and Post 2012). Fish condition is often used as a parameter to examine survival potential and recruitment processes (Heintz et al. 2013) and is often expressed as measures of energy density (kJ/g), which is driven by lipid content (Anthony et al. 2000). Stable isotopes have become a popular method for examining dietary sources and trophic structure within species and their ecosystems (DeNiro and Epstein 1978, 1981; Hobson and Welch 1992; Layman et al. 2007a; Peterson and Fry 2014). Many trophic studies focus on carbon and nitrogen isotopes, but they rarely consider simultaneous measures of energetic condition and growth. These latter measures offer important information describing the quality of prey items and consequently diet implications for fish condition and optimality of habitats within a system (Sherwood et al. 2007). Thus, food web structures and carbon sources that maximize productivity can be identified by combining isotopic analysis with indices of growth and energetic condition. This is important in the Arctic and sub-Arctic where marine conditions are changing rapidly (Grebmeier et al. 2006), and those changes are likely to affect prey availability and quality.
As coastal waters in the Arctic become increasingly warmer and ice-free due to shifting climate conditions, it is likely that species will extend, restrict, or shift their ranges as a function of changing tolerable conditions, eliciting change in coastal food web structures of northern oceans (Grebmeier et al. 2006; Moline et al. 2008; Eisner et al. 2013). These coastal food webs provide critical subsistence fisheries and serve as a food source for numerous endangered and protected marine mammals and sea birds (Hobson and Welch 1992). With these changes occurring, high-latitude species may see intrusions from lower-latitude species, introducing new predators and competition for resources and space (Gilman et al. 2010; Sorte et al. 2010; Grebmeier 2012; Litzow and Mueter 2014). The life-history strategies of species that have wide tolerance of environmental conditions may serve as proxies to examine fish condition with changes in food web structure across extensive spatial domains and offer insight into predicting how these important forage fish may fare in the Arctic as climatic conditions change.
The Western Coast of Alaska is bordered by the Bering Sea in the southwest and the Chukchi Sea in the northwest. The high-nutrient, high-salinity Bering Shelf Water (BSW) and the freshwater runoff and wind-driven Alaska Coastal Current (ACC) run along the entire length of these coasts, and the front between them typically occurs between 30 and 60 km from shore (Stabeno et al. 1995; Steele et al. 2010). The front between these two distinct water masses may generate optimal growth conditions to support an abundance of planktivorous fish in coastal waters (Coyle and Pinchuk 2005; Weingartner et al. 2005; Hopcroft et al. 2010; Eisner et al. 2013). Because of the extensive latitudinal range of this persistent front, it presents the opportunity to study the effects of latitude on trophic interactions and energy allocation strategies of coastal planktivorous fish. This water mass provides gradients of temperature and freshwater influx, two primary conditions that are expected to increase significantly with climate change (Peterson et al. 2002; Chan et al. 2011; Dunton et al. 2014). The proximity of this coastal current to land allows for the examination of fish trophodynamics and condition in response to freshwater/terrestrial influence.
Capelin (Mallotus villosus) are an abundant planktivorous forage fish found throughout western Alaskan waters and serve as an important link between lower and higher trophic levels (Gjøsæter and Båmstedt 1998; Johnson et al. 2010; Sherwood et al. 2007). Capelin’s wide geographical distribution suggests plasticity in its life-history strategies. Because of capelin’s trophic position and value as a prey resource, their ability to adapt to different conditions and prey resources will probably play an important role in the response of larger, economically important or endangered members of higher trophic levels, including fish, marine mammals, and sea birds. Previous research has shown that great variability exists in planktonic assemblages throughout the ACC and BSW (Schell et al. 1998; Dunton et al. 2006; Eisner et al. 2013) and it is likely that distinct planktonic assemblages coincide and interact with distinct food webs. Capelin can be used as a model species for the adaptability of mid trophic levels (forage fish) to changing climatic conditions in the Arctic by examining their energy allocation patterns and trophodynamics across gradients in climate and latitudes in which they are abundant.
Past studies have examined the latitudinal dependence of energy allocation strategies of fish, but to date they have largely been restricted to smaller scales or closed freshwater and have not incorporated stable isotope analysis (SIA) to examine the effects of variations in diet (Shoji et al. 2011; Mogensen and Post 2012; Rypel 2012; Siddon et al. 2013). The use of a combination of energetics and stable isotope data may offer further insight into the adaptability and sensitivity of these coastal food webs to changing conditions throughout the Alaskan coastline. We aim to quantify energy density, growth rates, and isotope ratios among juvenile capelin along the entire west coast of Alaska within a single water body, the ACC/BSW front, to examine how variation in condition and growth rate varies as a function of latitude, temperature, riverine influence, and trophic and dietary composition.
Materials and methods
Fish collections
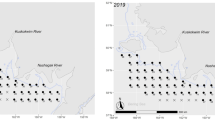
Juvenile capelin (n = 62) were collected during the summer fall of 2012 using three different vessels spanning 13.9° of latitude (57.5°N–71.4°N; Fig. 1). Capelin were selectively subsampled from expansive fish surveys from stations between 25 and 75 km from the coastline (with exception of the stations in Bristol Bay that were approximately 100 km from the coast) and thus likely to be near the ACC/BSW front (Stabeno et al. 1995; Steele et al. 2010). Capelin analyzed for this project are also limited to those <100 mm length and are assumed to be juveniles based on their size (Vesin et al. 1981; Hop and Gjøsæter 2013) and lack of adult pigmentation. Capelin were sampled using a 198-m-long surface trawl towed behind a 54.9-m chartered fishing vessel between August 8th and September 21st, 2012. The trawl had hexagonal mesh on the wings and body, a 1.2 cm mesh cod end liner, and a 50 m × 25 m mouth (horizontal × vertical). Each tow lasted for 30 min at approximately 8.3 km h−1 at stations within a 103 km2 grid along the western Alaskan coast between 60°N and 71.4°N. Four more sites were sampled using the same methods by the NOAA ship Oscar Dyson between 57.5°N and 60°N (August 20th–October 9th, 2012). Capelin along the nearshore were sampled with beach seines near Barrow, AK (~71°N), during August 7–20, 2012. The seine was 37 m long with variable mesh sizes (10 m of 32 mm outer panels, 4 m of 6 mm middle panels, and 9 m of 3.2 mm blunt panel). Each set was round-haul style, paid out of a 7-m skiff following methods used by Johnson et al. (2010). All collections occurred during daylight hours. Physicochemical parameters from offshore stations were averaged from the top 20 m of the water column using a CTD at each sampling station at the time of collection. Water temperature and salinity from beach seine sites were measured from the top 0.5 m of water using a thermometer and refractometer. Fish were measured to fork length (FL) and kept frozen until analyzed in the laboratory.
Map of Alaskan waters showing sampling locations of juvenile capelin collected. Filled shapes represent the Chukchi Sea stations, and open shapes represent Bering Sea stations. Like symbols represent regional groups of fish at similar latitudes. The numbers in parentheses after each river represent the individual annual discharge (km3 year−1) of each river (Benke and Cushing 2006; Nemeth et al. 2014). This map was produced with the CRAN-R package “Rgooglemaps” (Loecher and Ropkins 2015)
Individual capelin were randomly subsampled within each site (Table 1). In the laboratory, individual fish wet weights were measured, stomach contents removed, and a sample of white muscle of ~0.01 g was dissected and frozen (−80 °C) for RNA/DNA analysis. Individual capelin were dried to a constant weight using a LECO Thermogravimetric Analyzer (TGA) 601/701 and homogenized using mortar and pestle until a uniform consistency was reached. Dry homogenates of individual juvenile capelin were stored in a desiccator prior to SIA and bomb calorimetry analyses.
Bomb calorimetry
Energy density of juvenile capelin (kJ g−1 dry mass) was quantified using bomb calorimetry. A Parr Instrument 6725 semi-micro bomb calorimeter was used to combust pellets of dry fish homogenate following standard instrument operating protocols from the manufacturer. Precision and accuracy of measurements were assessed by evaluating duplicate benzoic acid standards, replicate samples, and a tissue reference material of Pacific herring or walleye pollock homogenate. Error limits were set for the quality assurance samples, where precision from replicate benzoic acid standards was not allowed to vary by more than 1.5 % coefficient of variation and must have been within 2.0 % of the target value. Sample replicates were not allowed to vary by more than 1.5 standard deviations, and tissue reference samples were not allowed to vary by more than 3.0 % from target reference values.
RNA/DNA analysis
Instantaneous growth rates were estimated from RNA/DNA ratios following methods outlined in Sreenivasan (2011). A ~10 mg of frozen muscle sample was taken from each fish. RNA/DNA ratios were quantified fluorometrically using one dye and two enzymes (RNase and DNase; Caldarone et al. 2001). Nucleic acids were isolated from the smaller muscle samples and dyed using 75 µL ethidium bromide (5 µg ml−1) according to the protocol outlined by Caldarone et al. (2001). Total fluorescence at excitation and emission wavelengths of 355 and 600 nm, respectively, was recorded, then the samples were sequentially treated with RNase and DNase, and the resulting reduced fluorescence was measured to obtain RNA and DNA fluorescence, respectively. Standard curves were constructed using serial dilutions of 18 s–28 s rRNA (Sigma R-0889) and calf thymus DNA (Sigma D-4764) standards. DNA concentrations in tissues are stable, but RNA concentrations vary greatly depending on the rate of protein synthesis where a high RNA/DNA ratio indicates a high growth rate (Weber et al. 2003).
Stable isotopes analysis
SIA of carbon and nitrogen is used to examine the origins and type of dietary sources assimilated by fish (DeNiro and Epstein 1978, 1981). All subsamples of dried fish homogenate were weighed to 0.55 ± 0.15 mg. In between every four samples, a standard or duplicate sample was analyzed to examine precision of measurements. Samples were analyzed at the Florida International University SERC Stable Isotope Laboratory using elemental analysis–isotope ratio mass spectrometry (EA-IRMS), with a NA1500 NC (EA) coupled to a Delta C (IRMS). Error based on internal glycine standards ranged 0.09–0.21 ‰ for δ15N and 0.07–0.10 ‰ for δ13C.
Lipid corrections were computed using C/N ratios (4.45 ± 0.66) following “Eq. 1” outlined by Logan et al. (2008). This equation requires the assumption that the difference between bulk δ13C and lipid-free δ13C approximates 6 ‰ as suggested by McConnaughey and McRoy (1979). Later work by Post et al. (2007) pointed out that these methods are suitable for organisms with 15 % or less lipid content, and that caution should be used at higher lipid contents because they had insufficient samples with such high-lipid content to adequately model the relationship. Thus, using the relationship presented by Post et al. (2007), we estimate that approximately 82 % of our samples contain less than 15 % lipids, 15 % of our samples contain <17.5 % lipid, and the remaining 3 % contain <20 % lipid. Based on this information, we deemed this method of lipid correction appropriate for our samples.
Stomach contents
Prey from the stomach contents of juvenile capelin was identified to species and life-history stage where possible using methods outlined by Sturdevant et al. (2012). Adult Calanus spp. were classified by size: small ≤2.4 mm in length, medium = 2.5–2.9 mm, and large ≥ 3 mm. Aggregate wet weights of separated prey groups were measured from each fish. Weights were converted into percent contributions to the total mass of prey found in each stomach to standardize against the unevenness in fullness. Percent contributions from individual capelin were averaged over regional groups (see Data analysis section below) to standardize against unevenness in sample size.
Data analysis
The 62 juvenile capelin from 17 sampling stations were separated into 7 regional groups (Table 1) based on latitude and distance from each other (Fig. 1). General Additive Models (GAMs) were used for qualitative assessment to identify patterns of dependent variables with latitude as the independent variables. Linear regressions were used to assess the relationship between dependent variables (δ15N, δ13C, energy density, RNA/DNA ratios, length, and weight) and to offer a quantitative approach to assessing the relationships of energetics and SIA with latitude.
Results
Energy allocation
Across the sample set, energy density ranged from 20.77 to 26.49 kJ g−1 (Mean ± SD = 22.85 ± 1.33 kJ g−1). RNA/DNA ratios ranged from 13.10 to 31.61 (22.89 ± 4.33). Linear regression indicated a weak positive correlation between energy density and RNA/DNA (R 2 = 0.32, p < 0.0001; Fig. 2a). Energy density increased significantly with latitude, but RNA/DNA did not (energy density: estimated degrees of freedom [edf] = 4, R 2 = 0.34, p < 0.0001, Fig. 3a; RNA/DNA: edf = 4, R 2 = 0.14, p = 0.01, Fig. 3b). The models for energy density and RNA/DNA suggest that the Point Barrow group may be an outlier relative to other samples, as the values rapidly decrease at this region group. In addition, capelin from Point Barrow were significantly smaller than all other groups (FL: p = 0.0002; wet weight: p < 0.0001) and were the only fish collected by beach seines in the very near shore. When the Point Barrow group was removed from the analysis, energy density was linearly correlated with latitude (R 2 = 0.36, p < 0.0001, Fig. 4a); however, the linear model between RNA/DNA and latitude was not significant (R 2 = 0.06, p = 0.05, Fig. 4b). Furthermore, linear regressions indicated that energy density was strongly positively correlated with fish length (FL: R 2 = 0.47, p < 0.0001) and full body wet weight (R 2 = 0.63, p < 0.0001).
General additive models for energy density (a), RNA/DNA ratios (b), δ13C (c), and δ15N (d) for all juvenile capelin with a smoothing function on latitude. All models created using cubic splines were used with five knots (edf = 4) for energy density and RNA/DNA ratios, and four knots (edf = 3) for δ13C and δ15N. The dashed lines represent the 95 % confidence interval. Each model was significant with varying levels of deviance explained (A: R 2 = 0.34, p < 0.0001; B: R 2 = 0.14, p = 0.01; C: R 2 = 0.54, p < 0.0001; D: R 2 = 0.67, p < 0.0001). The bold tick marks above the x-axis represent latitudes at which fish samples were collected
Linear regressions of energy density (a), RNA/DNA ratios (b), δ13C (c), and δ15N (d) with latitude. The northernmost sampling stations (Point Barrow group: filled square) and the southernmost sampling stations (Bristol Bay group: open diamond) were removed as outliers for the analysis. Energy density was strongly correlated (R 2 = 0.36, p < 0.0001) with latitude, but RNA/DNA ratios were not (R 2 = 0.06, p = 0.05). δ13C is negatively correlated, and δ15N is positively correlated with latitude (R 2 = 0.55, p < 0.0001; R 2 = 0.75, p < 0.0001; respectively). Station symbology: Wainwright Inlet (filled circle), Point Hope (filled triangle), Kotzebue Sound (filled diamond), Norton Sound (inverted triangle), and Nunivak Island (open square)
In contrast, GAM models using sea surface temperature as a predictor indicated that more variability in RNA/DNA ratios was explained by surface temperature than in energy density (RNA/DNA: edf = 3, R 2 = 0.28, p < 0.0001; energy density: edf = 4, R 2 = 0.15, p < 0.0001, Fig. 5a–b); however, both models suggest that these measures vary slightly with increasing temperature until a threshold of approximately 9 °C is reached, at which point they both decreased rapidly. In turn, a GAM using surface temperature with latitude as the predictor indicates that these factors have a nonlinear relationship (edf = 4, R 2 = 0.29, p < 0.0001, Fig. 6).
General additive models for energy density (a), RNA/DNA ratios (b), δ13C (c), and δ15N (d) for all juvenile capelin with a smoothing function on temperature. All models created using cubic splines were used with five knots (edf = 4) for energy density, and four knots (edf = 3) for RNA/DNA ratios, δ13C, and δ15N. The dashed lines represent the 95 % confidence interval. All models were significant with varying levels of deviance explained (A: R 2 = 0.15, p < 0.0001; B: R 2 = 0.28, p < 0.0001; C: R 2 = 0.31, p < 0.0001; D: R 2 = 0.30, p < 0.0001). The bold tick marks above the x-axis represent latitudes at which fish samples were collected
GAM of surface temperature with a smoothing function on latitude. The nonlinear relationship shows that there is no discernible trend between surface temperature and latitude. The dashed lines represent the 95 % confidence intervals. Cubic splines were used with five knots (edf = 4), the GAM explains 28.8 % of the deviance, and the relationship was found to be significant (p < 0.0001). The bold tick marks above the x-axis represent latitudes at which fish samples were collected
Stable carbon and nitrogen isotopes
Stable δ13C and δ15N isotope ratios of juvenile capelin were analyzed to identify changes in the origins of dietary material and trophic position across latitudes. Across this range, δ13C ranged from −22.47 to 17.89 ‰ (mean ± SD = −20.33 ± 1.10 ‰) and δ15N ranged from 11.76 to 17.09 ‰ (14.31 ± 1.29 ‰). The linear correlation between δ13C and δ15N was strongly negative (R 2 = 0.67, p < 0.0001; Fig. 2B).
The relationships between δ13C and δ15N with latitude were highly significant, and much of the variability in both SIA measures could be explained by latitude (δ13C: edf = 3, R 2 = 0.54, p < 0.0001; δ15N: edf = 3, R 2 = 0.67, p < 0.0001; Fig. 3C-D). Linear regressions indicated that δ13C was positively correlated and δ15N was negatively correlated with latitude (R 2 = 0.55, p < 0.0001; R 2 = 0.75, p < 0.0001, respectively; Fig. 4c–d) when anomalies at the northernmost sampling stations (Point Barrow group) and the southernmost sampling stations (Bristol Bay group; Fig. 1) are removed. A similar pattern for each isotope was seen with temperature, suggesting that peak energy content and growth rate at the high latitudes coincide with feeding at higher trophic levels and incorporation of depleted δ13C.
Stomach contents
Juvenile capelin diets were dominated by Calanus spp. copepods, with the average proportion of copepods in non-empty stomachs being 79.8 %. Stomach contents in Bristol Bay consisted solely of large- and medium-sized Calanus spp. copepods, but stations near the Bering Strait had increased prey diversity, including important contributions from decapod larvae, and smaller contributions of small copepods, cladocerans, and chaetognaths. At the Point Barrow stations, the diets consist completely of small copepods and Themisto libellula, a predatory hyperiid amphipod (Auel and Werner 2003; Pinchuk et al. 2013). Of the capelin caught at Point Barrow, 56 % of them had empty stomachs, while empty stomachs were not observed at any other station (Fig. 7).
Average contributions by weight of prey type in the stomach contents of the analyzed capelin for six of the seven regional groups identified in Table 1. Cal = Calanus spp. copepod. No stomach content data were available for the Norton Sound group
Discussion
Energy allocation
Given that capelin occupy a wide latitudinal range, it is expected that they exhibit plasticity not only in their diet but also the allocation of energy obtained to cope with differences in climatic conditions. When prey resources are limited, we expect to see a trade-off between energy storage and growth rate as energy availability is generally not great enough to allow for concurrent processes of high growth rates and energy storage in juvenile fish (Post and Parkinson 2001). We saw evidence for increased energy provisioning for more severe winters in juvenile capelin in higher latitudes, with a positive correlation between energy density and latitude. This phenomenon has been observed for other fish species where the longer high-Arctic winters require a larger store of energy to survive than shorter, low-latitude winter locations (Biro et al. 2005).
It is also plausible that though the fish were sampled at approximately the same time of year, season could play a factor in the greater energy densities at higher latitudes. Many species of fish undergo seasonal changes in their energy content as a factor of ontogenetic changes and maturation, particularly pelagic species such as capelin (Vollenweider et al. 2011). In general, fish increase energy during summer periods of high productivity, peak in the fall, and decline overwinter when prey can be scarce and gametes start developing for later spawning. The onset of winter will come much sooner to the fish in the Arctic. Therefore, it could be that higher energy densities observed at higher latitudes are a factor of the accelerated onset of winter, while further to the south there is additional time to prepare.
Increased energy storage at high latitudes could be expected to impair growth rates relative to southern areas. Another reason to expect lower growth rates in the Arctic is that capelin, as are many species of fish, are smaller at age at higher latitudes (Chambers and Leggettt 1987; Chambers et al. 1989; Olsen et al. 2005). However, measurements of RNA/DNA remained relatively constant across latitudes. In concert, higher energy densities in the Arctic and equivalent RNA/DNA across latitudes imply that fish in higher latitudes have greater accessibility to energy resources that can be simultaneously allocated to growth and energy storage. An alternate explanation is that RNA/DNA ratios in juvenile capelin are driven more by sea surface temperature. Comparisons across a temperature range have been shown to require laboratory-based calibration studies (Caldarone et al. 2001). Without these studies to validate the temperature effect on RNA/DNA and growth relationship, it is conceivable that temperature is confounding our use of RNA/DNA as an index to growth.
However, RNA/DNA ratios varied with temperature and were greatest just above 9 °C, which occurred in the middle range of the Chukchi coast near Wainwright Inlet. This indicates that RNA production is maximized at this temperature. However, it is unclear whether this translates to increased growth. It is possible that increased RNA synthesis is a compensation for reduced efficiency in protein synthesis enzymes (Houlihan et al. 1995; Smith and Ottema 2006). Coincidently, this is also the location where energy density reached a maximum. This could suggest that growing conditions are maximized at 9 °C. High growth rates near the end of the growing season have been associated with increased lipid reserves in high-latitude perch and subsequent winter survival (Huss et al. 2008). A summer-long sampling effort of capelin in the Point Barrow region during ice-free periods in 2013 and 2014 shows that capelin were more abundant at 8–9 °C (Barton et al. unpublished data). Capelin distribution, abundance, and diet are impacted by water temperature. In cold years (1–2 °C below average), capelin are distributed across a much broader area in higher numbers in the Bering Sea, and energy-rich Calanus spp. are important diet items (Andrews et al. pers. comm.). In warm years (1–1.5 °C above average), capelin distribution is relatively restricted to the cooler, northern reaches of the Bering Sea, and energy-poor Pseudocalanus spp. and Oikopleura spp. were more abundant in their diet. During August 2012, when these samples were collected, an average temperature anomaly near Point Barrow stations of 5 to 7.5 °C above average was measured, suggesting that an abundance of energy-poor prey items was likely present (Parkinson and Comiso 2013).
Similarly, capelin distribution in Glacier Bay in Southeast Alaska was highly correlated with water temperatures, but in contrast to our results, these capelin were most abundant in the colder (6–7 °C) glacial waters over warmer (7–8 °C) estuarine central bay waters (Arimitsu et al. 2008). In this case, the glacial waters must have offered an advantage over the central bay waters. Glacial runoff brings with it high concentrations of nutrients (Hood and Scott 2008) and creates stratification by forming a freshwater lens, thus promoting plankton blooms to occur and providing an abundant food source for resident capelin. Additionally, Arimitsu et al. (2008) demonstrated that these waters were highly turbid, which may offer protection from sight-based predation, and increased feeding opportunities for capelin. Though we expect that temperature plays an important role in the life-history strategies of capelin, different temperature preferences in Southeast Alaska compared to the Western coast of Alaska in our study suggest that other factors such as predator abundance, prey availability, and turbidity may be more important drivers than temperature.
Stable carbon and nitrogen isotopes
Relationships between δ13C and δ15N can be used to identify a number of factors that describe the variation of assimilated materials obtained through capelin diets as a function of latitude. It is common to observe positive correlations between δ13C and δ15N isotope ratios because both become enriched (δ13C ≈ +1 ‰; δ15N ≈ +3.8 ‰) with increasing trophic level provided that the basal resources remain the same (Hobson and Welch 1992; Layman et al. 2007a). The negative correlation seen in our results may suggest that within a single species, carbon sources and trophic structure may differ spatially. In order to better understand these differences in dietary composition of juvenile capelin, δ13C and δ15N ratios across the latitudinal range must be investigated individually.
Stable carbon isotopes
Basal resources in the BSW and Anadyr Water (AW) are mostly derived of pelagic production, but in the ACC basal resources consist mostly of terrestrial materials contributed by freshwater runoff (Grebmeier et al. 1988). This concept is consistent with the pattern of δ13C throughout our sampling stations, suggesting that the majority of the variation in δ13C may be explained by riverine inputs along the path of the ACC. It is expected that the ACC is less depleted in δ13C when entering Bristol Bay as it is derived from relatively marine waters from the Gulf of Alaska moving through Unimak Pass (Kline 1999). As it travels north between 58 and 63°N, a number of rivers discharge a substantial amount of fresh water into the ACC, including the Kvichak, Nushagak, Kuskokwim, and Yukon which cumulatively average 310 km3 year−1 (40 % of the average annual freshwater runoff into the ACC; Fig. 1) (Weingartner et al. 2005; Benke and Cushing 2006). As these rivers discharge, δ13C-depleted labile organic material accumulates and is incorporated into primary producers and eventually secondary consumers like capelin (Dunton et al. 2005; Helfield and Naiman 2016). As the ACC continues northward, it converges and mixes with the benthic-derived marine BSW and the AW (Grebmeier et al. 1988; Dunton et al. 2005), causing isotopic ratios to become less depleted again. The lack of major rivers that drain into the southern Chukchi coast of Alaska limits riverine inputs of terrestrial organic material north of the Bering Strait, and δ13C continues to become less depleted as the ACC travels further north.
The pattern described above might suggest that δ13C correlates with salinity; however, when this relationship was investigated, we found neither discernible patterns nor any significant correlation. This may be explained by the relative differences of salinity and isotopic content between riverine waters and ACC waters. We posit that the difference in salinity between riverine and coastal waters may be relatively small compared to the difference in abundance of materials that may be incorporated as basal resources, thus explaining why riverine discharge may elicit a change in δ13C, but not in salinity. Given that the ACC is known to be driven by freshwater discharge and has relatively low levels of in situ production (Grebmeier et al. 1988; Weingartner et al. 2005), this phenomenon may serve as a possible explanation for the observed trends.
Freshwater inputs support observations for δ13C between 58 and 70°N, but an anomaly exists at the northernmost regional groups (Wainwright Inlet and Point Barrow) of the data set where δ13C becomes more depleted again. It is possible that this could be attributed to the incorporation of high-lipid content in prey items that are depleting the carbon signature. However, if this were the case we would expect capelin in these regions to show an increase in energy density, which is not the case. A more likely explanation is that the series of small streams and rivers along the Chukchi coast of the North Slope of Alaska, as well as two substantial estuaries (Wainwright Inlet and Peard Bay) that are likely to carry large loads of labile terrestrial carbon from permafrost meltwater runoff into coastal waters, could be responsible for the depletion of δ13C ratios in capelin at the northernmost stations (Dutta et al. 2006; Schuur et al. 2008). Though this runoff is relatively small compared to major rivers in the Bering Sea, when combined, these terrestrial inputs may be large enough to cause a significant shift in dietary δ13C of capelin.
Stable nitrogen isotope
δ13C and δ15N have inverse relationships with latitude, where δ15N is less enriched in Bristol Bay, and then rapidly becomes more enriched at Nunivak Island (17.1 ‰). The ratios became gradually less enriched with latitude, reaching a minimum at Point Hope (11.8 ‰), at which point they become enriched again at the northernmost groups (Wainwright Inlet and Point Barrow). When investigating carbon isotope patterns, we found that our results resembled broad-scale patterns described by Schell et al. (1998); however, the fluctuations we found in nitrogen isotopes do not. The difference between our maximum and minimum δ15N surpasses the commonly accepted trophic enrichment value of 3.8 ‰ (Hobson and Welch 1992; Post 2002; Hansen et al. 2012) and suggests that capelin along this latitudinal gradient are feeding at different trophic levels. When this pattern is compared to that of δ13C, it becomes clear that δ15N is more enriched where terrestrial inputs are increased. This difference may be attributed to one or both of two scenarios: (1) The prey that capelin feed on are depending on different basal resources; (2) and capelin are feeding on different prey types in relation to latitude.
One possible explanation is that the basal resources vary with latitude and may elicit a cascading effect on the isotopic ratios of capelin. Fractionation of δ15N differs depending on the type of nitrogen compounds used by primary producers. Atmospheric nitrogen (N2) fixers such as phytoplankton generally have a small range of δ15N (−2 to 2 ‰), whereas nitrate, nitrite, ammonia, and ammonium fixers such as benthic marine plants and terrestrial plants have a much greater range (−8 to 3 ‰), leading to a wide range of possible values of coastal basal resources (Fry 2007). As mentioned previously, the relationship between δ13C and δ15N suggests that δ15N becomes more enriched where terrestrial inputs are highest. It is likely that the increased diversity in basal resources caused by the increased terrestrial inputs led to an increase in trophic level variation, thus supporting more trophic levels than areas with less terrestrial input (Layman et al. 2007b). This suggests that Arctic coastal food webs may gain complexity and productivity as Arctic warming continues to increase the magnitude of freshwater discharge (Peterson et al. 2002).
This logic leads us to consider the types of prey items being consumed as an explanation for patterns in nitrogen isotopes. The majority of capelin analyzed may be classified into the lower half of the size class defined as juveniles (75–100 mm) by Vesin et al. (1981), a life stage at which gape and stomach size limits consumable prey items and variability in their diet. Dietary composition of these small capelin was dominated by Calanus copepods, with the average proportion of copepods in non-empty stomachs being 79.8 % (Fig. 7). Less common prey items including decapod larvae, chaetognaths, and the hyperiid amphipod T. libellula are only seen at the Point Barrow group. Isotopic ratios of different size copepods are not likely to differ greatly because of their low trophic position (Schell et al. 1998), but decapod larvae, chaetognaths, and T. libellula feed on copepods, thus adding a trophic level between capelin and copepods (Saito and Kiørboe 2001; Auel and Werner 2003). The inclusion of these less common prey types may be responsible for the enrichment observed in δ15N. Feeding data suggest that at the latitudes where δ15N became suddenly enriched (Nunivak Island and Point Barrow), capelin included less common prey items in their diet that would be expected to feed at higher trophic levels (chaetognaths and T. libellula, respectively; Fig. 7). These results suggest that higher trophic level prey items are present where terrestrial nutrients are abundant and are at least partially responsible for variations in δ15N with latitude.
Fish collected at Point Barrow were anomalous in all dependent variables (δ13C, δ15N, energy density, and RNA/DNA). These fish were collected with beach seines and thus are inhabitants of the very nearshore where conditions can be extremely variable in comparison to the coastal offshore waters where the other fish were collected with surface trawls. If these nearshore samples are comparable with the offshore samples in lower latitudes, we may expect that the energy density of sub-Arctic forage fish in the Arctic may increase as future high-latitude conditions change to resemble current lower-latitude conditions. This may offer an alternate high-quality prey source for Arctic piscivores as climate change continues.
However, it is also possible that these nearshore fish are not comparable with offshore samples and thus were removed from most analyses as they were significantly smaller in size than all other samples. The Point Barrow fish were significantly lower in energy density (p = 0.0004) and RNA/DNA (p = 0.0004) than capelin examined from other areas. When examining the other regional groups, energy density increases from Bristol Bay to Wainwright Inlet and RNA/DNA remains relatively constant; however, at Point Barrow both of these measures fell to some of the lowest values observed. Furthermore, diet data indicate that most of the nearshore fish had empty stomachs (61 %) suggesting that prey are sparsely distributed, and these fish may be undernourished or have difficulty locating prey. The anomalous enrichment of δ15N in these fish may support this premise. During starvation, animals will metabolize their own fat and muscle tissue to survive; in essence, they are eating themselves. This causes trophic fractionation and will make it appear as though they are feeding at a higher trophic level (Vander Zanden and Rasmussen 2001). The combination of poor condition and growth, coupled with evidence of low food availability and potential starvation, suggests that the Arctic nearshore habitats near Barrow may not be an optimal environment for juvenile capelin.
If these nearshore habitats are suboptimal, why were capelin highly abundant? These had the highest surface temperatures (11 °C) and were much higher than the optimal growth temperatures (~9 °C), which is not likely to motivate capelin to inhabit these waters. One explanation for their abundance is that these shallow and turbid nearshore waters offer an advantage over nearby habitats such as refuge from abundant local predators (belugas, seals, and sea birds), which are commonly observed feeding in the nearshore. However, it is also possible that juvenile capelin are blown or advected into suboptimal nearshore areas by strong wind and currents, and therefore, their presence in the Arctic nearshore near Barrow is not by choice.
Conclusions
The combination of growth and condition indices with SIA is a useful method to understand how isotopic food sources contribute to fish production. We suggest that capelin may be an ideal species for such analysis and can provide insight into how the ecosystem may restructure under different climate scenarios, as they have a key trophic position in the ecosystem and a wide latitudinal range. A benefit of using a wide-ranging species such as capelin is that their response to different environmental conditions can be considered a natural experiment; however, as we have pointed out, this also requires additional information to interpret at regional scales. The Chukchi Sea is a sink for biota advected from the Bering Sea, and therefore, communities associated with warm conditions in the Bering Sea may serve as proxies to examine Arctic community level dynamics in the face of warming conditions (Walsh et al. 2004; Woodgate et al. 2012; Coyle et al. 2013). Consequently, examining the response of capelin to a range of physiological conditions may offer a better understanding of how populations of these important forage fish and the community in the Chukchi will respond to climate change. This study has revealed large-scale variation in condition of capelin, but the mechanisms behind this variation need to be further explored.
Varying terrestrial inputs are one mechanism that likely affected capelin condition. Habitats with greater terrestrial inputs are likely to have higher trophic diversity and more basal resources based on ranges of isotopic ratios. This is an important premise as freshwater inputs are expected to increase with climate change, and thus, we may expect to see more complex and more productive Arctic coastal food webs to develop in response. Two important questions arise from our results: (1) Will climate change lead to higher energy density of sub-Arctic forage fish in the Arctic and offer alternate high-quality forage to nearshore piscivores? (2) Are capelin utilizing suboptimal nearshore habitats near Barrow to avoid predation, or are they advected there through their ontogeny? We suggest that additional studies be developed to examine energetics and SIA of capelin in Arctic and sub-Arctic habitats to better elucidate the mechanisms that underlie these patterns.
Change history
11 April 2017
An erratum to this article has been published.
References
Anthony JA, Roby DD, Turco KR (2000) Lipid content and energy density of forage fishes from the northern Gulf of Alaska. J Exp Mar Biol Ecol 248:53–78. doi:10.1016/S0022-0981(00)00159-
Arimitsu ML, Piatt JF, Litzow MA et al (2008) Distribution and spawning dynamics of capelin (Mallotus villosus) in Glacier Bay, Alaska: a cold water refugium. Fish Oceanogr 17:137–146. doi:10.1111/j.1365-2419.2008.00470.x
Auel H, Werner I (2003) Feeding, respiration and life history of the hyperiid amphipod Themisto libellula in the Arctic marginal ice zone of the Greenland Sea. J Exp Mar Biol Ecol 296:183–197. doi:10.1016/S0022-0981(03)00321-6
Benke A, Cushing C (2006) Rivers of North America, 1st edn. Elsevier Academic Press, Burlington
Biro PA, Post JR, Abrahams MV (2005) Ontogeny of energy allocation reveals selective pressure promoting risk-taking behaviour in young fish cohorts. Proc R Soc B 272:1443–1448. doi:10.1098/rspb.2005.3096
Caldarone EM, Wagner M, Onge-burns JS, Buckley LI (2001) Protocol and guide for estimating nucleic acids in larval fish using a fluorescence microplate reader. Northeast Fish Sci Cent Ref Doc 1:1–22
Chambers RC, Leggett WC (1987) Size and age at metamorphosis in marine fishes: an analysis of laboratory-reared winter flounder (Pseudopleuronectes americanus) with a review of variation in other species. Can J Fish Aquat Sci 44:1936–1947
Chambers RC, Leggett WC, Brown JA (1989) Egg size, female effects, and the correlations between early life history traits of capelin, Mallotus villosus: an appraisal at the individual level. Fish Bull 87:515–523
Chan P, Halfar J, Williams B et al (2011) Freshening of the Alaska coastal current recorded by coralline algal Ba/Ca ratios. J Geophys Res: Biogeosci 116:1–8. doi:10.1029/2010JG001548
Conover RJ (1988) Comparative life histories in the genera Calanus and Neocalanus in high latitudes of the northern hemisphere. Hydrobiologia 167:127–142. doi:10.1007/BF00026299
Coyle KO, Pinchuk AI (2005) Seasonal cross-shelf distribution of major zooplankton taxa on the northern Gulf of Alaska shelf relative to water mass properties, species depth preferences and vertical migration behavior. Deep-Sea Res Part II 52:217–245. doi:10.1016/j.dsr2.2004.09.025
Coyle KO, Gibson GA, Hedstrom K et al (2013) Zooplankton biomass, advection and production on the northern Gulf of Alaska shelf from simulations and field observations. J Mar Syst 128:185–207. doi:10.1016/j.jmarsys.2013.04.018
DeNiro M, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Ac 42:495–506
DeNiro M, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Ac 45:341–351
Dunton KH, Goodall JL, Schonberg SV et al (2005) Multi-decadal synthesis of benthic–pelagic coupling in the western arctic: role of cross-shelf advective processes. Deep-Sea Res Part II 52:3462–3477. doi:10.1016/j.dsr2.2005.09.007
Dunton KH, Weingartner T, Carmack EC (2006) The nearshore western Beaufort Sea ecosystem: circulation and importance of terrestrial carbon in arctic coastal food webs. Prog Oceanogr 71:362–378. doi:10.1016/j.pocean.2006.09.011
Dunton KH, Grebmeier JM, Trefry JH (2014) The benthic ecosystem of the northeastern Chukchi Sea: an overview of its unique biogeochemical and biological characteristics. Deep-Sea Res Part II 102:1–8. doi:10.1016/j.dsr2.2014.01.001
Dutta K, Schuur EAG, Neff JC, Zimov SA (2006) Potential carbon release from permafrost soils of northeastern Siberia. Glob Change Biol 12:2336–2351. doi:10.1111/j.1365-2486.2006.01259.x
Eisner L, Hillgruber N, Martinson E, Maselko J (2013) Pelagic fish and zooplankton species assemblages in relation to water mass characteristics in the northern Bering and southeast Chukchi seas. Polar Biol 36:87–113. doi:10.1007/s00300-012-1241-0
Fry B (2007) Stable isotope ecology. Springer, Berlin
Gilman SE, Urban MC, Tewksbury J et al (2010) A framework for community interactions under climate change. Trends Ecol Evol 25:325–331. doi:10.1016/j.tree.2010.03.002
Gjøsæter H, Båmstedt U (1998) The population biology and exploitation of capelin (Mallotus villosus) in the Barents Sea. Sarsia 83:453–496
Grebmeier JM (2012) Shifting patterns of life in the Pacific Arctic and sub-Arctic seas. Annu Rev Mar Sci 4:63–78. doi:10.1146/annurev-marine-120710-100926
Grebmeier JM, Mcroy CP, Feder HM (1988) Pelagic-benthic coupling on the shelf of the northern Bering and Chukchi Seas. I. Food supply source and benthic biomass. Mar Ecol Prog Ser 48:57–67
Grebmeier JM, Overland JE, Moore SE et al (2006) A major ecosystem shift in the northern Bering Sea. Science 311:1461–1464. doi:10.1126/science.1121365
Hansen J, Hedeholm R, Sünksen K et al (2012) Spatial variability of carbon (δ13C) and nitrogen (δ15 N) stable isotope ratios in an Arctic marine food web. Mar Ecol Prog Ser 467:47–59. doi:10.3354/meps09945
Heintz RA, Siddon EC, Farley EV, Napp JM (2013) Correlation between recruitment and fall condition of age-0 pollock (Theragra chalcogramma) from the eastern Bering Sea under varying climate conditions. Deep-Sea Res Part II 94:150–156. doi:10.1016/j.dsr2.2013.04.006
Helfield J, Naiman R (2016) Salmon and alder as nitrogen sources to riparian forests in a boreal Alaskan watershed. Oecologia 133:573–582
Hobson K, Welch H (1992) Determination of trophic relationships within a high arctic marine food web using Delta-13C and Delta-15 N analysis. Mar Ecol Prog Ser 84:9–18
Hood E, Scott D (2008) Riverine organic matter and nutrients in southeast Alaska affected by glacial coverage. Nat Geosci 1:583–587. doi:10.1038/ngeo280
Hop H, Gjøsæter H (2013) Polar cod (Boreogadus saida) and capelin (Mallotus villosus) as key species in marine food webs of the Arctic and the Barents Sea. Mar Biol Res 9:878–894. doi:10.1080/17451000.2013.775458
Hopcroft RR, Kosobokova KN, Pinchuk AI (2010) Zooplankton patterns in the Chukchi Sea during summer 2004. Deep-Sea Res Part II 57:27–39. doi:10.1016/j.dsr2.2009.08.003
Houlihan DF, Pedersen BH, Steffensen JF, Brechin J (1995) Protein synthesis, growth, and energetics in larval herring (Clupea harengus) at different feeding regimes. Fish Physiol Biochem 14:195–208
Huss M, Byström B, Strand A, Eriksson L, Persson L (2008) Influence of growth history on the accumulation of energy reserves and winter mortality in young fish. Can J Fish Aquat Sci 65:2149–2156
Johnson SW, Thedinga JF, Neff AD, Hoffman CA (2010) Fish fauna in nearshore waters of a barrier island in the western Beaufort Sea, Alaska. US Dep Commer, NOAA Tech Memo NMFSAFSC 210:1–28
Kline TC (1999) Temporal and spatial variability of 13C/12C and 15 N/14 N in pelagic biota of Prince William Sound, Alaska. Can J Fish Aquat Sci 56:94–117
Layman CA, Arrington DA, Montaña CG et al (2007a) Can stable isotope ratios provide for community-wide measures of trophic structure. Ecology 88:42–48
Layman CA, Quattrochi JP, Peyer CM, Allgeier JE (2007b) Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol Lett 10:937–944. doi:10.1111/j.1461-0248.2007.01087.x
Litzow MA, Mueter FJ (2014) Assessing the ecological importance of climate regime shifts: an approach from the North Pacific Ocean. Prog Oceanogr 120:110–119. doi:10.1016/j.pocean.2013.08.003
Loecher M, Ropkins K (2015) R googlemaps and loa: unleashing R graphics power on map tiles. J Stat Softw 63:1–18
Logan JM, Jardine TD, Miller TJ et al (2008) Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. J Anim Ecol 77:838–846. doi:10.1111/j.1365-2656.2008.01394.x
McConnaughey T, McRoy CP (1979) Food-Web structure and the fractionation of carbon isotopes in the Bering sea. Mar Biol 53:257–262. doi:10.1007/BF00952434
Mogensen S, Post JR (2012) Energy allocation strategy modifies growth-survival trade-offs in juvenile fish across ecological and environmental gradients. Oecologia 168:923–933. doi:10.1007/s00442-011-2164-0
Moline MA, Karnovsky NJ, Brown Z et al (2008) High latitude changes in ice dynamics and their impact on polar marine ecosystems. Ann NY Acad Sci 1134:267–319. doi:10.1196/annals.1439.010
Nemeth MJ, Priest J, Degan DJ, Shippen K, Link MR (2014) Sockeye salmon smolt abundance and inriver distribution: results from the Kvichak, Ugashik, and Egegik rivers in Bristol Bay, Alaska, 2014
Olsen EM, Lilly GR, Heino M et al (2005) Assessing changes in age and size at maturation in collapsing populations of Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 62:811–823. doi:10.1139/f05-065
Parkinson CL, Comiso JC (2013) On the 2012 record low Arctic sea ice cover: combined impact of preconditioning and an August storm. Geophys Res Lett 40:1356–1361. doi:10.1002/grl.50349
Peterson BJ, Fry B (2014) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Peterson BJ, Holmes RM, McClelland JW et al (2002) Increasing river discharge to the Arctic ocean. Science 298:2171–2173. doi:10.1126/science.1077445
Pinchuk AI, Coyle KO, Farley EV, Renner HM (2013) Emergence of the Arctic Themisto libellula (Amphipoda: Hyperiidae) on the southeastern Bering Sea shelf as a result of the recent cooling, and its potential impact on the pelagic food web. ICES J Mar Sci 70:1244–1254
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718. doi:10.2307/3071875
Post JR, Parkinson EA (2001) Energy allocation strategy in young fish: allometry and survival. Ecology 82:1040–1051
Post DM, Layman CA, Arrington DA et al (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189. doi:10.1007/s00442-006-0630-x
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rypel AL (2012) Meta-analysis of growth rates for a circumpolar fish, the northern pike (Esox lucius), with emphasis on effects of continent, climate and latitude. Ecol Freshw Fish 21:521–532. doi:10.1111/j.1600-0633.2012.00570.x
Saito H, Kiørboe T (2001) Feeding rates in the chaetognath Sagitta elegans: effects of prey size, prey swimming behaviour and small-scale turbulence. J Plankton Res 23:1385–1398. doi:10.1093/plankt/23.12.1385
Schell D, Barnett B, Vinette K (1998) Carbon and nitrogen isotope ratios in zooplankton of the Bering, Chukchi and Beaufort seas. Mar Ecol Prog Ser 162:11–23. doi:10.3354/meps162011
Schuur EAG, Bockheim J, Canadell JG et al (2008) Vulnerability of permafrost carbon to climate change: implications for the global carbon cycle. Bioscience 58:701–714. doi:10.1641/B580807
Sherwood GD, Rideout RM, Fudge SB, Rose GA (2007) Influence of diet on growth, condition and reproductive capacity in Newfoundland and Labrador cod (Gadus morhua): insights from stable carbon isotopes (δ13C). Deep-Sea Res Part II 54:2794–2809. doi:10.1016/j.dsr2.2007.08.007
Shoji J, Toshito SI, Mizuno KI et al (2011) Possible effects of global warming on fish recruitment: shifts in spawning season and latitudinal distribution can alter growth of fish early life stages through changes in day length. ICES J Mar Sci 68:1165–1169. doi:10.1093/icesjms/fsr059
Siddon EC, Heintz RA, Mueter FJ (2013) Conceptual model of energy allocation in walleye pollock (Theragra chalcogramma) from age-0 to age-1 in the southeastern Bering Sea. Deep-Sea Res Part II 94:140–149. doi:10.1016/j.dsr2.2012.12.007
Smith RW, Ottema C (2006) Growth, oxygen consumption, and protein and RNA synthesis rates in the yolk sac larvae of the African catfish (Clarias gariepinus). Comp Biochem Physiol A 143:315–325
Sorte CJB, Williams SL, Carlton JT (2010) Marine range shifts and species introductions: comparative spread rates and community impacts. Glob Ecol Biogeogr 19:303–316. doi:10.1111/j.1466-8238.2009.00519.x
Sreenivasan A (2011) Nucleic acid ratios as an index of growth and nutritional ecology in Pacific cod (Gadus macrocephalus), walleye pollock (Theragra chalcogramma), and Pacific herring (Clupea pallasii). University of Alaska Fairbanks, Fairbanks
Stabeno PJ, Reed RK, Schumacher JD (1995) The Alaska coastal current: continuity of transport and forcing. J Geophys Res 100:2477–2485
Steele J, Thorpe S, Turekian K (2010) Ocean currents: a derivative of the encyclopedia of ocean sciences. Academic Press, London
Sturdevant MV, Orsi JA, Fergusson EA (2012) Diets and trophic linkages of epipelagic fish predators in coastal southeast Alaska during a period of warm and cold climate years, 1997–2011. Mar Coast Fish 4:526–545. doi:10.1080/19425120.2012.694838
Van Noordwijk AJ, De Jong G (2014) Acquisition and allocation of resources: their influence on variation in life history tactics. Am Nat 128:137–142
Vander Zanden M, Rasmussen J (2001) Variation in 15 N and 13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Vesin J, Legget W, Able K (1981) Feeding ecology of capelin (Mallotus villosus) in the estuary and Western Gulf of St. Lawrance and its multispecies implications. Can J Fish Aquat Sci 38:257–267
Vollenweider JJ, Heintz RA, Schaufler L, Bradshaw R (2011) Seasonal cycles in whole-body proximate composition and energy content of forage fish vary with water depth. Mar Biol 158:413–427. doi:10.1007/s00227-010-1569-3
Walsh JJ, Dieterle DA, Maslowski W, Whitledge TE (2004) Decadal shifts in biophysical forcing of Arctic marine food webs: numerical consequences. J Geophys Res C Ocean 109:1–30. doi:10.1029/2003JC001945
Weber LP, Higgins PS, Carlson RI, Janz DM (2003) Development and validation of methods for measuring multiple biochemical indices of condition in juvenile fishes. J Fish Biol 63:637–658. doi:10.1046/j.1095-8649.2003.00178.x
Weingartner TJ, Danielson SL, Royer TC (2005) Freshwater variability and predictability in the Alaska coastal current. Deep-Sea Res Part II 52:169–191. doi:10.1016/j.dsr2.2004.09.030
Woodgate RA, Weingartner TJ, Lindsay R (2012) Observed increases in Bering Strait oceanic fluxes from the Pacific to the Arctic from 2001 to 2011 and their impacts on the Arctic Ocean water column. Geophys Res Lett 39:2–7. doi:10.1029/2012GL054092
Acknowledgments
We thank Franz Mueter and the rest of the Arctic EIS crew for providing samples, as well as the scientists and crewmen aboard the R/V Oscar Dyson. Special thanks to Craig George, Leandra de Sousa, Todd Sformo, Robert Suydam, and the North Slope Borough Division of Wildlife, who provided significant logistical support for collection of samples in Barrow. Additional thanks to those NOAA affiliates who processed samples in the bioenergetics laboratory, including A Robertson, M Callahan, A Sreenivasan, E Fergusson, J Weems, and H Findley. Funding was provided in part by the North Pacific Research Board to KM Boswell (Project 1229) and in part from BOEM Cooperative Agreement Contract Nos. M12PG00024 (ACES) and M12PG00018 (Arctic EIS) of the US Department of the Interior, Bureau of Ocean Energy Management (BOEM), Alaska Outer Continental Shelf Region, Anchorage Alaska as part of the BOEM Environmental Studies Program. This report is funded in part with qualified outer continental shelf oil and gas revenues by the Coastal Impact Assistance Program, Fish and Wildlife Service, US Department of the Interior under Agreement Number 10-CIAP-010, F12AF00188. This is contribution No. 18 from the Marine Education and Research Center in the Institute for Water and Environment at Florida International University.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised with an updated Fig. 3.
An erratum to this article is available at https://doi.org/10.1007/s00300-017-2097-0.
Rights and permissions
About this article
Cite this article
Barton, M.B., Moran, J.R., Vollenweider, J.J. et al. Latitudinal dependence of body condition, growth rate, and stable isotopes of juvenile capelin (Mallotus villosus) in the Bering and Chukchi Seas. Polar Biol 40, 1451–1463 (2017). https://doi.org/10.1007/s00300-016-2041-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-2041-8