Abstract
The annual population dynamics (nauplii, old copepodites CIV–CV and adults) and seasonal variations in reproductive parameters of the cyclopoid copepod Oithona similis were investigated on the basis of the data 1999–2006 in Kola Bay, a large subarctic fjord in the Barents Sea. Population density of O. similis ranged from 110 to 9,630 ind m−3 and averaged 1,020 ± 336 ind m−3. The relative abundance of adults was high during winter (~60%). At the end of winter (mid-March), the population included a large percentage of later-stage copepodites (stage CIV 23% and stage CV 57%). There were two periods of mass spawning, in late June and September. Autumn and summer generations strongly differed in abundance, average prosome length (PL), clutch size (CS), egg diameter (D), egg production rates (EPR and SEPR) and female secondary production. Average PL decreased with increasing water temperature, while D and CS were strongly correlated with PL but unaffected by temperature. Annual average EPR and SEPR were 0.55 ± 0.18 eggs female−1 day−1 and 0.0011 ± 0.003 day−1, respectively. Female secondary production averaged 0.8 ± 0.3 μg C m−3 day−1 (range 0.001–3.58). There were positive relationships between abundance, EPR, SEPR, production and water temperatures. Reproductive parameters appeared to be controlled by hydrological factors and food conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Barents Sea is one of the most productive regions of the World Ocean (Timofeev 2000). Zooplankton play an important role in marine ecosystem structure and function of the Barents Sea, forming a main link between primary producers (phytoplankton) and higher trophic levels (e.g. fishes, sea birds and mammals) (Timofeev 2000). A large amount of data about composition, distribution and dynamics of planktonic animals are already available in the literature for this region (e.g. Kamshilov and Zelikman 1958; Degtereva 1979; Pedersen et al. 1995). In particular, the biology of the copepod Calanus finmarchicus and the euphausiids Thyssanoessa inermis and Thyssanoessa raschii has been investigated more intensively because they are the base food resource for commercial fishes (Timofeev 2000). However, there is little information concerning life cycles and reproductive dynamics of other copepods, especially those with small body sizes.
Recent studies suggest that small copepods may be a more significant component in marine food webs, than that was previously considered (Gallienne and Robins 2001; Turner 2004). Oithona similis Claus, 1866 (Cyclopoida) is among the most numerous small planktonic copepods in the Barents Sea. In some seasons, O. similis abundance and biomass can exceed that of C. finmarchicus (Degtereva 1979). Due to their small size (<1 mm) and their high mean abundance, O. similis are thought to have a crucial importance as a food source for other copepods, chaetognaths, fish larvae and even adult planktivorous fishes in cold years in the Barents Sea when C. finmarchicus abundance is low (Degtereva 1979). Nevertheless, O. similis life history is insufficiently studied in the Barents Sea, although some data on their seasonal population dynamics have been obtained in Eastern Murman coastal waters (Fomin 1978, 1991). The main cause of our restricted knowledge about true population composition, abundance and biomass has been the use of coarse (>200 μm mesh size) nets. Studies on the ecology of O. similis in the arctic and sub-arctic regions are rare, although in temperate waters the biology of Oithona spp. has been explored well (Kiørboe and Nielsen 1994; Sabatini and Kiørboe 1994; Uye and Sano 1995, 1998; Nielsen and Sabatini 1996; Williams and Muxagata 2006; Porri et al. 2007).
The most thorough investigation of the annual cycle of O. similis comes from the arctic Kongsfjorden (northern Svalbard waters) by Lischka and Hagen (2005).
In our study, we have summarized available data about O. similis distribution in Kola Bay during 1999–2006. We do not have data about the abundance of O. similis during a single period. Data compiled from different years, but with similar climatological conditions, were used in our investigation. The main aim of our paper is to describe “averaged” seasonal cycle and some reproductive characteristics of O. similis populations in the sub-arctic zone of the Barents Sea.
Materials and methods
Study area
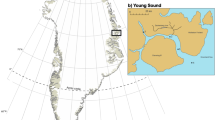
Kola Bay is typical of the fjords constituting the coastline in the Norwegian and Barents Seas (Fig. 1). The bay is about 51 km long, with its width gradually decreasing from 3.0–3.5 km near its mouth to 1.5–2.5 km in the centre to 1.0–1.5 km at its southern end (Matishov et al. 1997). The fjord’s dominant water source is the Atlantic Ocean and the Atlantic inflow entering from the Barents Sea shelf is characterized by high salinity (34.5 psu) and relatively high temperatures (1.5–2°C in April and 7–8°C in August–September). The influx of Atlantic waters keeps most of the fjord from freezing, although in cold winters the southern part is covered with ice (30–40 cm). Water dynamics in the fjord are determined by tidal currents and winds. The water is stratified during the spring–summer period (Fig. 2), so distinct thermocline and halocline are formed in surface layers (Matishov et al. 1997).
Location of sampled stations in Kola Bay. Numbers refer to numbers of sampling events as shown in Table 1
The climate is maritime, with maximal and minimal air temperatures recorded in August and February, respectively. Spring temperatures are relatively cold, while autumn temperatures are relatively warm (Matishov et al. 1997). Water temperatures rapidly increase during late spring and early summer (May–July) and slowly decrease during autumn and winter.
Sampling
Zooplankton samples were collected in Kola Bay during expeditions and cruises by the Murmansk Marine Biological Institute covering the years 1999–2006 (Table 1, Fig. 1). During cruises 2–5 and 11–12, the entire area including stations in the southern, central and northern parts was investigated. We used a Juday plankton net (mouth diameter: 0.37 m; length: 2.0 m; mesh opening: 168 μm) to determine stage composition of copepodites throughout the study period and OTE PVC bottles (5 l capacity) to estimate the abundance of nauplii during mass reproduction period (June–September) at two to three stations (two replicates at each station). Zooplankton net tow samples were taken from the bottom to the surface or from 100 to 0 m, while bottle samples were taken at 10 m. After the completion of each haul, nets were washed and the samples fixed in buffered 4% formaldehyde-seawater solution.
Processing
Each sample was divided into subsamples with a splitter (from 1/2 to 1/8) depending on the zooplankton abundance. A total of 500–700 individuals were taken from subsamples for subsequent analyses. Identification of nauplii, copepodites and sexes of O. similis was performed according to Gibbons and Ogilvie (1933) and Shuvalov (1980). All stages were counted and copepodites’ and adults’ prosome lengths (PL) were measured under a stereomicroscope MBS-10 (32–56 magnifications). A 168-μm mesh net is too coarse to sample nauplii and young copepodites (CI–CIII) quantitatively, and therefore only abundances of the older stages (PL > 400 μm) are considered. In most cases, there were females with intact egg sacs and intact detached egg sacs in plankton samples. The total number of ovigerous females at each station was determined as the sum of the number of females with egg sacs and the number of detached egg sacs divided by 2. A total of 40–50 females carrying egg sacs were taken from each sample. Clutch size (CS) was calculated as the sum of the eggs in both sacs. Average egg diameter (D) was obtained by measuring the diameter of each egg from a total of 250 egg sacs under a stereomicroscope (LOMO ES BIMAM R–11–1, 100× magnification). The abundance of eggs was calculated by multiplying the number of ovigerous females by the CS. Egg production rates (EPR, eggs female−1 day−1) were determined using the egg-to-female ratio (E/F) and hatching time (HT) according to Edmondson (1971):
Egg hatching time (HT, days) was calculated using the equation of Nielsen et al. (2002):
where T = water temperature (°C). Specific egg production of O. similis (SEPR per day) was estimated using the egg carbon content (conversion factor is 0.14 pg C μm−3; Kiørboe et al. 1985) and female carbon content based on length–weight relationship (Sabatini and Kiørboe 1994). The secondary production of females (SPF) was obtained by multiplying the biomass of females and SEPR.
Median CS, D, EPR, SEPR and SPF in autumn and summer generations of O. similis were compared by nonparametric procedures (Kruskal–Wallis tests), because variances for these parameters were heteroscedastic (Levene’s test a = 0.05) even after attempts at data transformation.
Linear regression analyses were used to reveal relationships between the physical variables (average temperature and salinity in upper 100 m layer or 0 in bottom layer) and the log(x + 1)-transformed abundance of each stage, PL and reproductive characteristics (proportion of females with egg sacs, CS, D, EPR, SEPR, SPF). In order to describe annual population dynamics of O. similis, we used average data for each month.
Results
Hydrology
The annual minimum temperature was usually registered in February or March (<+0.8°C). By July, the temperature of the surface layer rises up to 10.5°C. The salinity regime of Kola Bay is determined by interactions between a weak river run-off (only in a little area in the southern part) and the Barents Sea water masses. There are strong temporal and spatial salinity variations in the upper 10 m layer of water. Winter and spring salinity values ranged between 20 and 34 psu. In summer, salinity typically decreased to around 15 psu (in southern part), but increased again during autumn (up to 25–34 psu). Typical annual cycles in mean water temperature and salinity in Kola Bay, as recorded with our SBE 19plus SEACAT profiler, are presented in Fig. 2. Literature information (Terziev et al. 1990; Matishov et al. 1997) and unpublished cruise reports (temperature in upper 10 m and 10–100 m layer) were also used during those occasions in 2000, 2001 and 2004 when our in situ sensors were not registering hydrological parameters.
Phytoplankton dynamics
According to published data, during the winter season (from November 2004 to March 2005) in the surface layer of the southern bend of Kola Bay, the values of chlorophyll a concentration varied from 0.01 to 0.04 mg m−3 (0.02 ± 0.01 mg m−3). During the spring season (April–June 2005), the chlorophyll a concentration gradually increased from 0.06 to 0.78 mg m−3 (0.27 ± 0.22 mg m−3) (Trofimova 2007). Within summer (July–August 2005) the concentrations of chlorophyll a were considerably higher than spring values (0.14–4.07 mg m−3) (Trofimova 2007). During autumn (September–October 2005), the concentrations of chlorophyll a considerably dropped to 0.06–0.09 mg m−3 (0.07 ± 0.02 mg m−3). The chlorophyll a concentration reduction continued into winter (0.01 mg m−3) (Trofimova 2007) (Fig. 3).
Seasonal dynamics of chlorophyll a mean concentration (mg m−3) in the surface layer in Kola Bay based on the results 2004–2005 (after Trofimova 2007)
Seasonal variations of stage composition, abundance and PL in O. similis
During our “mean” year, population density of O. similis ranged between 110 and 9,630 ind m−3 (or between 110 and 560 ind m−3 with nauplii excluded) and averaged 1,020 ± 336 (or 208 ± 19) ind m−3.
In the beginning of winter (November–December), the population was dominated by adults (about 52%), late-stage copepodites accounted for 48% and nauplii were absent (Fig. 4). By March, the relative abundance of adults decreased to 32%, the percentage of late-stage copepodites increased to 68% (Fig. 4b, c) and young copepodite stages were rare. At the end of winter (the middle of March), the population was made up of 23% stage-CIV copepodites, 57% stage-CV copepodites, 18% adult females and 2% adult males (Fig. 4). By the end of March, the number of adult males increased slightly due to growth and development of copepodids CV. Thus, it is possible to speak of sex differentiation beginning during the March period. Mean total abundance of O. similis varied from 109 to 256 ind m−3.
Seasonal dynamics in absolute abundances of Oithona similis stages: a Nauplii abundance estimated from bottle samples. b–d Late copepodites and adults abundances estimated from net samples. The symbols correspond to the data points for different years. The line indicated mean value for all years studied
During April–May, adult male and female relative abundances increased (from 2% to 20% and from 18% to 71%, respectively) due to moulting of the copepodites from the previous year. Abundances of CV-stage copepodites decreased by 9%, and CIV were not found in plankton in mid-May, obviously, due to their development to CV stages (Fig. 4b, c). At the beginning of June, we saw mass reproduction of O. similis. Total abundance of nauplii sharply increased to where they accounted for 97% of O. similis population (Fig. 4a). By mid-to-late June, nauplii still dominated in the plankton (95%), however, all copepodite stages were found (Fig. 4). Total abundance of O. similis during this period was 120–2,670 ind m−3 (Fig. 4).
The beginning of the summer period (end of June–end of July) was characterized by a high proportion of nauplii. Nauplii abundances gradually fell from 96% to 87% (Fig. 4a), while relative abundance of old copepodids increased (from 0% to 4% CV and from 0% to 2% CIV, respectively) (Fig. 4b, c). Maximum population densities (including nauplii) for the “mean” year were recorded during this period (4,700–9,600 ind m−3). In the second half of summer season (the end of July–the end of August), nauplii relative abundance decreased from 87% to 49% of the population (Fig. 4a). However, proportions of old copepodites and adults increased by 25% and 26%, respectively (Fig. 4b, c, d). There were some young copepodites present during this period. The species total abundance varied between 410 and 1,300 ind m−3.
Mass spawning of the new generation of females was observed in September. All stages of O. similis were recorded in the samples. Subsequently, nauplii made up 70–73% of the O. similis population (Fig. 4a), adults accounted for 18–20% (Fig. 4d) and old copepodids accounted for 7–12% (Fig. 4b, c), respectively. By the beginning of October, the abundance of nauplii was 7%, old copepodids CIV–CV reached 36% and adults dominated the population (57%). The total population density had decreased from 1,300 to 300 ind m−3 over this month (Fig. 4). In the end of autumn, the population was made up of 60% adult males and females, and 40% old copepodites. There were no nauplii in the plankton. In winter, the total abundance varied from 300 to 400 ind m−3.
Variations in PL in O. similis were observed over the year. In Fig. 5, seasonal variations in body size are presented for adults and stage-CV copepodites (the only stages that were present in the whole year). The average sizes (PL) for all groups were largest in May: 505 μm in females, 415 μm in males and 493 μm in CV copepodites. Average PL of females decreased somewhat, reaching minimal values by August (469 μm) then again increased to 480 μm by December. Males were smallest in September (395 μm), their average PL increased to 415 μm by November and remained at that size during the winter period. Similar yearly patterns of PL variability were observed in CV-stage copepodites, with minimum PL observed in July (456 μm) and the second maximum in October (473 μm).
Seasonal variations of reproductive characteristics in O. similis
Oithona similis apparently reproduced year-round in Kola Bay, as females with egg sacs were found in all seasons (single females carrying eggs found during winter season were not included in Fig. 6). Number of females carrying egg sacs in May was 1–2% the total female number. Two peaks in the abundance of ovigerous females were found, one in the beginning of July (39%) and one in the beginning of September (28%) (Fig. 6). The average percentage of the females with egg sacs was 12 ± 2% during the reproductive period (May–November).
The mean CS of O. similis varied from 20 to 26 eggs per female. Peaks in CS were observed in June, September and November, with the largest values found in the beginning of June (Fig. 7a). CS in the autumn generation noted in the summer period (22 ± 1) was lower, than CS in the summer generation noted in autumn (24 ± 1).
The mean egg diameter (D) of O. similis differed across seasons. Maximum egg diameter was registered in May (65–70 μm), while minimum egg sizes (51–44 μm) were noted in July–August (Fig. 7b). In late autumn and early winter, D varied between 48 and 52 μm. Mean egg diameter was 52 ± 2 μm over the period when female with egg sacs occurred in the plankton.
Oithona similis EPR and SEPR were lowest in May and from October to December (no more than 0.04 eggs female−1 day−1 and 0.0006 day−1) (Fig. 8a, b). Maximum values reached 2.2 and 1.2 eggs female−1 d−1 (0.046 and 0.023 day−1) in July (autumn generation) and in September (summer generation), respectively (Fig. 8a, b). Average EPR and SEPR were 0.6 (±0.2) eggs female−1 day−1 and 0.0011 (±0.003) day−1, respectively.
Secondary production of females ranged between 0.001 and 3.58 (0.8 ± 0.3) μg C m−3 day−1. The maxima and minima were found during autumn and winter, respectively (Fig. 8c).
According to Kruskal–Wallis tests, PL, CS, D, EPR and SEPR significantly differed in autumn and summer generations of O. similis (Table 2).
Analysis of size and reproductive characteristics revealed differences in female PL, CS and D of both generations (Fig. 9, Table 3). These parameters were correlated with one another. In the autumn generation females, CS increased with increasing PL. The inverse relationship was found between CS and PL in summer generation females (Fig. 9, Table 3). Egg sizes were positively correlated with PL (Fig. 9, Table 3). There were negative relationships between CS and D in both generations (Fig. 9, Table 3).
Length–reproductive relationships in autumn and summer generations of Oithona similis. a, d Relationships between prosome length and clutch size. b, e Relationships between prosome length and mean egg diameter. c, f Relationships between clutch size and mean egg diameter. Statistical parameters are presented in Table 3
Relationships between hydrological conditions and population parameters
According to linear regression analyses, oceanographic factors strongly influenced population characteristics of O. similis. The mean abundance of all stages (except copepodites CIV and CV) increased with increasing water temperature. PLs of adults and stage-CV copepodites were negatively correlated with water temperature and positively with salinity, the abundances were also negatively correlated to water salinity excepting stage CV (Table 4). In contrast, PLs of nauplii and CIV copepodites were positively scaled with temperature and negatively to salinity. The reproductive characteristics CS and D were only poorly related to hydrological parameters. Proportion of females with egg sacs, EPR, SEPR and SPF significantly increased with increasing water temperature and decreasing water salinity (Table 4).
Discussion
Method
Although complete and accurate sampling of cyclopoid copepods requires the use of large water bottles or nets with a mesh size <100 μm (Fransz and Gonzalez 1997), we think that our nets adequately characterized the distribution pattern of late copepodites (CIV–CV) and adults. The 168-μm net and bottles used in our study are appropriate to investigate the abundance of mesozooplankton, i.e. species having a total length of 0.5–3.0 mm (Vinogradov and Shushkina 1987). For this reason, young copepodites (CI–CIII) of O. similis with total length <420 μm were excluded from consideration in our studies. Moreover, in some cases coarse nets may be more suitable for Oithona sampling than very fines ones. For example, Hansen et al. (2004) found that a 150-μm net better retained all developmental stages of O. similis, except nauplii than a 50-μm net. They suggested that the smaller mesh size in conjunction with the towing speed may have created a dynamic pressure wave in front of the net opening that reduced the sampling efficiency. On the other hand, Nielsen and Andersen (2002) found that O. similis abundances estimated from the WP-2 net (200 μm) samples were 2.7–10.5 times lower compared with the values estimated from Niskin bottle samples. However, these authors investigate all the stages of O. similis while we studied the copepodites IV–VI only. In addition, the net used in our study was too coarse for us to have confidence that detached egg sacs (100–125 μm in width and 300–350 μm in length) would be sampled quantitatively and therefore there would be some underestimations in the proportion of ovigerous females, EPR and SEPR. However, we suggest that such underestimations were low because we registered detached egg sacs very rarely in the net samples. Unfortunately, we sampled only the top 10-m layer of water to collect O. similis nauplii (although other life stages were collected over the entire water column). We cannot assume that we know the true nauplii depth distribution because the upper layer strongly differed in hydrographical characteristics from the underlying water layers. Nevertheless, we suggest that our data on O. similis nauplii represent the general pattern of their distribution because O. similis is a true epiplanktonic species preferred to inhabit in the upper water layers (e.g. Shuvalov 1980; Satapoomin et al. 2004; Madsen et al. 2008).
Dynamics of population characteristics in O. similis
Oithona similis is one of the most common copepods in the Barents Sea and is found in the coastal zone the whole year-round (Fomin 1991). However, prior to our study, the early life history of O. similis in Kola Bay has not been sufficiently investigated. A few studies of O. similis life history have been conducted in the Arctic region (Nielsen and Andersen 2002; Thor et al. 2005, 2008). However, only two studies have been conducted to investigate the population dynamics and annual cycle of the species in the Atlantic influenced Svalbard waters, the productive Greenland Sea region (Lischka and Hagen 2005) and in Disco Bay in western Greenland waters (Madsen et al. 2008). We found that there were two peaks of O. similis abundance, one in July and one in September, and that the species apparently reproduced mainly from May to November because females carrying egg sacs were largely absent at other times. Our findings are similar to the population dynamics pattern observed by Fomin (1978, 1991) in a coastal zone of the Eastern Murman (southern part of the Barents Sea). The author noted that the number of copepodites and adults was very high during all seasons (mean annual abundance 200–250 ind m−3) and that O. similis abundance peaks were reached in June, September, November and late December (1,154, 1,604, 1,344 and 926 ind m−3, respectively).
In an earlier study of the Barents Sea, juvenile stages of O. similis were recorded in the second half of summer and in late autumn. Females with egg sacs were found at the end of June (Fomin 1991). In Balsfjorden (northern Norway waters), Oithona spp. had two peaks of abundance, in the spring and in autumn, the mean annual abundance varied between 289 (in shallow waters) and 939 ind m−3 (in deeper waters) (Barthel 1995). Maximal biomass of O. similis (about of 1.4 g dry weight m−2) was registered in the first half of July in the coastal waters of Balsfjorden (Pasternak et al. 2000). A similar pattern of seasonal variation of Oithona biomass has been recorded in Lindaspollene, western Norway (Magnesen 1989). Spring–summer and autumn maxima of abundance were connected with mass reproduction of O. similis (Fomin 1978; Hopkins 1981) as in our investigation. In Disco Bay, Oithona spp. were the most numerically abundant copepod all year round and reached their maximum abundance (~1,500 ind m−3) in late August (Madsen et al. 2008).
It is worth noting that summer O. similis abundance apparently varies considerably among years and among coastal sites in the Barents Sea. For example, in July–August 1987, average population density of the species in Yarnyshnaya Bay, a typical fjord of the Barents Sea was only 7,150–15,440 ind m−2 (Timofeev 1994), considerably lower in comparison with our finding (385 ind m−3 or ca. 42,000 ind m−2). On the other hand, our recent research has shown that in summer of 2004–2006, mean abundance of O. similis varied from 42,340 ind m−2 or 386 ind m−3 to 100,200 ind m−2 or 916 ind m−3 in the open waters of the southern Barents Sea (Dvoretsky 2008), exceeding the concentrations that we found in Kola Bay.
The population dynamics of females and males suggests the presence of two generations of O. similis during the year. One peak of adult abundance occurred in late summer and resulted from development of a new O. similis generation which in July was present as nauplii and in August as copepodite stages. The developmental time of the summer generation was therefore ~2 months. On the other hand, maturing of the autumn generation was not complete until late-spring or early-summer of the next year, so the life cycle of the autumn generation was ~9–10 months from the previous September maximum in nauplii numbers to the May–June increases in proportions of adult female and males in the current year. Low abundances of adults in winter plankton indicated that only a small portion of the autumn generation of copepodites were able to finish their development by November–December. Comparing our study to others suggests that O. similis life cycles are similar across the Polar regions. The basic differences among populations are related to spawning time and occurrence of abundance peaks, and also to generation development durations. In Kongsfjorden (north-western Svalbard waters), O. similis reproduced year round with two main reproductive periods in May–June and in August–September (Lischka and Hagen 2005). However, the maximum abundance was in November (>704,633 ind m−2) and the minimum abundance was found in June (4,483 ind m−2) in contrast to our data. We think that these differences between regions might be explained by colder temperatures in Kongsfjorden in comparison with Kola Bay. In Kola Bay, the life cycle of O. similis is quite similar to the White Sea where mass spawning has been found in May–June and July–August (Dvoretskii 2007). Madsen et al. (2008) also reported that all stages of Oithona spp. were present year round in Disco Bay and the females constituted 40% of the population in spring and summer.
The mean annual abundance in the White Sea was 2,560 ind m−3 (Prygunkova 1974), which greatly exceeds values registered in our study. Developmental times of the first and second generation were 2 and 9–10 month, respectively (Prygunkova 1974). Two reproduction peaks also appear to be present in western Greenland waters (in June and August–September) because of high abundance of adults in these periods (Ussing 1938). However, according to Digby (1954), only one generation was present in Greenland Sea. The author noted that only a small part of the new generations appearing in June was able to develop into adults. In shelf waters of south-western Greenland, the maximum biomass of O. similis (65 mg C m−2) was registered in July (Pedersen et al. 2005) which is similar to our data.
In Antarctic seas, O. similis also formed two generations during the year (Atkinson 1998), and the same patterns have been observed in Canadian Arctic waters (Hopcroft et al. 2005) and in the northern part of the Bering Sea (Shaginyan 1982). In contrast, only one generation is most likely to occur in the Laptev Sea during all seasons (Lischka et al. 2001).
Hydrological conditions strongly influence population dynamics and generations times. Elevated temperatures can accelerate development of O. similis and increase the number of generations in a year, while depressed salinity reduces the species breeding potential in the White Sea (Shuvalov 1980) and in the Barents Sea (Timofeev 1994). In the Arctic regions, ice cover is usually present during most of the year although intensive melting takes place in May–June. During similar periods of decreasing salinity in the upper water column, declining abundance has been observed in Kongsfjorden (Lischka and Hagen 2005). The authors suggested that decreasing water salinity might reduce the population density because their data showed that in the Baltic Sea, O. similis concentrated in the zone of the halocline with high salinity (Hansen et al. 2004). In Kola Bay, the upper water layer (0–10 m) is affected by late spring freshening (in May salinity is about 28–30 psu), but in the other seasons surface water salinity did not drop below 32 psu, and salinity in the deeper water layers varies between 32 and 34 psu during all year round (Matishov et al. 1997 and our observations) (Fig. 2). We therefore think it unlikely that salinity strongly influences the abundance of O. similis in Kola Bay.
According to the authors’ data and past literature data (Sabatini and Kiørboe 1994; Uye and Sano 1995; Nielsen et al. 2002), water temperature has a pronounced effect on Oithona biology (especially, duration of reproduction, HT, EPR and SEPR). If water were warmer, then the number of generations occurring within a year increases. In the Gulf of Maine, there appeared to be at least three and possibly four broods (generations) of O. similis in March, May, July and September (at 6.08–9.38°C). A developmental period of 2 months during the early season and about 6 weeks in summer has been found (Fish 1936). O. similis reproduced continuously throughout the year with two main abundance peaks forming no more than three generations in the Loch Striven (the Clyde Sea area) (Marshall 1949). According to Kozhevnikov (1975), in the northern Japan Sea the developmental cycle of O. similis (from egg to egg) was about 31–37 days (at 15–17°C). In the Japan Sea, O. similis populations produced at least 4–5 generations during the year (at temperature range from 6 to 22°C) (Kasyan 2001). In the warm Andaman Sea (Thailand waters), Oithona spp. produced five generations in all seasons (at >15°C) (Satapoomin et al. 2004). Positive correlations between water temperature and abundance of O. similis confirm the role of this factor in the species dynamics in Kola Bay (Table 4), in the southern Barents Sea (Fomin 1991; Timofeev 1994), in the Arctic waters in the Greenland Sea (Richter 1994), in Iceland waters (Gislason and Astthorsson 2004) and near northern Svalbard (Daase and Eiane 2007).
We found that the average length of O. similis varied within season, and that mean body size was larger in summer than autumn; because water temperatures were lower in autumn, there was a negative correlation between PL and temperature. A similar pattern has been noted for O. similis in the White Sea (Shuvalov 1965), in the Clyde Sea (Marshall 1949), in the Irminger Sea (Castellani et al. 2007), in the Svalbard waters (Daase and Eiane 2007) and for O. davisae in the Japan Sea (Uye and Sano 1995). In addition to water temperature, food conditions are important factors determining copepod sizes (Timofeev 2000). The finding that the presence of largest O. similis occurs in Kola Bay during summer can be explained by longer generation time in the autumn brood (10 months). In contrast, summer generation (2 months old) had smaller PL. There is high food availability due to a phytoplankton bloom starting in late April (Matishov et al. 1997) in the Barents Sea, when development of the previous autumn’s generation is finished. Significant relationship between O. similis abundance and chlorophyll a concentrations have been found in Antarctic waters (Ward and Hirst 2007) although some authors showed that protoozoplankton played a more important role in feeding of O. similis (Castellani et al. 2005).
Mean annual PL of O. similis females (484 μm) found in our study was higher than in such Arctic areas as the Greenland Sea, Gulf of Alaska and the White Sea (Shuvalov 1965; Nielsen et al. 2002) but lower than in the North Sea (Nielsen and Sabatini 1996). Shuvalov (1975) delineated two different size-morphological forms of O. similis. The Atlantic-White Sea group consists of the North Atlantic population with a modal female body size of about 730 μm (PL = 480–490 μm) and a White Sea population with a modal female body size 700 μm (PL of about 460–470 μm). The Arctic–Okhotsk Sea group consists of Arctic populations (the Central Arctic Basin) with a modal body size of 970 μm (PL = 520–530 μm) and an Okhotsk Sea population with a modal body size of 850 μm (PL = 510–520 μm). We think the population of O. similis existing in Kola Bay together with populations from the Greenland Sea, Svalbard waters and from others regions affected by warm Atlantic waters are intermediate in female PL between the small boreal form and the large Arctic form.
Variations in O. similis reproductive characteristics
In the present study, females with egg sacs were present year round. Similar results have been reported previously for others arctic and temperate coastal water (Kiørboe and Nielsen 1994; Madsen et al. 2008). The maximum proportion of ovigerous females was observed in July and September in Kola Bay. We think that females carrying egg sacs in June and July belonged to the autumn generation of the previous year, while egg-sac carrying females found in September are part of the summer generation of the current year. It is noteworthy that that the proportion of females carrying egg sacs in Kola Bay (0–39%) is rather low fraction compared to other studies. For example, in Yarnyshnaya Bay during the summer of 1987, more than 48% of O. similis females carried out egg sacs (a 168 μm net; Timofeev 1994). In the Fukuyama Harbor, more than 60% of O. davisae females had egg sacs in November when peak reproduction occurred (a 62-μm mesh net; Uye and Sano 1995). The maximum proportion of O. similis females with egg sacs reached 67% in the Southern Ocean (a 100-μm mesh net; Ward and Hirst 2007).
Relatively low rates of reproducing females in Kola Bay may be an artefact produced by losses of egg sacs due to using a coarse mesh net for the sampling. This is supported by occasional findings of females with one or even half of an egg sac. On the other hand, differences in environmental conditions (hydrology and interspecific interactions) might also be responsible for low proportions of reproducing females. This reason is complex, and includes hydrological conditions and inter–specific interactions. For example, low salinity in an estuarine zone might cause losses of egg sacs and high concentrations of specimens might reduce the proportions of ovigerous females in copepods (Timofeev 2000). Kola Bay is a strongly polluted water area, while Yarnyshnaya Bay has practically no anthropogenous influence.
According to our data and other recent investigations in the southern Barents Sea (Dvoretsky 2008), the mean CS in O. similis did not vary much (22–24 eggs per sacs). In the Kara Sea, the North Sea, the White Sea, the Irminger Sea and the western Bering Sea, summer CSs (22–24 egg per sacs) were comparable to the values we observed in Kola Bay (Belousova 1977; Fomin 1989; Nielsen and Sabatini 1996; Castellani et al. 2007; Dvoretskii 2007). However, CS obtained in our study was higher in comparison with CS measured during other seasons in other regions. For example, in the Irminger Sea winter CS was 18 ± 0.6 egg per sacs (Castellani et al. 2007), and in the White Sea CS was 8 eggs per sac during autumn (Shuvalov 1980). Our results suggest that CS is rather stable within the Arctic Seas and, therefore, little dependent on environmental factors. Non-significant relationships between physical variables and CS (Table 4) verify this assumption.
Mean egg diameter is an important functional parameter in marine animals. This parameter directly correlates with energy reproductive investment in the future generations (Timofeev 2000). We found that D was 52 ± 2 μm in Kola Bay. This value is in a good accordance with data from the southern Barents Sea (55 ± 0.2 μm, Dvoretsky 2008), the White Sea (60 ± 0.2 μm, Dvoretskii 2007), the Irminger Basin (60 ± 0.2 μm, Castellani et al. 2005) and the North Sea (57 μm, Sabatini and Kiørboe 1994). In contrast, in Arctic waters D was more variable and high (58–67 μm, Nielsen et al., 2002). Thus, within subarctic and temperate waters, variability of egg size is low and apparently stable. Water temperature and salinity did not influence D (Table 4) as was the case with CS.
The lack of effect of hydrological variables on CS and D is probably due to how these parameters are related with other aspects of O. similis biology. The duration of the lifespan determines CS and D in O. similis. For example, in the White Sea the large long-lived females of the autumn generation that spawned during May–June were characterized by lower CS and higher D (Shuvalov 1980), perhaps because bigger animals (longer PL) are capable of producing bigger eggs. Length-CS, length-D and CS-D relationships obtained for the different generations (Table 3, Fig. 9) provide support for similar patterns in Kola Bay. Similar data have been obtained in the southern Barents Sea and the White Sea (Timofeev 1994; Dvoretskii 2007; Dvoretsky 2008), although Castellani et al. (2007) concluded that another main factor determining the CS is the food condition and reported that the mean CS increased in periods with low temperature, but high protozooplankton concentrations. As noted above, food availability is highest in summer in Kola Bay. The females of the autumn generation that produce eggs in late June–July might take advantage of good food conditions (chlorophyll a varied from 0.78 to 4.07 mg m−3, Fig. 3, Trofimova 2007) to gain high production rates, whereas females producing eggs in September (summer generation) might be more food limited (chlorophyll a = 0.06–0.09 mg m−3, Fig. 3, Trofimova 2007).
We found the highest mean EPR and SEPR in July and in September during the periods of O. similis mass spawning. Our results are similar to those obtained by Madsen et al. (2008) who reported that SEPR were highest in July (3.5% day−1) and in September (5.9% day−1). In the present study, EPR and SEPR in O. similis were strongly scaled with temperature (Table 4). Ward and Hirst (2007) also indicated that in situ EPR (fecundity per female) was significantly and positively related to temperature in the Southern Ocean. SEPR of O. davisae females increased linearly with increasing temperature between November 1986 and June 1987, when temperature was less than ca. 22°C in the Japan Sea (Uye and Sano 1995).
In contrast, there were no relationships between EPR, SEPR and water temperature in the Irminger and the North Seas (Sabatini and Kiørboe 1994; Nielsen and Sabatini 1996; Castellani et al. 2005), instead fecundity in O. similis was correlated with food conditions and especially protozooplankton concentrations. According to Castellani et al. (2007), the water temperature effect on EPR and SEPR in O. similis is indirectly mediated by body size. It seems likely that a similar pattern exists in the Kola Bay because of the strong correlations observed between PL and temperature.
Thus, our study has demonstrated that over the course of a year, two generations of O. similis were present in Kola Bay. These broods strongly differed in mean CS, D, EPR and SEPR. Population dynamics of the species was characterized by the presence of two abundance peaks (in July and September) corresponding to periods of O. similis mass spawning. Temporal differences in reproductive characteristics and production seem to be determined by temperature, food conditions and variations in female body size.
References
Atkinson A (1998) Life cycles strategies of epipelagic copepod in the Southern Ocean. J Mar Syst 15:289–311. doi:https://doi.org/10.1016/S0924-7963(97)00081-X
Barthel KG (1995) Zooplankton dynamics in Balsfjorden, northern Norway. In: Skjoldal HR, Hopkins C, Erikstad KE, Leinaas HP (eds) Ecology of fjords and coastal waters. Elsevier Science, Amsterdam, pp 113–126
Belousova SP (1977) Some data about fecundity of Oithona similis and Pseudocalanus elongatus (Crustacea, Copepoda) in the western part of the Bering Sea. Izv TINRO 101:29–30 (in Russian)
Castellani C, Irigoien X, Harris RP, Lampitt RS (2005) Feeding and egg production of Oithona similis in the North Atlantic. Mar Ecol Prog Ser 288:173–182. doi:https://doi.org/10.3354/meps288173
Castellani C, Irigoien X, Harris RP, Holliday NP (2007) Regional and temporal variation of Oithona spp. biomass, stage structure and productivity in the Irminger Sea, North Atlantic. J Plankton Res 29:1051–1070. doi:https://doi.org/10.1093/plankt/fbm079
Daase M, Eiane K (2007) Mesozooplankton distribution in northern Svalbard waters in relation to hydrography. Polar Biol 30:969–981. doi:https://doi.org/10.1007/s00300-007-0255-5
Degtereva AA (1979) The regularities in plankton quantitative development in the Barents Sea. Tr PINRO 43:22–53 (in Russian)
Digby PSB (1954) The biology of marine plankton copepods of Scoresby Sound, East Greenland. J Anim Ecol 23:298–338. doi:https://doi.org/10.2307/1984
Dvoretskii VG (2007) Characteristics of the Oithona similis (Copepoda: Cyclopoida) in the White and Barents Seas. Dokl Biol Sci 414:223–225. doi:https://doi.org/10.1134/S0012496607030167
Dvoretsky VG (2008) Distribution and reproductive characteristics of Oithona similis (Copepoda, Cyclopoida) in the southern part of the Barents Sea. Probl Fish 9:66–82 (in Russian)
Edmondson WT (1971) Reproductive rates determined directly from egg ratio. In: Edmondson WT, Winberg GG (eds) A manual on methods for assessment of secondary production in fresh waters. Blackwell Scientific, Oxford, pp 165–169
Fish CJ (1936) The biology of Oithona similis in the Gulf of Maine and Bay of Fundy. Biol Bull 71:168–187. doi:https://doi.org/10.2307/1537414
Fomin OK (1978) Some temporal characteristics of zooplankton in the coastal Murman waters. In: Bryazgin VF (ed) Regularities of biological production in the Barents Sea. Kola Branch AS USSR Press, Apatity, pp 72–91 (in Russian)
Fomin OK (1989) Some structural characteristics of zooplankton. In: Matishov GG (ed) Ecology and bioresources of the Kara Sea. KSC AN USSR Press, Apatity, pp 65–85 (in Russian)
Fomin OK (1991) Seasonal dynamics of abundance and seasonal distribution of the dominant species in the southern Barents Sea. In: Matishov GG (ed) Production and decomposition in the Barents Sea pelagic zone. KSC AN SSSR Press, Apatity, pp 72–80 (in Russian)
Fransz HG, Gonzalez SR (1997) Latitudinal metazoan plankton zones in the antarctic circumpolar current along 6°W during austral spring 1992. Deep Sea Res Part II Top Stud Oceanogr 44:395–414. doi:https://doi.org/10.1016/S0967-0645(96)00065-3
Gallienne CP, Robins DB (2001) Is Oithona the most important copepod in the world’s oceans? J Plankton Res 23:1421–1432. doi:https://doi.org/10.1093/plankt/23.12.1421
Gibbons SG, Ogilvie HS (1933) The develpoment stages of Oithona helgolandica and Oithona spinirostris, with a note on the occurrence of body spines in cyclopoid nauplii. J Mar Biol Assoc UK 18:529–550
Gislason A, Astthorsson OS (2004) Distribution patterns of zooplankton communities around Iceland in spring. Sarsia 89:467–477. doi:https://doi.org/10.1080/00364820410009256
Hansen FC, Mollmann C, Schutz U, Hinrichsen HH (2004) Spatio-temporal distribution of Oithona similis in the Bornholm Basin (Central Baltic Sea). J Plankton Res 26:659–668. doi:https://doi.org/10.1093/plankt/fbh061
Hopcroft RR, Clarke C, Nelson RJ, Raskoff KA (2005) Zooplankton communities of the Arctic’s Canada Basin: the contribution by smaller taxa. Polar Biol 28:198–206. doi:https://doi.org/10.1007/s00300-004-0680-7
Hopkins CCE (1981) Ecological investigations on the zooplankton community of Balsfjorden, northern Norway: changes in zooplankton abundance and biomass in relation to phytoplankton and hydrography, March 1976–February 1977. Kiel Meeresforsch 5:124–139
Kamshilov MM, Zelikman EA (1958) Zooplankton species list in the East Murman coastal zone. Tr MBS AN SSSR 4:41–44 (in Russian)
Kasyan VV (2001) Distribution and dynamics of Oithona similis Claus (Copepoda: Cyclopoida) in the Amur Bay of the Japan Sea. Izv TINRO 139:271–281 (in Russian)
Kiørboe T, Nielsen TG (1994) Regulation of zooplankton biomass and production in a temperate, coastal ecosystem. I. Copepods. Limnol Oceanogr 39:493–507
Kiørboe T, Mohlenberg F, Hamburge K (1985) Bioenergetics of the planktonic copepod Acartia tonsa: relation between feeding, egg production and respiration and composition of specific dynamic action. Mar Ecol Prog Ser 26:85–97. doi:https://doi.org/10.3354/meps026085
Kozhevnikov BP (1975) Duration of Oithona similis (Copepoda, Cyclopoida) development in the northern Japan Sea. Izv TINRO 69:87–90 (in Russian)
Lischka S, Hagen W (2005) Life histories of the copepod Psedocalanus minutus, P. acuspes (Calanoida) and Oithona similis (Cyclopoida) in the Arctic Kongsfjorden (Svalbard). Polar Biol 28:910–921. doi:https://doi.org/10.1007/s00300-005-0017-1
Lischka S, Knickmeier K, Hagen W (2001) Mesozooplankton assemblages in the shallow Arctic Laptev Sea in summer 1993 and autumn 1995. Polar Biol 24:186–199. doi:https://doi.org/10.1007/s003000000195
Madsen SD, Nielsen TG, Hansen BW (2008) Annual population development of small sized copepods in Disko Bay. Mar Biol (Berl) 155:63–77. doi:https://doi.org/10.1007/s00227-008-1007-y
Magnesen T (1989) Vertical distribution of size-fractions in the zooplankton community in Lindaspollene, western Norway. 1. Seasonal variations. Sarsia 74:59–68
Marshall SM (1949) On the biology of the small copepods in Loch Striven. J Mar Biol Assoc UK 28:45–122
Matishov GG, Denisov VV, Dzhenyuck SL, Chinarina AD, Timofeeva SV (eds) (1997) The Kola bay: oceanography, biology, ecosystems, pollutants. KSC RAS Press, Apatity, 265 pp (in Russian)
Nielsen TG, Andersen CM (2002) Plankton community structure and production along a freshwater influenced Norwegian fjord system. Mar Biol (Berl) 141:707–724. doi:https://doi.org/10.1007/s00227-002-0868-8
Nielsen TG, Sabatini M (1996) Role of cyclopoid copepods Oithona spp. in North Sea plankton communities. Mar Ecol Prog Ser 139:79–93. doi:https://doi.org/10.3354/meps139079
Nielsen TG, Møller EF, Satapoomin S, Ringuette M, Hopcroft RR (2002) Egg hatching rate of the cyclopoid copepod Oithona similis in arctic and temperate waters. Mar Ecol Prog Ser 236:301–306. doi:https://doi.org/10.3354/meps236301
Pasternak A, Arashkevich E, Wexels Riser C, Ratkova T, Wassmann P (2000) Seasonal variation in zooplankton and suspended faecal pellets in the subarctic Norwegian Balsfjorden, in 1996. Sarsia 85:439–452
Pedersen G, Tande KS, Nilssen EM (1995) Temporal and regional variation in the copepod community in the central Barents Sea during spring and early summer 1988 and 1989. J Plankton Res 17:263–282. doi:https://doi.org/10.1093/plankt/17.2.263
Pedersen SA, Ribergaard MH, Simonsen CS (2005) Micro- and mesozooplankton in Southwest Greenland waters in relation to environmental factors. J Mar Syst 56:85–112. doi:https://doi.org/10.1016/j.jmarsys.2004.11.004
Porri F, McQuaid CD, Froneman WP (2007) Spatio-temporal variability of small copepods (especially Oithona plumifera) in shallow nearshore waters off the south coast of South Africa. Estuar Coast Shelf Sci 72:711–720. doi:https://doi.org/10.1016/j.ecss.2006.12.006
Prygunkova RV (1974) Certain peculiarities in the seasonal development of zooplankton in the Chupa Inlet of the White Sea. Exp Fauna Seas 13:4–55 (in Russian)
Richter C (1994) Regional and seasonal variability in the vertical distribution of mesozooplankton in the Greenland Sea. Ber Polarforsch 154:1–90
Sabatini M, Kiørboe T (1994) Egg production, growth and development of the cyclopoid copepod Oithona similis. J Plankton Res 16:1329–1351. doi:https://doi.org/10.1093/plankt/16.10.1329
Satapoomin S, Nielsen TG, Hansen PJ (2004) Andaman sea copepods: spatio-temporal variations in biomass and production, and role in the pelagic food web. Mar Ecol Prog Ser 274:99–122. doi:https://doi.org/10.3354/meps274099
Shaginyan ER (1982) Population dynamics and age structures of Oithona similis (Claus) and Pseudocalanus elongatus (Boeck) in the Bay of Oliyutorsk. Izv TINRO 106:123–126 (in Russian)
Shuvalov VS (1965) Geographical size variability and some aspects of biology of Othona similis Claus (Copepoda, Cyclopoida) in the White Sea (Kandalaksha Bay). Okeanologia 5:338–347 (in Russian)
Shuvalov VS (1975) Geographical variability of some species of the family Oithonidae (Copepoda, Cyclopoida). In: Zvereva ZA (ed) Geographical and seasonal variability of marine plankton. Keter Publishing House Jerusalem Ltd, Jerusalem, pp 169–185
Shuvalov VS (1980) Copepod cyclopoids of the family Oithonidae of the World Ocean. Nauka Press, Leningrad (in Russian)
Terziev FS, Girdyuk GV, Zykova GG (eds) (1990) Hydrometeorology and hydrochemistry of the seas of the USSR. V.1. The Barents Sea. Issue 1. Hydrometeorological conditions. Gidrometeoizdat Press, Leningrad (in Russian)
Thor P, Nielsen TG, Tiselius P, Juul-Pedersen T, Michel C, Møller EF, Dahl K, Selander E, Gooding S (2005) Post spring bloom community structure of copepods in the Disko Bay, Western Greenland. J Plankton Res 27:341–356. doi:https://doi.org/10.1093/plankt/fbi010
Thor P, Nielsen TG, Tiselius P (2008) Post-spring bloom mortality rates of epipelagic copepods in the Disko Bay, Western Greenland. Mar Ecol Prog Ser 359:151–160. doi:https://doi.org/10.3354/meps07376
Timofeev SF (1994) Zooplankton of Yarnyshnaya Bay (Barents Sea) in summer (July–August 1987). In: Matishov GG (ed) Hydrobiological investigations in the bays and fjords of the northern seas of Russia. KSC RAS Press, Apatity, pp 19–31 (in Russian)
Timofeev SF (2000) Ecology of the marine zooplankton. Murmansk State Pedagogical Institute Press, Murmansk (in Russian)
Trofimova VV (2007) Photosynthetic pigments of phytoplanktonic assemblage of the southern bend of Kola Bay: seasonal dynamics and distribution. PhD Thesis, Murmansk Marine Biological Institute, Murmansk (in Russian)
Turner JT (2004) The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool Stud 43:255–266
Ussing HH (1938) The biology of some important plankton animals in the fjords of East Greenland. Medd Gronl 100:1–108
Uye SI, Sano K (1995) Seasonal reproductive biology of the small cyclopoid copepod Oithona davisae in a temperate eutrophic inlet. Mar Ecol Prog Ser 118:121–128. doi:https://doi.org/10.3354/meps118121
Uye SI, Sano K (1998) Seasonal variations in biomass, growth rate and production rate of the small cyclopoid copepod Oithona davisae in a temperate eutrophic inlet. Mar Ecol Prog Ser 163:37–44. doi:https://doi.org/10.3354/meps163037
Vinogradov ME, Shushkina EA (1987) Function of plankton communities in epipelagic zone of the ocean. Nauka Press, Moscow (in Russian)
Ward P, Hirst AG (2007) Oithona similis in a high latitude ecosystem: abundance, distribution and temperature limitation of fecundity rates in a sac spawning copepod. Mar Biol (Berl) 151:1099–1110. doi:https://doi.org/10.1007/s00227-006-0548-1
Williams JA, Muxagata E (2006) The seasonal abundance and production of Oithona nana (Copepoda: Cyclopoida) in Southampton Water. J Plankton Res 28:1055–1065. doi:https://doi.org/10.1093/plankt/fbl039
Acknowledgments
Thanks to our colleagues Dr. V.V. Larionov, Dr. E.I. Druzhkova, I.V. Berchenko, O.V. Druzhinina, E.F. Marasaeva, A.A. Oleynik, E.A. Garbul for help in sampling. We are grateful to the crew of the R/V ‘GSS-440’ for their valuable assistance. We are indebted to Dr. D.V. Moiseev for providing hydrological data. We thank especially Dr. A. Trebitz (U.S. EPA Mid Continent Ecology Division) for correcting the English and invaluable help during the manuscript preparation. Two anonymous referees provided helpful comments to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by X. Irigoien.
Rights and permissions
About this article
Cite this article
Dvoretsky, V.G., Dvoretsky, A.G. Life cycle of Oithona similis (Copepoda: Cyclopoida) in Kola Bay (Barents Sea). Mar Biol 156, 1433–1446 (2009). https://doi.org/10.1007/s00227-009-1183-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1183-4