Abstract
In order to understand the migratory history and habitat use of the tropical anguillid eels Anguilla celebesensis, A. marmorata, and A. bicolor bicolor, the otolith strontium (Sr) and calcium (Ca) concentrations were examined for eels collected in Indonesian waters. In A. bicolor bicolor collected in a lagoon, the change in Sr:Ca ratios outside the high Sr:Ca core generally indicated two patterns of habitat residence: (1) constant living in either brackish or sea waters with no freshwater life (25%) and (2) habitat shifts from fresh water to brackish or sea waters (75%). No A. bicolor bicolor had a general life history as a freshwater resident. A. celebesensis and A. marmorata from the uppermost freshwater lake showed freshwater life history patterns. The wide range of otolith Sr:Ca ratios in A. bicolor bicolor indicated that the habitat use of this tropical eel was facultative among fresh, brackish, and marine waters during the growth phase after recruitment to coastal areas similar to that for temperate eels. Thus, the migration of anguillid eels into fresh water is clearly not an obligatory.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The eels of the genus Anguilla, being catadromous, migrate between freshwater growth habitats and offshore spawning areas. Fifteen species of Anguilla have been reported worldwide, ten of which occur in tropical regions (Ege 1939). Of the latter, seven species/subspecies occur in the western Pacific around Indonesia, i.e. A. celebesensis, A. interioris, A. nebulosa nebulosa, A. marmorata, A. borneensis, A. bicolor bicolor, and A. bicolor pacifica (Ege 1939; Castle and Williamson 1974; Arai et al. 1999). Mitochondrial DNA analysis has revealed that A. borneensis from Borneo Island was closest to the ancestral form among the 15 presently known species (Aoyama et al. 2001). Furthermore, the tropical species seem to be more closely related to the ancestral form than their temperate counterparts. Aoyama et al. (2001) suggested that freshwater eels originated in the present-day Indonesian region during the Cretaceous. Thus, studying the life history and migration of tropical eels may provide some clues to understanding the nature of primitive forms of catadromous migration in anguillid eels and how the migration of the genus became established. The results may also contribute to understanding the evolutionary pathway of migration in the genus as well as other diadromous fish species that migrate between freshwater and seawater habitats.
Examinations of otolith microchemistry have revealed considerable information on the life history of temperate Anguilla species including A. japonica, A. anguilla, A. rostrata, A. australis, and A. dieffenbachii (Tzeng et al. 2000; Tsukamoto and Arai 2001; Arai et al. 2004, 2006; Shiao et al. 2003; Kotake et al. 2003, 2005; Daverat et al. 2006; Chino and Arai 2009). Those studies have revealed that some yellow and silver stage-eels of temperate anguillid eels never migrate into fresh water but spend their entire life in the ocean. Further, the use of otolith Sr:Ca ratios to trace the migratory history of eels also revealed otolith signatures that were intermediate to those of temperate eels that were marine residents and those that were freshwater residents appeared to reflect estuarine residence or showed clear evidence of switching between different salinity environments. It thus appears that a proportion of eels move frequently between different environments during their growth phase. Therefore, because individuals of several anguillid species have been found to remain in estuarine or marine habitats, it appears that anguillid eels do not all enter freshwater environments, and these species may be facultatively catadromous (Tsukamoto and Arai 2001). However, such information has been obtained mainly from temperate species, and little is known about the life history and environmental habitat use of tropical species.
It is not clear why during the growth phase some eels migrate to fresh water and others do not. The occurrence of fish migration is generally explained by a difference in food abundance between marine and freshwater habitats, and Gross (1987) proposed that diadromy occurs when the gain in fitness from using a second habitat minus the migration costs of moving between habitats exceeds the fitness from staying in only one habitat. Juvenile anadromous salmon utilize freshwater habitats at high latitudes with low productivity, and they migrate to higher productivity habitats in the ocean for growth before returning to fresh water for breeding. In contrast, catadromous freshwater eels that recruit at low latitudes might migrate upstream into freshwater habitats of higher productivity for growth before returning to the ocean for breeding. Thus, to understand comprehensive migration strategy in the anguillid eels, the details of migration of tropical eels must be revealed.
This study examines the otolith microchemistry of the tropical species Anguilla celebesensis, A. marmorata, and A. bicolor bicolor collected in Indonesian waters. Habitat preference and movements between habitats were examined and compared with previous studies on eel migration by tropical and temperate eels in an attempt to understand the occurrence and evolution of migration of Anguilla species.
Materials and methods
Fish
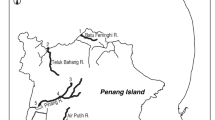
A total of 59 specimens of three tropical eel species, Anguilla celebesensis, A. marmorata, and A. bicolor bicolor, were collected in Indonesian waters. A total of 42 Anguilla bicolor bicolor were collected in Segara Anakan, central Java Island, Indonesia in December 2007 (around 7°35′–7°48 S, 108°46–109°03′E) (Fig. 1). The eels were collected by angling and bamboo traps at night. The Segara Anakan consists of a lagoon isolated from the Indian Ocean by Nusakambangan Island (Fig. 1) and comprises a mangrove forest area, a settlement area, and rice field areas. This lagoon is the only major estuarine-mangrove area in the central part of Java. Two seasons affect the area, the rainy or wet season, from November to April, and the dry season, from July to September. This equatorial climate has a high average monthly temperature between 28.0°C (dry season) and 31.3°C (wet season) (White et al. 1989). The water temperature fluctuates throughout the year between 27.5°C (dry season) and 35.0°C (wet season). The average monthly rainfall ranges from 100 to 180 mm during the dry season, and from 180 to 400 mm during the wet season (White et al. 1989). Therefore, salinity fluctuates highly between these seasons ranging from 8 to 28 ppt in the dry season, and from 1 to 20 ppt in the wet season (White et al. 1989).
A total of 15 Anguilla celebesensis and 2 A. marmorata were collected by bamboo trap in Lake Poso, central Sulawesi Island, Indonesia (around 1°03′–1°58 S, 119°57–120°22′E) (Fig. 1). This lake is the third biggest freshwater lake in Indonesia and is approximately 30-km long and 16-km wide. The water is approximately 360-m deep in the south and 500-m in the north. The lake is connected to the sea by a single river, the Poso River, which drains the water from an elevation of 512 m past a waterfall and down to the sea along a 40-km stretch. The water temperature in the lake ranges from 27 to 31°C (Haffiner et al. 2001), and its salinity is 0 ppt.
Otolith preparation and microchemical analysis
After the measurement of the morphological characteristics in each specimen, sagittal otoliths were extracted from each fish, embedded in epoxy resin (Struers, Epofix), and mounted on glass slides. The otoliths were then ground and polished, as described by Arai et al. (2004, 2006). They were then cleaned in an ultrasonic bath and rinsed with deionized water prior to being examined. For electron microprobe analyses, all otoliths were Pt–Pd coated by a high vacuum evaporator. All otoliths were used for “life-history transect” analysis of Sr and Ca concentrations using a JEOL JXA-8900R Electron Probe Microanalyzer as described by Arai et al. (2004, 2006). “X-ray intensity maps” of both elements were also made following Arai et al. (2004, 2006). Otoliths of five of the specimens of Anguilla bicolor bicolor used for the life-history transects were also used in the X-ray intensity map analyses. We calculated the average Sr:Ca ratios for the values outside the elver mark and according to the criteria of Tsukamoto and Arai (2001). We categorized the specimens into “marine resident” (Sr:Ca ≥ 6.0 × 10−3) and “estuarine resident” (2.5 × 10−3 ≤ Sr:Ca < 6.0 × 10−3).
Age estimation and data analyses
Following the microchemistry analyses, the otoliths were repolished to remove the coating. Otoliths were then etched with 1% HCl for 60 s, stained with 1 % toluidine blue, and aged by counting the number of blue-stained transparent zones, as reported in Arai et al. (2004). The ages given in this study are up to the last annuli and do not include any additional daily age of less than 1.
Results
Biological characteristics
The total length (TL) of Anguilla bicolor bicolor collected from Segara Anakan ranged from 485 to 746 mm, with a mean ± SD of 564.6 ± 64.3 mm (n = 42) (Table 1). The wet body weight (BW) ranged from 161.0 to 962.6 g, with a mean ± SD of 310.6 ± 177.8 g (n = 42). The TL of A. celebesensis collected from Lake Poso ranged from 601 to 1,039 mm, with a mean ± SD of 771.7 ± 99.46 mm, and BW ranged from 500 to 2,550 g, with a mean ± SD of 970.6 ± 500.76 g (n = 15), respectively. The TLs of A. marmorata collected from the lake were 832 mm and 925 mm, and BWs were 2,050 and 2,360 g (n = 2).
The annuli in the otoliths of A. bicolor bicolor ranged from 8 to 18 years, with a mean ± SD of 12.9 ± 3.23 year (n = 16). Annuli in the otoliths of A. celebesensis ranged from 12 to 23 years, with a mean ± SD of 16.8 ± 3.31 year (n = 10). The annuli of A. marmorata were 11 and 12 year (n = 2). The criteria used to interpret the otolith annuli in temperate eels were adopted for use in the present study. However, the determination of annuli in tropical eels is very difficult due to the existence of some false annuli. In this study, we counted annuli just for easily countable specimens.
Otolith strontium distribution
Two-dimensional images of the Sr concentration in otoliths showed remarkable variation among the specimens examined (Fig. 2) in Anguilla bicolor bicolor. However, the centers of the otoliths of all specimens had a high-Sr area (a red oval spot about 120–220 μm from the otolith center) that was surrounded by an elver mark that could be observed with a light microscope.
Two-dimensional images made using X-ray electron microprobe analysis of the Sr concentrations in the otoliths of Anguilla bicolor bicolor collected in Segara Anakan, central Java, Indonesia. a Consistent yellowish-green color between the otolith core and the edge suggesting medium Sr concentration; b two contrasts involving a bluish color (low Sr concentration) surrounding a yellowish color (medium Sr concentration); c several contrasts involving bluish (low Sr concentration) and yellowish (medium Sr concentration) colors
Anguilla bicolor bicolor collected in Segara Anakan showed two apparent patterns from outside the high-Sr area in the otolith center region to the edge. One of the patterns showed a uniformly yellowish-green color throughout the otolith, suggesting a medium Sr concentration (Fig. 2a). The other pattern showed two contrasts involving a bluish color (low Sr concentration) surrounding a yellowish-green color (medium Sr concentration) (Fig. 2b). Further, the latter pattern showed another narrow bluish phase in the yellowish area (Fig. 2c).
Life-history transects
The Sr:Ca ratios in the transects along the radius of each otolith showed a common feature in all specimens around the center of the otolith (Fig. 3) of a peak of high values of Sr:Ca ratios (12.7 × 10−3–16.4 × 10−3) from the otolith core out to approximately 160 μm, which corresponded to the high Sr core visible in the two-dimensional images in Fig. 2.
Plots of the otolith Sr:Ca ratios along transect lines from the core to the edge of the otolith of representative cases from all specimens (59 specimens) collected from Segara Anakan and Lake Poso in Indonesia. a The constant habitat residence type lived in estuarine/marine water (A. bicolor bicolor); b the switch type shifted residence from fresh water to marine water (A. bicolor bicolor); and c a typical catadromous life history pattern (A. celebesensis, A. marmorata)
Outside of the high Sr:Ca core, there was great variation in the change of the Sr:Ca ratios in the otoliths of eels from different habitats. The migratory patterns that revealed the life history of A. bicolor bicolor in the Segara Anakan were generally classified into two types (Fig. 3a, b). The first pattern can be labeled the constant type, with constant Sr:Ca ratios (range: 5.39–7.40 × 10−3; mean ± SD 6.27 × 10−3 ± 0.74 × 10−3 Fig. 3a) along the life-history transect, which suggests that these specimens (n = 5) lived in the same environment throughout their lives. The second pattern can be labeled the switch type with Sr:Ca ratios shifting from low phase (range: 0.77–2.34 × 10−3; mean ± SD 1.61 × 10−3 ± 0.46 × 10−3) to high phase (range: 6.01–7.62 × 10−3; mean ± SD 6.53 × 10−3 ± 0.50 × 10−3) between 390 and 890 μm (mean ± SD 708 ± 153.31 μm) from the otolith core (Fig. 3b), which suggests that these specimens (n = 15) shifted their residence environment. Further, two specimens of this switch type showed multiple shifts, with several low and high Sr:Ca ratio phases along the life-history transect, which suggests that these specimens moved to different environments several times in their lives. There were significant differences between the low and high Sr:Ca ratio phases (Mann–Whitney U-test P < 0.0001) for all switch-type specimens. A. celebesensis and A. marmorata specimens collected from Lake Poso showed uniformly low Sr:Ca ratios along the life-history transect (Fig. 3c). This suggests that they spent their entire lives in a freshwater environment. We excluded the Sr:Ca ratio results in A. marmorata from further discussion due to the limited sample size (n = 2).
Anguilla bicolor bicolor had a wide range of otolith Sr:Ca ratios, which ranged from 0.33 × 10−3 to 12.00 × 10−3 (Fig. 4). The wide range of otolith Sr:Ca ratios indicated that the habitat use of A. bicolor bicolor was variable after their recruitment to the coastal waters as glass eels. However, all specimens from Lake Poso showed less variability with low Sr:Ca values ranging from 0.59 × 10−3 to 2.26 × 10−3 with a mean ± SD of 1.06 × 10−3 ± 0.52 × 10−3 (Fig. 4). Most of these values were <2.5 × 10−3, and thus the Sr:Ca values were a useful indicator of the partitioning of freshwater residence in the present study (Fig. 4).
Discussion
The most significant finding of this study was that both X-ray map and line history transect analyses showed that the otolith Sr contents and Sr:Ca ratios were remarkably variable among tropical eel Anguilla bicolor bicolor specimens collected in Segara Anakan, Indonesia. Recently, the migratory histories of temperate anguillid eels have been studied using the same microchemical techniques on their otoliths. The Sr:Ca ratio in the otoliths of those fishes differed according to the time they spent in fresh water, brackish water, and sea water (Tzeng et al. 2000; Tsukamoto and Arai 2001; Arai et al. 2004, 2006; Kotake et al. 2003, 2005; Chino and Arai 2009). Studies of the incorporation of strontium into otoliths have found that the Sr:Ca values in otoliths strongly correlated with the salinity of the ambient water (Tzeng 1996). Thus, the Sr:Ca ratios of otoliths could help in determining whether or not individual eels actually enter fresh water at the elver stage and remain in freshwater, brackish water, or seawater environments until the silver eel stage, or whether they move among different habitats with differing salinity regimes. These findings support the observations of previous studies that the influence of salinity on the Sr content or Sr:Ca ratios in the otoliths of anguillid eels can be used to evaluate habitat residence. In A. bicolor bicolor, the change in Sr:Ca ratios outside the high Sr:Ca core was generally divided into two patterns: (1) the constant type (25% of the eels examined) (Fig. 3a) and (2) the switch type (75%) (Figs. 3b, 5). To determine the Sr:Ca value that corresponds to freshwater residence, we used the mean Sr:Ca values of the eels A. celebesensis collected in the freshwater Lake Poso. All A. celebesensis eels showed constantly low Sr:Ca values(<2.5 × 10−3), which could thus be a useful indicator of freshwater residence (Fig. 4). Based on the criteria, it was observed that constant-type A. bicolor bicolor resided in two different constant environments throughout their lives. The first pattern was marine residence throughout their lives, and the second was estuarine residence. Further, the switch-type eels were confirmed to shift habitats from freshwater areas to seawater areas (Figs. 2b, 3b). Also, two of 15 fishes of the switch type showed multiple movements between freshwater and seawater environments (Fig. 2c). The occurrence of a fully marine resident life history for some and largely marine resident history for most A. bicolor bicolor is the first finding in the tropical eels. These findings strongly suggested that the tropical eel A. bicolor bicolor also has a flexible migration strategy with a high degree of behavioral plasticity and an ability to utilize the full range of salinity. Such diverse behavior is similar to that of temperate eels, A. japonica (Tsukamoto and Arai 2001; Kotake et al. 2003, 2005; Chino and Arai 2009), A. anguilla (Tzeng et al. 2000; Arai et al. 2006), A. rostrata (Daverat et al. 2006), A. australis, and A. dieffenbachii (Arai et al. 2004), which migrate flexibly among freshwater, brackish water, and seawater environments.
In this study, the migratory patterns of A. bicolor bicolor were mainly of the switch type involving moving from freshwater to seawater environments (75%) (Fig. 5). The other eels resided consistently in either seawater or brackish water. No A. bicolor bicolor showed constant freshwater residence in Segara Anakan, Indonesia (Fig. 5). Unlike this study, some A. bicolor bicolor in the Philippines showed freshwater residence (37%), while most showed estuarine residence (63%) (Briones et al. 2007) (Fig. 5).
Fish migration is generally explained by a difference in food abundance between marine and freshwater habitats (Gross 1987). Based on this theory, catadromous freshwater eels that recruit at low latitudes might migrate upstream into freshwater habitats with higher productivity for growth before returning to the ocean for breeding. However, some A. bicolor bicolor spent their entire lives in seawater and brackish water environments, with no occurrence of general freshwater residence in the tropical equatorial region of the present study. Therefore, the hypothesis may not apply that a latitudinal gradient in freshwater residency of anguillid eels occurs, with a higher frequency of freshwater residency at lower latitudes where the productivity is higher in freshwater than in the ocean.
Freshwater eels of the genus Anguilla are considered to have originated from a marine ancestor (Tesch 1977). All anguilliform fishes except Anguilla are marine species, and the marine breeding habits of Anguilla are probably a conserved trait. This suggests the hypothesis that some tropical and temperate species of catadromous eels have never lost the ability to reside in marine habitats during the juvenile growth phase.
References
Aoyama J, Nishida M, Tsukamoto K (2001) Molecular phylogeny and evolution of the freshwater eel, genus Anguilla. Mol Phyl Evol 20:450–459
Arai T, Aoyama J, Limbong D, Tsukamoto K (1999) Species composition and inshore migration of the tropical eels, Anguilla spp., recruiting to the estuary of the Poigar River, Sulawesi Island. Mar Ecol Prog Ser 188:299–303
Arai T, Kotake A, Lokman PM, Miller MJ, Tsukamoto K (2004) Evidence of different habitat use by New Zealand freshwater eels, Anguilla australis and A. dieffenbachii, as revealed by otolith microchemistry. Mar Ecol Prog Ser 266:213–225
Arai T, Kotake A, McCarthy TK (2006) Habitat use by the European eel Anguilla anguilla in Irish waters. Estuar Coast Shelf Sci 67:569–578
Briones AA, Yambot AV, Shiao JC, Iizuka Y, Tzeng WN (2007) Migratory pattern and habitat use of tropical eels Anguilla spp. (Teleostei: Anguilliformes: Anguillidae) in the Philippines, as revealed by otolith microchemistry. Raffl Bull Zool 14:141–149
Castle PHJ, Williamson GR (1974) On the validity of the freshwater eel species Anguilla ancestralis Ege from Celebes. Copeia 2:569–570
Chino N, Arai T (2009) Relative contribution of migratory type on the reproduction of migrating silver eels, Anguilla japonica, collected off Shikoku Island, Japan. Mar Biol 156:661–668
Chino N, Arai T (2010) Migratory history of the giant mottled eel Anguilla marmorata in the Bonin Islands of Japan. Ecol Freshw Fish (in press)
Daverat F, Limberg KE, Thibault I, Shiao JC, Dodson JJ, Caron F, Tzeng WN, Iizuka Y, Wickström H (2006) Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Mar Ecol Prog Ser 308:231–241
Ege V (1939) A revision of the genus Anguilla Shaw. Dana Rep 16(13):8–256
Gross MR (1987) Evolution of diadromy in fishes. Am Fish Soc Symp 1:12–25
Haffiner GD, Hehanussa PE, Hartoto D (2001) The biology and physical processes of large lakes of Indonesia: lakes Matano and Towuti. In: Munawar M, Hecky RE (eds) The Great Lakes of the World (GLOW). Food web, health, and integrity. Backhuys Publishers, Leiden, pp 183–192
Kotake A, Arai T, Ozawa T, Nojima S, Miller MJ, Tsukamoto K (2003) Variation in migratory history of Japanese eels, Anguilla japonica, collected in coastal waters of the Amakusa Islands, Japan, inferred from otolith Sr/Ca ratios. Mar Biol 142:849–854
Kotake A, Okamura A, Yamada Y, Utoh T, Arai T, Miller MJ, Oka HP, Tsukamoto K (2005) Seasonal variation in migratory history of the Japanese eel, Anguilla japonica, in Mikawa Bay, Japan. Mar Ecol Prog Ser 293:213–221
Shiao JC, Iizuka Y, Chang CW, Tzeng WN (2003) Disparities in habitat use and migratory behaviour between tropical eel Anguilla marmorata and temperate eel A. japonica in four Taiwanese rivers. Mar Ecol Prog Ser 261:233–242
Tesch FW (1977) The Eel. Biology and management of anguillid eels. Chapman and Hall, London
Tsukamoto K, Arai T (2001) Facultative catadromy of the eel, Anguilla japonica, between freshwater and seawater habitats. Mar Ecol Prog Ser 220:365–376
Tzeng WN (1996) Effects of salinity and ontogenic movements on strontium:calcium ratios in the otoliths of the Japanese eel, Anguilla japonica Temminck & Schlegel. J Exp Mar Biol Ecol 199:111–122
Tzeng WN, Wang CH, Wickström H, Reizenstein M (2000) Occurrence of the semi-catadromous European eel Anguilla anguilla in the Baltic Sea. Mar Biol 137:93–98
White AT, Martosubroto P, Sadorra MSM (1989) The coastal environmental profile of Segara Anakan-Cilacap, South Java, Indonesia. ICLARM technical reports 25. International Center for Living Aquatic Resources Management, Manila
Acknowledgments
We are grateful to Dr. Daniel Limbong and Ms. Terry Louis Kepel for their kind assistance with the field survey. The authors also would like to thank Ms. Mayumi Otsuki for assistance with the chemical analysis of Ca and Sr. We thank the anonymous reviewers for their constructive comments. This work was supported in part by Grant-in-Aid No. 20688008 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. A. Peck.
Rights and permissions
About this article
Cite this article
Chino, N., Arai, T. Occurrence of marine resident tropical eel Anguilla bicolor bicolor in Indonesia. Mar Biol 157, 1075–1081 (2010). https://doi.org/10.1007/s00227-009-1388-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1388-6