Abstract
In order to understand the reproductive contribution among migratory types in the Japanese eel, Anguilla japonica, otolith strontium (Sr) and calcium (Ca) concentrations by X-ray electron microprobe analysis were examined for 37 silver eels collected in Kii Channel off Shikoku Island during the spawning migration season. The wide range of otolith Sr:Ca ratios indicated that the habitat use of A. japonica was not obligatory but facultative among fresh, brackish and marine waters during their growth phases after recruitment to the coastal areas as glass eels. Three migratory types, which were categorized as river eels, estuarine eels and sea eels were found. The estuarine eels were dominant (59%), followed by sea eels (22%) and river eels (19%). The low proportion of river eels from the spawning migration season suggested that the estuarine and sea eels inhabiting the nearby coastal areas might make a larger reproductive contribution to the next generation in this area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Japanese eel Anguilla japonica Temminck and Schlegel is widely distributed in East Asia, from Taiwan in the south, through eastern China and Korea, up to the Sanriku Coast of northern Honshu Island, Japan (Tesch 1977). A. japonica generally has been considered a catadromous fish species (McDowall 1988), which spawns in the north equatorial current to the west of the Mariana Islands. Their transparent leaf-like larvae (leptocephali) are transported from the spawning area toward the coastal waters of east Asia by the north equatorial and Kuroshio currents, where they metamorphose into glass eels. In general, the glass eels migrate upstream to grow into the elver and yellow eel stages in freshwater. At maturation, the yellow eels metamorphose into silver eels, which migrate downstream to the ocean to begin their spawning migration (Tesch 1977).
Otolith microchemistry studies have revealed that some yellow and silver eels of temperate A. japonica never migrate into freshwater, but spend their entire life history in the ocean (Tsukamoto and Arai 2001). The application of otolith Sr:Ca ratios to trace the migratory history of eels has also revealed otolith signatures intermediate to those of marine and freshwater residents of several anguillid species (Tzeng et al. 2000; Tsukamoto and Arai 2001; Arai et al. 2003a, 2003b, 2004, 2006; Shiao et al. 2003; Kotake et al. 2003, 2005; Daverat et al. 2006), all of which appeared to reflect estuarine residence or showed clear evidence of switching between different salinity environments. It thus appears that a proportion of eels move frequently between different environments during their growth phase. Therefore, because individuals of several anguillid species have been found to remain in estuarine or marine habitats, it appears that anguillid eels do not all enter into freshwater environments and that these species display more of a facultative catadromy (Tsukamoto and Arai 2001).
Although Sr:Ca ratios have been studied in the otoliths of yellow and silver eels of the five species of temperate anguillid eels, there have only been several studies of this nature on these species including A. japonica. Therefore, it is not known if all populations display the same utilization of both estuarine and marine environments in addition to the typical freshwater environments in the species. Furthermore, due to the difficulty of collecting silver eels in the open ocean, there is little information available on estimating the relative contribution of the migratory type to reproduction. To address this question, we analyzed the Sr:Ca ratios in the otoliths of silver eels of A. japonica that were caught offshore on the Pacific side of Japan.
In order to examine the variation of the migratory history of the Japanese eel, we determined the Sr:Ca ratios in the otoliths of silver eels collected during their spawning migration season. We discuss the implications of these findings in relation to the possible contribution of their migratory types, i.e., sea eels, estuarine eels and river eels to the spawning population of A. japonica.
Materials and methods
Fish
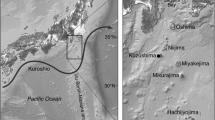
A total of 37 eels were collected in the Kii Channel off the eastern part of Shikoku Island, central Japan (around 33°00′–33°50′N, 134°80′–135°00′E; Fig. 1). The eels were collected at night by bottom trawling between 20 December 2007 and 9 January 2008.
After measurement of total length (TL), the sex of each eel was determined by visual observation of the gonads (Matsui 1972). Body color was observed to distinguish the developmental stage, and each individual was classified as a silver eel, after Matsui (1972). The gonad and body weights were measured to determine the gonad somatic index (GSI) of each eel. The GSI value was calculated as follows:
Otolith preparation
Sagittal otoliths were extracted from all specimens, and the otoliths were embedded in epoxy resin (Struers, Epofix). These otoliths were then ground to expose the core along the anterior–posterior direction in the frontal plane, using a grinding machine equipped with a diamond cup wheel (Struers, Discoplan-TS), and polished further with oxide polishing suspension on an automated polishing wheel (Struers, PdM-Force-20). Finally, they were cleaned using distilled water and ethanol, and dried at 50°C in an oven prior to examination. The ground surfaces of the otoliths were examined at 200× with a light microscope, and photographs were taken to measure the “radius” of the elver mark (the distance from the otolith core to the elver check).
Otolith X-ray microprobe analysis
For electron microprobe analyses, all otoliths were Pt–Pd coated by a high vacuum evaporator. Otoliths from all specimens were used for life-history transect analyses of Sr and Ca concentrations, which were measured along a line down the longest axis of each otolith from the core to the edge using a wavelength dispersive X-ray electron microprobe (JEOL JXA-8900R), as described in Arai et al. (2006). Wollastonite (CaSiO3) and Tausonite (SrTiO3) were used as standards. The accelerating voltage and beam current were 15 kV and 1.2 × 10−8 A, respectively. The electron beam was focused on a point 10 μm in diameter, with measurements spaced at 10 μm intervals.
We calculated the average Sr:Ca ratios for the values outside the elver mark. Following the criteria of Tsukamoto and Arai (2001), these specimens were categorized into “sea eels” (Sr:Ca ≥ 6.0 × 10−3), “estuarine eels” (2.5 × 10−3 ≤ Sr:Ca < 6.0 × 10−3) and “river eels” (Sr:Ca < 2.5 × 10−3).
Age determination
Following the electron microprobe analysis, the otoliths were repolished to remove the coating, etched with 1% HCl and thereafter stained with 1% toluidine blue. The age of the specimens was determined by counting the number of blue-stained transparent zones following the method of Arai et al. (2003a, b). The positions of the transparent zones were then correlated to elemental analysis points. The relative ages at particular elemental analysis points could then be assigned. The otolith radii for each age of the specimens were also measured. The total lengths of each age of the specimens were estimated from the growth rate and age of each fish. The growth rate was calculated as follows: growth rate = (TL − 6.0)/age, where 6.0 is the mean length (cm) of glass eels when they are recruited to a coastal area (Arai et al. 1997).
Statistics
Differences between data were tested using the Mann–Whitney U-test. Differences among data were tested using the Kruskal–Wallis test. The significance of the correlation coefficient and regression slope were tested by Fisher’s Z-transformation and an analysis of covariance (ANCOVA) (Sokal and Rohlf 1995).
Results
Biological characteristics
The eels collected in Kii Channel were predominantly females (89%, n = 33), with only 11% (n = 4) of the total catch of males in this area (Table 1).
The total length (TL) of the females ranged from 48.4 to 84.5 cm, with a mean ± SD of 67.0 ± 7.7 cm (n = 33), while the TL of males ranged from 44.0 to 56.4 cm, with a mean of 49.2 ± 5.4 cm (n = 4; Fig. 2a). The body weight (BW) of females ranged from 224 to 1080 g with a mean of 463 ± 174.8 g (n = 33), and the BW of males ranged from 127 to 249 g, with a mean of 172 ± 53.5 g (n = 4 Fig. 2b). The body size of most females was clearly larger in both TL and BW than that of the males, and significantly different (Mann–Whitney U-test, P < 0.005).
The GSI of all the females (n = 33) ranged from 1.21 to 3.98 (mean: 2.57 ± 0.57). The GSI of the males (n = 4) ranged from 0.25 to 0.60 with a mean of 0.37 ± 0.16. The GSI was larger in females (mean: 2.57) than in males (mean: 0.37; Mann–Whitney U-test, P < 0.005; Fig. 2c).
Examinations of the annuli in the otoliths of the eels caught in Kii Channel indicated that the females reached greater ages than the males. The ages of the females ranged from 5 to 16 years with a mean ± SD of 8 ± 2.1 years, and their growth rate (GR) ranged from 3.8 to 11.1 cm year−1, with a mean of 7.7 ± 1.8 cm year−1. The ages of the males ranged from 4 to 5 years with a mean of 5 ± 0.6 years, and their GR ranged from 7.6 to 12.6 cm year−1, with a mean of 9.8 ± 2.4 cm year−1. Females were significantly older than males (Mann–Whitney U-test, P < 0.05), and the GR of females was significantly higher than that of males (Mann–Whitney U-test, P < 0.05). Close relationships were found between TL and BW, between age and TL and between age and BW in females (ANCOVA P < 0.0001), but not for males due to the limited number of specimens (four specimens).
Migratory history
The Sr:Ca ratios in the transects along the radius of each otolith showed the same common feature in all specimens, but there were generally three different patterns outside the otolith core. All otoliths had a common peak of high values of Sr:Ca ratios at the center of the otolith inside the elver mark (ca 150 μm), which roughly corresponded to the leptocephalus and early glass eel stages during their oceanic life (Arai et al. 1997). Outside of the high Sr core, there was a great variation in the change of the Sr:Ca ratios in the otoliths of eels from different habitats. The change in Sr:Ca values outside the elver mark was generally divided into three types (Fig. 3): (1) relatively high values of around 6.00–6.88 × 10−3 (mean: 6.22 ± 0.30), but larger than 6 × 10−3 with no movement into freshwater (Fig. 3a), (2) intermediate values of 2.65–5.91 × 10−3 (mean: 4.73 ± 0.36; Fig. 3b), and (3) constantly low values of 1.33–1.81 × 10−3 (mean: 1.61 ± 0.17; Fig. 3c). Each transect had sudden spikes and/or sharp decreases. These were probably measurement errors caused by tiny flaws on the surface of the otolith or an unevenness of the Pt–Pd coating that could greatly reduce the reflection of the X-ray from the sample and would have no biological significance in relation to migration or habitat.
Typical changes in otolith Sr:Ca ratio along line transects from the core (0 μm) to the edge in the frontal plane of sagittal otoliths of the Japanese eels collected in Kii Channel off Shikoku Island, Japan. a Sea eel. b Estuarine eel. c River eel. The specimens were classified based on the Sr:Ca average ratio outside the elver mark (ca 150 μm from the core)
The specimens of silver eel stages of A. japonica collected off Shikoku Island had a wide range of otolith Sr:Ca ratios. The otolith Sr:Ca ratios ranged from 0.04 to 14.2 × 10−3 (Fig. 4). The wide range of otolith Sr:Ca ratios indicated that the habitat use of A. japonica was variable among fresh, brackish and marine waters after recruitment to the coastal waters.
The mean Sr:Ca ratio value outside of 150 μm from the core of all otoliths ranged from 1.33 to 6.88 × 10−3, with a peak at 5.0–6.0 × 10−3 (Fig. 5), and based on those mean values each specimen was categorized as either sea, estuarine or river eels. Of the specimens examined, sea eels (Sr:Ca ≥ 6.0 × 10−3) comprised 22% (n = 8) of all specimens, river eels (Sr:Ca < 2.5 × 10−3) were 19% (n = 7) and estuarine eels (2.5 × 10−3 ≤ Sr:Ca < 6.0 × 10−3) were the most abundant (59%, n = 22) of the three types (Fig. 5). In females, the number of river, estuarine and sea eels was 6, 19 and 18 specimens, respectively. Those in males were 1, 3 and 0, respectively. Among the sea eels, estuarine eels and river eels, there were no significant differences in TL, BW, GSI and age at maturation in females (Kruskal–Wallis test, P > 0.05).
Discussion
It is noteworthy that the proportion of eels with an ordinary diadromous pattern was lowest (19%; Fig. 5), and sea eels and estuarine eels were abundant in the study area. The wide range of otolith Sr:Ca ratios indicated that the habitat preference of A. japonica during their growth phases just before spawning migration to the open ocean would be facultative and not obligatory. These findings strongly suggested that A. japonica has a flexible migration strategy with a high degree of behavioral plasticity and an ability to utilize the full range of salinity. A similar phenomenon was indicated in the otoliths of yellow and silver eels of A. japonica at other localities in the Japanese coastal waters (Tsukamoto and Arai 2001; Arai et al. 2003a, b; Kotake et al. 2003, 2005). Otolith analyses of European, American and Australasian yellow and silver eels have also shown evidence of marine and estuarine residencies (Tzeng et al. 2000; Arai et al. 2004, 2006; Daverat et al. 2006).
The migratory type of female eels included a high percentage of sea eels (24%), while the migratory male eels were either river or estuarine eels with no occurrence of sea eels (Fig. 5). Kotake et al. (2003) also reported that female silver eels caught near the Amakusa Islands of western Japan included many sea eels (44%) and that males were mainly river eels (43%). A similar finding, a high percentage of sea eels (45%), while the migratory male eels were mainly river (42%) and estuarine eels (42%), is also reported in Mikawa Bay along the east coast of central Japan (Kotake et al. 2005). Krueger and Oliveira (1999) concluded that increases in population density, and the resulting slower growth, favored the production of males in A. rostrata. Wiberg (1983) concluded that warm temperatures induce maleness in A. anguilla, but long-term experiments in Sweden produced a small but significant increase in the number of females with increasing temperature (Holmgren and Mosegaard 1996). In A. japonica, Sasai et al. (2001) reported that in the East China Sea, females were more abundant (n = 71) than males (n = 18). Tzeng et al. (1995) also reported that A. japonica collected near the estuary and at down-, mid- and up-stream sites of rivers in northern Taiwan were mainly made up of smaller individuals whose sex was undetermined, whereas the eels whose sex could be determined were mainly female. They concluded that overcrowding and poor feeding would give rise to male eels, and low population densities with rich feeding would favor females. Thus, the potentially food-rich and low population density environment in the coastal waters of Shikoku Island might favor the production of females, while river habitats with poor food and higher population density might favor males, although population density could not be examined in the present study.
The characteristics and timing of the capture of the eels examined in this study indicated that they were caught during their spawning migration. The GSI values of the females at this sampling site (mean: 2.57, 1.21–3.98) were similar or a little higher than those of female silver eels collected during their downstream migration in freshwater in Japan (range 1.1–2.5; Sasai et al. 2001) or close to the coast of southern (mean: 1.98; range 1.4–2.8; Kotake et al. 2003) and eastern Japan (mean 1.76; range 0.1–3.9; Okamura et al. 2002; mean 2.1; range 0.2–4.3; Kotake et al. 2005). This suggests that these fish are not residents in this fishing area, but are migrants from somewhere in the region of the Kii Channel and were on their way to the spawning area offshore. Because the sampling can be assumed to be random, the low proportion of river eels in the sample from the spawning migration season suggested that the estuarine and sea eels inhabiting this coastal area might make a larger reproductive contribution to the next generation. However, for further conclusions to be made, more specimens collected at different localities covering the whole range of geographic distribution of the species need to be examined.
The GSI values of the male eels examined in this study (0.25 to 0.60) were similar to those in the studies mentioned above. However, due to the difficulty in collecting migrating eels around coastal and offshore areas of Japan and the highly skewed sex ratio of the specimens collected, information on the maturity of male Japanese eels at the beginning of their spawning migration is scarcer than for females (Sasai et al. 2001; Okamura et al. 2002; Kotake et al. 2003, 2005, 2007). All reported GSI values for males have been under 1.0 (0.2–0.6) during the spawning migration season (Sasai et al. 2001; Kotake et al. 2003, 2005). These results suggest that the male eels collected in the Kii Channel were also beginning their spawning migration.
All of the river eels as well as estuarine and sea eels were caught during the same spawning migration season in the present study. The GSI values of some of the female river eels were the highest among the three migratory patterns. These eels must have move downstream from rivers to the channel at the onset of maturation, while estuarine and sea eels begin their migration in estuaries and coastal areas. It is possible that most eels begin their spawning migration in the ocean at about the same maturity level. Thus, this suggests that river eels may begin their spawning migration earlier than most individuals of the other two migratory types. Alternatively, the catches of the matured river eels would presumably be later than those of the other types because they inhabit freshwater areas further from the sampling location. The earlier start of downstream migration in river eels is probably why some had higher GSI values than the other types. However, the GSI level reaches 30–60 at the final stage of maturation in Japanese eels that are artificially matured with hormone treatments (Satoh et al. 1992; Sato et al. 2003), so minor differences in maturity at the beginning of the spawning migration should not be an impediment to reproduction.
Diadromous fish migration is generally explained by a difference in food abundance between marine and freshwater habitats (Gross 1987). Juvenile anadromous salmon are born in freshwater habitats at high latitudes with low productivity, and they migrate to higher productivity habitats in the ocean for better growth before returning to their freshwater habitats for breeding. With an opposite migratory pattern to anadromous salmon, catadromous eels that originate in low population tropical ocean spawning areas then migrate upstream into freshwater for better growth in higher productivity habitats. Therefore, a latitudinal cline in the productivity of the habitats might predict that marine resident freshwater eels would occur more frequently at higher latitudes where the productivity of freshwater habitats are lower compared to that in the ocean (Tsukamoto and Arai 2001). The Japanese eel is a temperate species, and in the present study the eels collected in the Kii Channel were mostly sea and estuarine eels and not river eels. This observation seems to support the above prediction. To test this hypothesis, analyses of the otolith Sr:Ca ratios of silver phase tropical eels during their spawning migration in the ocean needs to be made, and their degree of seawater residence should be compared between tropical and temperate eels.
Both river, estuarine and sea eels began their spawning migration toward the open ocean at about the same time. This type of synchronization of migration and gonadal maturation, and the apparent predominance of estuarine and marine habitats in the central region of Japan, has important implications for the conservation of this species. It implies that eels from both freshwater and marine habitats can mix together during the spawning migration and potentially contribute to the next generation, and that estuarine and marine habitats may be very important for eels around Japan.
References
Arai T, Otake T, Tsukamoto K (1997) Drastic changes in otolith microstructure and microchemistry accompanying the onset of metamorphosis in the Japanese eel Anguilla japonica. Mar Ecol Prog Ser 161:17–22
Arai T, Kotake A, Ohji M, Miller MJ, Tsukamoto K, Miyazaki N (2003a) Occurrence of sea eels of Anguilla japonica along the Sanriku Coast of Japan. Ichthyol Res 50:78–81
Arai T, Kotake A, Ohji M, Miyazaki N, Tsukamoto K (2003b) Migratory history and habitat use of Japanese eel Anguilla japonica in the Sanriku Coast of Japan. Fish Sci 69:813–818
Arai T, Kotake A, Lokman PM, Miller MJ, Tsukamoto K (2004) Evidence of different habitat use by New Zealand freshwater eels, Anguilla australis and A. dieffenbachii, as revealed by otolith microchemistry. Mar Ecol Prog Ser 266:213–225
Arai T, Kotake A, McCarthy TK (2006) Habitat use by the European eel Anguilla anguilla in Irish waters. Estuar Coast Shelf Sci 67:569–578
Daverat F, Limberg KE, Thibault I, Shiao JC, Dodson JJ, Caron F, Tzeng WN, Iizuka Y, Wickström H (2006) Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Mar Ecol Prog Ser 308:231–241
Gross MR (1987) Evolution of diadromy in fishes. Amer Fish Soc Symp 1:12–25
Holmgren K, Mosegaard H (1996) Implications of individual growth status on the future sex of the European eel. J Fish Biol 49:910–925
Kotake A, Arai T, Ozawa T, Nojima S, Miller MJ, Tsukamoto K (2003) Variation in migratory history of Japanese eels, Anguilla japonica, collected in coastal waters of the Amakusa Islands, Japan, inferred from otolith Sr/Ca ratios. Mar Biol 142:849–854
Kotake A, Okamura A, Yamada Y, Utoh T, Arai T, Miller MJ, Oka HP, Tsukamoto K (2005) Seasonal variation in migratory history of the Japanese eel, Anguilla japonica, in Mikawa Bay, Japan. Mar Ecol Prog Ser 293:213–221
Kotake A, Arai T, Okamura A, Yamada Y, Utoh T, Oka HP, Miller MJ, Tsukamoto K (2007) Ecological aspects of the Japanese eel, Anguilla japonica, collected from coastal areas of Japan. Zool Sci 24:1213–1221
Krueger WH, Oliveira K (1999) Evidence for environmental sex determination in the American eel, Anguilla rostrata. Environ Biol Fish 55:381–389
Matsui I (1972) Eel biology biological study. Koseisha-Koseikaku, Tokyo
McDowall RM (1988) Diadromy in fishes. Cromm Helm, London
Okamura A, Yamada Y, Tanaka S, Horie N, Utoh T, Mikawa N, Akazawa A, Oka HP (2002) Atmospheric depression as the final trigger for the seaward migration of the Japanese eel Anguilla japonica. Mar Ecol Prog Ser 234:281–288
Sasai S, Aoyama J, Watanabe S, Kaneko T, Miller MJ, Tsukamoto K (2001) Occurrence of migrating silver eels, Anguilla japonica, in the East China Sea. Mar Ecol Prog Ser 212:305–310
Sato N, Kawazoe I, Suzuki Y, Aida K (2003) Induction of vitellogenesis. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 387–399
Satoh H, Yamamori K, Hibiya T (1992) Induced spawning of the Japanese eel. Nippon Suisan Gakkai Shi 58:825–832
Shiao JC, Iizuka Y, Chang CW, Tzeng WN (2003) Disparities in habitat use and migratory behaviour between tropical eel Anguilla marmorata and temperate eel A. japonica in four Taiwanese rivers. Mar Ecol Prog Ser 261:233–242
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practice of statistics in biological research, 3rd edn edn. Freeman, New York
Tesch FW (1977) The eel biology and management of anguillid eels. Chapman and Hall, London
Tsukamoto K, Arai T (2001) Facultative catadromy of the eel, Anguilla japonica, between freshwater and seawater habitats. Mar Ecol Prog Ser 220:365–376
Tzeng WN, Cheng PW, Lin FY (1995) Relative abundance, sex ratio and population structure of the Japanese eel Anguilla japonica in the Tanshui River system of northern Taiwan. J Fish Biol 46:183–201
Tzeng WN, Wang CH, Wickström H, Reizenstein M (2000) Occurrence of the semi-catadromous European eel Anguilla anguilla in the Baltic Sea. Mar Biol 137:93–98
Wiberg UH (1983) Sex determination in European eel (Anguilla anguilla L.): a hypothesis based on cytogenetic results, correlated with findings of skewed sex ratio. Cytogenet Cell Genet 36:589–598
Acknowledgments
We are grateful to Ms. M. Otsuki for assistance with the chemical analysis of Ca and Sr. This work was supported in part by Grants-in-Aid Nos. 18780141 and 20688008 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Rights and permissions
About this article
Cite this article
Chino, N., Arai, T. Relative contribution of migratory type on the reproduction of migrating silver eels, Anguilla japonica, collected off Shikoku Island, Japan. Mar Biol 156, 661–668 (2009). https://doi.org/10.1007/s00227-008-1116-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-1116-7