Abstract
Zooplankton was sampled at ten stations in the Canada Basin during August 2002 using both 53- and 236-μm mesh nets to examine the contribution by smaller and less studied species. Copepod nauplii, the copepods Oithona similis, Oncaea borealis and Microcalanus pygmaeus, and the larvacean Fritillaria borealis typica dominated the upper 100 m of the water column numerically, while biomass was dominated by the copepods Calanus hyperboreus, Calanus glacialis and Paraeuchaeta glacialis, and the chaetognath Eukrohnia hamata. Zooplankton biomass ranged from 3.7 to 14.5 mg AFDW m−3, with a mean of 9.6 mg AFDW m−3 . While the three microcopepods contributed less than 5% of the biomass, estimates of their potential growth rates suggest they might contribute upwards of 25% of the metazoan zooplankton production. The true rates of growth and development of these microcopepods in the Arctic need to be determined to conclusively ascertain their importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Relative to most of the world’s oceans, our knowledge of zooplankton communities in the Arctic Ocean is deficient, especially so in the central basins due to their thick cover of multiyear ice. Over the past 50 years, zooplankton research in the basins has either taken the form of drifting ice stations that describe seasonal cycles or, more recently, ice-breaker transits that provide more extensive geographic coverage, but are generally confined to late summer when the ice is thinnest. Due to their high abundance and ease of capture, the taxonomic composition (Brodsky 1983; Sirenko 2001) and life history of the larger, more common copepods in the Arctic Ocean is now relatively well understood (see review by Smith and Schnack-Schiel 1990). The same cannot be said for the smallest copepod species that are variably missed by collection techniques (e.g. Auel and Hagen 2002), deep-water taxa (e.g. Kosobokova and Hirche 2000), or the more fragile gelatinous forms (see Raskoff et al. 2004).
Historically, effort has concentrated on copepods of the genus Calanus because they appear to dominate the zooplankton biomass (e.g. Smith and Schnack-Schiel 1990; Mumm et al. 1998; Thibault et al. 1999; Ashjian et al. 2003). They feed on algae and protozoa in the surface layers and accumulate surplus energy in the form of lipids. This chemical energy is utilized for over-wintering at depth and to fuel reproduction in the following spring. The life cycles of these species have been estimated to take 2–4 years (e.g. Hirche 1997; Kosobokova 1999). As in most oceans, smaller copepod taxa are actually numerically dominant (e.g. Conover and Huntley 1991; Kosobokova and Hirche 2000; Auel and Hagen 2002), yet only a few studies have used sufficiently fine meshes to fully assess their contribution (i.e., Kosobokova 1980; Pautzke 1979; Ashjian et al. 2003). These smallest species exhibit shorter generation time and more sustained reproduction (Ashjian et al. 2003), suggesting that their importance in ecosystem productivity could be greater than that implied by their biomass alone.
Although copepods are typically predominant in the basins, there is a broad assemblage of other holoplanktonic groups in the Arctic (e.g. Sirenko 2001) that are either ignored or briefly summarized (Thibault et al. 1999), and only occasionally reported in full detail (e.g. Kosobokova and Hirche 2000). Larvaceans (Appendicularians), for example, have been shown to be abundant in Arctic polynyas (Ashjian et al. 1995; Acuna et al. 1999) and common in the central Arctic (Kosobokova and Hirche 2000; Auel and Hagen 2002), although none of these studies has used sufficiently fine meshed nets to fully assess their importance. These soft-bodied filter-feeders are capable of much higher rates of ingestion, growth and reproduction than crustaceans (Hopcroft et al. 1998; Gorsky and Fenaux, 1998; R. R. Hopcroft, unpublished data), allowing them to respond more rapidly to shifts in primary productivity. During the times when larvaceans are numerically abundant, the efficiency with which primary production is exported to the benthos may be greatly increased compared to when copepods abound (Gorsky and Fenaux 1998).
Similarly, important and common predatory groups, such as the chaetognaths, amphipods, ctenophores and cnidarians, have received detailed reports in only a few surveys (Kosobokova and Hirche 2000; Auel and Hagen 2002). Arctic chaetognaths may represent considerable biomass (Kosobokova and Hirche 2000), have long life cycles (e.g. 2 years—Welsh et al. 1996) and are thought to be important in controlling Calanus populations (Falkenhaug and Sakshaug 1991). Hyperiid amphipods can also be common in Arctic waters (Mumm 1993; Auel and Werner 2003), with 2- to 3-year life cycles, and a similar potential to graze a notable proportion of the Calanus population (Auel and Werner 2003). The importance of ctenophores and cnidarians is under-appreciated. This is considered elsewhere in this issue (Raskoff et al. 2004).
In recent years, we have come to recognize that the Arctic may be more rapidly influenced by climate change than elsewhere on the globe (e.g. Sturm et al. 2003). Thus, there is a critical need for improved knowledge of its communities if any climatic effects are to be observed. To better understand the zooplankton communities of the Canada Basin, we employed both standard and fine-meshed nets to better establish the contribution of the smaller and less studied taxa.
Materials and methods
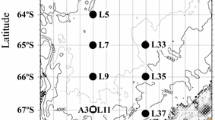
Zooplankton samples were collected from 21 August to 5 September 2003, between 0700 and 2100 hours by vertical hauls to 100 m depth of two separate sets of Bongo nets. Collections were made at a total of ten stations (Fig. 1), with sampling generally executed in ice-free zones to simplify deployment. Water temperatures in the upper 100 m varied between ~0 and −1.5°C with salinity from 26 psu at the surface to ~32 psu deeper (for details of physics, see McLaughlin et al. 2004). The larger Bongo set consisted of 60-cm-diameter black MARMAP-style nets of 236 μm mesh. The smaller set consisted of 22 cm diameter nets designed with a 1-m cylinder followed by a 1.6-m conical section, with one Bongo being of 150 μm mesh (used for other purposes) and the other of 53 μm mesh. One of the larger nets was fitted with a Karl uni-directional flowmeter. Both nets in the smaller Bongo were fitted with GO flowmeters mounted such that the net immobilized the rotor during descent. Upon retrieval, the contents were preserved in 10% formalin.
Preserved samples were processed for quantitative determination of species composition using a combination of Folsom and Beaker splits. A maximum of 100 animals per species were identified and measured using a computer-assisted measuring system (Roff and Hopcroft 1986), with increasingly larger subsamples examined for the rarer taxa. For the copepods, the developmental stages and the sex of the adults was also noted, except for Oncaea, where staging of the subadults proved problematic. It is likely that for some congeneric species where earlier copepodites cannot be distinguished, they have been incorrectly grouped with the sibling species for which adults were identified. In the case of Calanus, the two species were distinguished largely by differences in prosome length (e.g. Unstad and Tande 1991; Hirche et al. 1994). The weight of each individual was predicted from species-specific relationships, or from those of a morphologically similar species (Table 1). Notably, although a relationship has been reported for Oithona similis (Sabatini and Kiørboe 1994), its slope is unusually shallow, and we have elected to use that for another congender. The data for smaller-bodied taxa were taken entirely from the 53-μm mesh nets, while data for larger-bodied taxa were taken from the 236-μm net because they filtered larger volumes and hence yielded better estimates of abundances (see Results).
Although it was not a goal of this study, where it could be easily observed, the reproductive condition of the various species was noted because such information is rare in the literature. In the case of the copepods, such observations are confined to egg-carrying species, where the eggs and egg sacs remain characteristically identifiable, even after separation from the female.
Results
A total of 24 species of copepods and 13 non-copepod taxa were collected during the cruise (Table 2). In general, most taxa were adequately collected by the 236-μm net, with the notable exception of copepods (and nauplii) whose prosome length was less than 500 μm (Fig. 2). Curiously, after the rapid drop in abundance of copepod size classes to ~1,000 μm prosome length, the abundance spectra remained surprisingly flat. In terms of biomass, the 236-μm net missed relatively little of the total, with the overall spectra characterized by a depressed region between ~600 and 3,000 μm and a peak between 6,000 and 7,000 μm that corresponds to the size of Calanus hyperboreus females.
On average, the 53-μm net captured 1,447 copepods m−3 (including nauplii), while the 236-μm net captured only 149 m−3 . In terms of composition, this difference was due largely to the extrusion of virtually all nauplii, all stages of Oncaea borealis, Microcalanus pygmaeus below stage C5, and Oithona similis below C5. In terms of the non-copepods, the 53-μm net on average captured 43.9 individuals m−3, while the 236-μm net captured 32.6 m−3, with this difference due in part to better retention of larvaceans by the finer mesh. In terms of corresponding biomass, the 53-μm net on average captured 6.7 and 1.4 mg AFDW m−3 of copepods and non-copepods, respectively, while the 236-μm net captured 8.39 and 1.3 mg AFDW m−3 of copepods and non-copepods, respectively. These biomass differences arise in part because at two stations (al10 and nw1) 53-μm nets were not collected, and also due to the greater accuracy of the larger net in estimating the biomass of larger organisms. Ultimately, these biomass differences are not statistically significant between the nets (t-test and Mann–Whitney Rank). From this point forward we will, therefore, combine the data on all stages of these smaller species taken exclusively from the 53-μm net, while all other data will be employed from the 236-μm net, with the exception of the missing samples for which the 236-μm data will be employed for those species where the bias is less extreme. Once the data from the two nets are combined, total zooplankton biomass ranges from 3.7 to 14.5 mg AFDW m−3 (Figs. 3, 4), with a mean of 9.6 mg AFDW m−3 . Although calanoid nauplii (average 76 m−3) and non-calanoid nauplii (average 984 m−3) contributed significantly to the community, due to the difficulty in identifying them to the species, and their low biomass (0.02 and 0.08 mg AFDW m−3, respectively), they shall not be considered further.
Of the 24 copepod taxa observed (Table 2), 14 species were present at more than half the stations. Seven of these copepods warrant further attention (Fig. 3) because they dominated in terms of abundance (Oithona similis, Oncaea borealis and Microcalanus pygmaeus) or biomass (Calanus hyperboreus, C. glacialis, Paraeuchaeta glacialis and Metridia longa). The cyclopoid Oithona similis was the most abundant copepod on average (142 m−3), displaying no trends across the study area. All stages were well represented in the collections, with their readily identifiable egg sacs found loose in the collections at all stations (Table 3). The poecilostomatoid Oncaea borealis was the next most abundant taxon throughout the study area (average 129 m−3) at all but the shallowest stations on the Northwind Ridge (i.e. na5, nw1, nw5). Its absolute numbers were greatest over the deepest waters of the basin. Although this species was not staged routinely, lengths suggest a mix of all developmental stages. The calanoid Microcalanus pygmaeus was third in abundance (average 89 m−3) and, like Oncaea, its numbers tended to be greater over the deeper parts of the study area. All stages were present in the samples, but notably adults were relatively scarce, with C4 and C5 dominating at most stations (Fig. 5). Despite their high abundances, these three “micro”-copepods contributed relatively little to biomass (Fig. 2) due to their size even though they rank fifth through seventh in terms of copepod biomass.
In contrast, Calanus hyperboreus (average 4.6 m−3, 5.5 mg AFDW m−3 —Table 2) represented the majority of the copepod community biomass. There were no spatial trends in its abundance or biomass (Fig. 3). Females, and to a lesser extent C5s, generally predominated, but both tended to be more prevalent over the central Basin, while C3 and younger were most common near and over the Northwind Ridge (Fig. 5). Calanus glacialis (average 2.6 m−3, 1.2 mg AFDW m−3 —Table 2) ranked second for biomass and tended to be slightly more common at the most shoreward stations. Female C. glacialis were not as common as C5 throughout the study area, although like C. hyperboreus, C3 and younger were most common near and over the Northwind Ridge (Fig. 5). Like the calanids, Metrida longa (average 2.0 m−3, 0.2 mg AFDW m−3 —Table 2) showed no spatial trends in terms of abundance, but the biomass was higher over the Northwind Ridge. This was related to an increase from east to west in average developmental stage (Fig. 5). Despite lower abundances, Paraeuchaeta glacialis (average 0.9 m−3, 0.7 mg AFDW m−3—Table 2) ranked third in terms of biomass. The numbers were too low and variable to establish an across-region stage distribution, but all stages were present (Table 3), and a number of females were observed carrying egg sacs. Notably, Microcalanus pygmaeus tied Metrida longa in terms of biomass, and both were followed closely by Oithona similis.
Nine species of non-copepods warrant attention (Fig. 4) because of their abundance (Oikopleura vanhoeffeni, Fritillaria borealis, Limacina helicina) or biomass (Eukrohnia hamata, Sagitta elegans, Conchoecia borealis, Themisto abysorrum, Themisto libellula, Aglantha digitale). The larvacean F. borealis clearly dominated the abundance of non-copepods at all stations, but contributed little to biomass due to its small size (mean trunk length 400–500 μm). The larvacean O. vanhoeffeni was present as two distinct size classes, predominately juveniles with trunks <700 μm, and limited numbers of individuals of 2–4 mm trunk length. Unlike F. borealis, which frequently had well-developed gonads, gonads in even the largest O. vanhoeffeni were virtually undeveloped (see Shiga 1976). The pteropod L. helicina was present at all stations only as small juveniles (<1 mm). The chaetognath E. hamata dominated the non-copepod biomass at all stations, with notable contribution from S. elegans at the two shallowest stations on the Northwind Ridge. The majority of the chaetognaths were larger than 10 mm body length, and on several occasions female E. hamata were observed to have broods carried in their marsupium. Similarly, the ostracod C. borealis (0.6–3 mm carapace) was found at all stations in low numbers, and on several occasions with a clutch of large eggs incubated within the valves of the larger individuals. Both the amphipods T. abysorrum and T. libellula were more variable in terms of presence and size (3–10 mm), with the former being more common. The reproductive condition could not be judged for either species because of the limited number of animals and the potential liberation of juveniles within the marsupium upon preservation. A. digitale (3–13 mm axial length) was the only cnidarian found in the nets, and it occurred at all but one of the stations. Gonadal development was variable, but never extensive in this hydromedusae.
Discussion
In general, the species observed in this study are simply characteristic of those reported by other studies working in the deeper waters of the Arctic (e.g. Mumm 1991; Kosobokova et al. 1998; Kosobokova and Hirche 2000; Auel and Hagen 2002). Consistent with these studies, Calanus species dominate the biomass, followed by the Metridia longa and then Paraeuchaeta species, while smaller species dominate numerically. Furthermore, the number of unique species observed, and their relative abundance/biomass (accounting for mesh-size biases), are also relatively similar to those described for the upper 100 m by taxonomically detailed studies (Mumm 1991; Kosobokova et al. 1998; Kosobokova and Hirche 2000; Auel and Hagen 2002) in deeper waters. The most notable differences in species composition between those studies that emphasize the Eurasian Basins and this study is the absence of the Atlantic Calanus fimarchicus, which is not normally present in the Amerasian Basins (e.g. Kosobokova 1982; Smith and Schnack-Schiel 1990). Reciprocally, single individuals of the Pacific Eucalanus bungii and Neocalanus cristatus were not normally observed in the Eurasian Basins but were found as far into the Canada Basin as sb2 and sb1, respectively.
It is much harder to compare the composition, abundance and biomass of the non-copepods because they are not often reported in detail, or the sampling depth strata are not directly comparable to this study. Nonetheless, other studies in Arctic Basins have indicated that Eukrohnia hamata dominates the biomass of the non-copepods, contributing 7–18% of the epipelagic biomass (Kosobokova et al. 1998; Kosobokova and Hirche 2000). It is replaced by the neritic Sagitta elegans as one moves into shallower waters (Kosobokova et al. 1998). This study does indicate a higher abundance of larvaceans than previously reported for the basins, although this may be due to the use of the 53-μm net that sampled Fritillaria borealis relatively undamaged. At times when larger Oikopleura vanhoeffeni are common, larvacean biomass may be relatively more important in the Basins than observed here (e.g. Kosobokova and Hirche 2000). Interestingly, while F. borealis populations appeared to be reproductively active, O. vanhoeffeni appeared to exist as two distinct cohorts, the larger of which appeared to be in an arrested reproductive development that is as yet undocumented for this group. The standard growth mode in larvaceans involves simultaneous development of both somatic and reproductive tissue (Fenaux 1998), such that gonads are extremely obvious in larger individuals. Aside from basic information on abundance and biomass (e.g. Kosobokova et al. 1998; Kosobokova and Hirche 2000; Auel and Hagen 2002), and occasionally detailed information on size–structure (Mumm 1991; Richter 1994), information on the reproductive patterns of most of these non-copepod groups is absent from the literature.
Overall, the abundance and biomass of most metazoan zooplankton species, and the community in aggregate, was surprisingly stable over the entire region of the study (>1,000 km), and similar to that of a number of other deeper-water Arctic studies previously cited. Nonetheless, there exists a significant range in the observations between these studies, and given the concerns about climate change impacts in the Arctic, it is worth considering if these are regional, year-to-year, or systematic differences. Given that the biomass peak may be relatively brief in the Arctic (e.g. Pautzke 1979), and that species and biomass can be extremely stratified (e.g. Kosobokova 1982; Kosobokova and Hirche 2000; Auel and Hagen 2002; Ashjian et al. 2003), it becomes critical that inter-study comparisons be made from the same depth intervals and the same seasonal periods. Although mesh size can also represent a significant bias in biomass estimates in most ecosystems (Hopcroft et al. 2001), the bias resulting from differences in mesh sizes below 236 μm is relatively small in the central Arctic, and therefore not of major consequence in comparing studies. Notably, the values from this study are three to fourfold lower than those observed in the upper 100 m during August 1994 on, and north of, the Chukchi Plateau (Thibault et al. 1999), and those from August 1998 on the Chukchi Plateau (Ashjian et al. 2003). Despite this, the stage distributions of the dominant copepods are very similar to those observed in August 1998 (Ashjian et al. 2003). The biomass values reported in this study are, however, often very similar to the central basin values reported for August 1995 (Mumm et al. 1998), and the summer of 1975 (Kosobokova 1982; Kosobokova and Hirche 2000). Like all of these more contemporary studies, historical biomass values of 1 mg DW m−3 reported for the Canada Basin in August 1966 (Hopkins 1969) and August 1975 (Pautzke 1979) are not supported. Both these latter ice-camp studies surveyed a region similar to those of this study. Notably, in both cases, unmetered nets of 62–73 μm were employed, and overestimation of the volume filtered could account for their low numbers. Interestingly, when unmetered 223-μm mesh nets were employed during August 1970–1973, the biomass was estimated as 2–8 mg DW m−3 (Pautzke 1979) overlapping the values observed within this study. Without a more standardized set of locations, more regular occupation of them and more standardized methods, it would be impossible to disentangle the effect of location and year.
What has not been previously well established is the actual contribution of these smaller species to community biomass because a substantial proportion, if not the bulk, of these species has been extruded through the collection nets. Pautzke (1979) reached conclusions similar to those of this study, in terms of which stages of Microcalanus, Oncaea and Oithona were missed by his 223-μm mesh compared to the 62- or 73-μm nets, but did not estimate the impact on biomass. Our data, which collected even the smallest metazoans, support the longstanding belief that during Arctic summer, the biomass of these smaller taxa in the epipelagic realm is trivial in comparison to the larger taxa. This appears to be a feature unique for Arctic waters, because in most other pelagic systems zooplankton biomass tends to decline with increasing size (Quinones et al. 2003, and references therein). This pattern appears to hold even when restricted to just the copepods for both tropical and temperate waters (Hopcroft et al. 2001). It is therefore remarkable that over deep waters in the Arctic, abundance in the upper 100 m remains relatively flat across much of the spectra, while biomass actually increases with size along the spectra. The curious depression in the biomass spectra between 600 and 3,000 μm is, however, likely not a permanent or consistent feature of Arctic waters, because earlier in the year it would be better occupied by earlier stages of larger species. In coastal waters, this gap would also be filled by medium-bodied species such as Pseudocalanus (Conover and Huntley 1991), and perhaps by a greater contribution of Metridia longa (Conover and Huntley 1991; Ashjian et al. 2003), which although generally characterized as a strong diel migrator, is commonly observed during summer in the Arctic’s upper 100 m (Pautzke 1979; Kosobokova and Hirche 2000; Auel and Hagen 2002). Seasonally, the Calanus species will migrate from the upper mixed layer to enter diapause at depth, and during such times Microcalanus, Oncaea and Oithona will contribute more significantly to the biomass spectra. These small species continue active, albeit reduced, growth and recruitment outside of the summer period, as suggested by their stage distribution in this and other studies (e.g. Pautzke 1979; Ashjian et al. 2003). Thus, some of our impression of biomass and production patterns in the Arctic may be biased by emphasis on the late summer periods. It would therefore be interesting to see what a seasonally averaged size spectra of biomass and production actually looks like during future efforts.
Elsewhere, it has been argued that the biomass present in these smaller zooplankton size fractions may be misleading because smaller animals tend to grow faster than larger ones (Hirst and Lampitt 1998; Hirst and Bunker 2003). To explore such a premise in the Arctic, it is useful to undertake a crude estimation of the growth rates and productivity of the Calanus species that dominate the biomass, and with Oithona similis that dominates numerically. The simplest approach is to take the weight of the egg and the adult female and the typical generation time it takes to move between the two (Huntley and Lopez 1992), ignoring the fact that some species undergo diapause periods. C. hyperboreus appears to have a 3- to 4-year life cycle (Conover 1967; Hirche 1997), has eggs that weigh 1.4 μg DW (Conover 1962) and are up to 5 mg DW as adult females in the Canada Basin (Ashjian et al. 2003). In a genus such as Calanus, where diapause occupies a large proportion of the year, the growth rate will be much greater during the active periods, and zero (or negative) during diapause. However, we can calculate an average instantaneous growth rate over their lifetime of ~0.006 day−1 (i.e. 0.6% day−1), which compares nicely to an independent specific egg production rate estimate of 0.7% day−1 (Hirche and Niehoff 1996).
Oithona similis eggs are ~60 μm in diameter (Nielsen et al. 2002), so assuming an egg density of 0.14 pg C μm−3 (Kiørboe and Sabatini 1994), and carbon as 40% AFDW, the eggs weigh 0.04 μg AFDW, while females in this study would weigh 2.6 μg AFDW on average. The generation lengths of Oithona, Oncaea and Microcalanus in the Arctic are not well established, with the suggestion from field observations being that only one generation occurs per year (McLaren 1969; Kosobokova 1980). The broad representation of all stages, including females carrying sacs and high numbers of nauplii, in this and other studies (e.g. Ashjian et al. 2003) is, however, suggestive of multiple overlapping generations per year. If we assume that as many as three generations occur per year, the instantaneous growth rate would be ~0.035 day−1 for O. similis. This also agrees with the maximum reproductive rate of 7.1% at 15°C (Hirst and Bunker 2003), which corrected by his Q10 of 1.49 for sac spawning adults, would predict a rate of 0.039 day−1 at 0°C, although caution is warranted in extrapolating his relationships below 5°C. Thus, growth rates in O. similis could be approximately sixfold greater than that those of C. hyperboreus.
Assuming that these crude estimates of growth rate are correct, this raises the aggregate importance of the three smallest species from ~5% of the total copepod biomass to ~25% of the total copepod production. Although the production by the Calanus species would still predominate, the production of these smaller genera (i.e. Microcalanus, Oncaea and Oithona) would be worth serious consideration. If such high productivity by small species is in fact true, it implies that these small species must be under intense predatory pressure, which keeps them from making more substantial contributions to community biomass as is common in most other oceanic regions. This would help explain the unusual biomass size spectra we have observed. Alternatively, the growth in small species is not disproportionately faster than larger species, and their importance in terms of both biomass and production is therefore not significant. This suggests that future efforts would be well spent determining the true rates of growth and development of these smaller Arctic species to distinguish between these possibilities.
References
Acuna JL, Deibel D, Bochdansky AB, Hatfield E (1999) In situ ingestion rates of appendicularian tunicates in the Northeast Water Polynya (NE Greenland). Mar Ecol Prog Ser 186:149–160
Ashjian CJ, Smith SL, Lane PVZ (1995) The Northeast Water Polynya during summer 1992: distribution and aspects of secondary production of copepods. J Geophys Res 100:4371–4388
Ashjian CJ, Campbell RG, Welch HE, Butler M, Keuren DV (2003) Annual cycle in abundance, distribution, and size in relation to hydrography of important copepod species in the western Arctic Ocean. Deep Sea Res I 50:1235–1261
Auel H, Hagen W (2002) Mesozooplankton community structure, abundance and biomass in the central Arctic Ocean. Mar Biol 140:1013–1021
Auel H, Werner I (2003) Feeding, respiration and life history of the hyperiid amphipod Themisto libellula in the Arctic marginal ice zone of the Greenland Sea. J Exp Mar Biol Ecol 296:183–197
Brodsky KA (1983) Copepod Crustacea (Copepoda: Calanoida) of the seas of the USSR and adjacent waters (Part 1). Zoological Institute of the Academy of Sciences of the USSR, Leningrad
Chisholm LA, Roff JC (1990) Size–weight relationships and biomass of tropical neritic copepods off Kingston, Jamaica. Mar Biol 106:71–77
Conover RJ (1962) Metabolism and growth in Calanus hyperboreus in relation to its life cycle. Rapp PV Reun Cons Int Explor Mer 153:190–197
Conover RJ (1967) Reproductive cycle, early development, and fecundity in laboratory populations of the copepod Calanus hyperboreus. Crustaceana 13:61–72
Conover RJ, Huntley M (1991) Copepods in ice-covered seas—distribution, adaptations to seasonally limited food, metabolism, growth patterns and life cycle strategies in polar seas. J Mar Syst 2:1–41
Deibel D (1986) Feeding mechanism and house of the appendicularian Oikopleura vanhoeffeni. Mar Biol 93:429–436
Falkenhaug I, Sakshaug E (1991) Prey composition and feeding rate of Sagitta elegans var. arctica (Chaetognatha) in the Barents Sea in early summer. Polar Res 10:487–506
Fenaux R (1976) Cycle vital, croissance et production chez Fritillaria pellucida (Appendicularia), dans la baie de Villefranche-sur-Mer, France. Mar Biol 34:229–238
Fenaux R (1998) Life history of the Appendicularia. In: Bone Q (ed) The biology of pelagic tunicates. Oxford University Press, New York, pp 151–159
Gorsky G, Fenaux R (1998) The role of Appendicularia in marine food chains. In: Bone Q (ed) The biology of pelagic tunicates. Oxford University Press, New York, pp 161–169
Hirche H-J (1997) Life cycle of the copepod Calanus hyperboreus in the Greenland Sea. Mar Biol 128:607–618
Hirche H-J, Niehoff B (1996) Reproduction of the Arctic copepod Calanus hyperboreus in the Greenland Sea—field and laboratory observations. Polar Biol 16:209–219
Hirche H-J, Hagen W, Mumm N, Richter C (1994) The Northeast Water Polyna, Greenland Sea. III. Meso- and macrozooplankton distribution and production of dominant herbivorous copepods during spring. Polar Biol 14:491–503
Hirst AG, Bunker AJ (2003) Growth of marine planktonic copepods: global rates and patterns in relation to chlorophyll a, temperature, and body weight. Limnol Oceanogr 48:1988–2010
Hirst AG, Lampitt RS (1998) Towards a global model of in situ weight-specific growth in marine planktonic copepods. Mar Biol 132:247–257
Hopcroft RR, Roff JC, Bouman HA (1998) Zooplankton growth rates: the larvaceans Appendicularia, Fritillaria and Oikopleura in tropical waters. J Plankton Res 20:539–555
Hopcroft RR, Roff JC, Chavez FP (2001) Size paradigms in copepod communities: a re-examination. Hydrobiologia 453/454:133–141
Hopcroft RR, Clarke C, Chavez FP (2002) Copepod communities in Monterey Bay during the 1997 to 1999 El Niño and La Niña. Prog Oceanogr 54:251–263
Hopkins TL (1969) Zooplankton standing crop in the Arctic Basin. Limnol Oceanogr 14:80–85
Huntley ME, Lopez MDG (1992) Temperature-dependent production of marine copepods: a global synthesis. Am Nat 140:201–242
Ikeda T, Shiga N (1999) Production, metabolism and production/biomass (P/B) ratio of Themisto japonica (Crustacea: Amphipoda) in Toyama Bay, southern Japan Sea. J Plankton Res 21:299–308
Kiørboe T, Sabatini M (1994) Reproductive and life cycle strategies in egg-carrying cyclopoid and free-spawning calanoid copepods. J Plankton Res 16:1353–1366
Kosobokova KN (1980) Seasonal variations in the vertical distribution and age composition of Microcalanus pygmaeus, Oithona similis, Oncaea borealis and O. notopus populations in the central Arctic basin. Biologiya Tsentral’nogo Arkicheskogo Basseyna. Nauka, Moscow, pp 167–182
Kosobokova KN (1982) Composition and distribution of the biomass of zooplankton in the central Arctic Basin. Oceanology 22:744–750
Kosobokova KN (1999) The reproductive cycle and life history of the Arctic copepod Calanus glacialis in the White Sea. Polar Biol 22:254–263
Kosobokova KN, Hirche H-J (2000) Zooplankton distribution across the Lomonosov Ridge, Arctic Ocean: species inventory, biomass and vertical structure. Deep Sea Res I 47:2029–2060
Kosobokova KN, Hanssen H, Hirche H-J, Knickmeier K (1998) Composition and distribution of zooplankton in the Laptev Sea and adjacent Nansen Basin during summer, 1993. Polar Biol 19:63–73
Matthews JBL, Hestad L (1977) Ecological studies on the deep-water pelagic community of Korsfjorden, Western Norway. Length/weight relationships for some macroplanktonic organisms. Sarsia 63:57–63
McLaren IA (1969) Population and production ecology of zooplankton in Ogac Lake, a landlocked fjord on Baffin Island. J Fish Res Bd Can 26:1485–1559
McLaughlin F, Shimada K, Carmack E, Ito M, Nishino S (2004) The hydrography of the deep Canada Basin, 2002. Polar Biol (in press)
Mumm N (1991) On the summerly distribution of mesozooplankton in the Nansen Basin, Arctic Ocean (in German). Rep Polar Res 92:1–173
Mumm N (1993) Composition and distribution of mesozooplankton in the Nansen Basin, Arctic Ocean, during summer. Polar Biol 13:451–461
Mumm N, Auel H, Hanssen H, Hagen W, Richter C, Hirche H-J (1998) Breaking the ice: large-scale distribution of mesozooplankton after a decade of Arctic and transpolar cruises. Polar Biol 20:189–197
Nielsen TG, Møller EF, Satapoomin S, Ringuette M, Hopcroft RR (2002) Egg hatching rate of the cyclopoid copepod Oithona similis in arctic and temperate waters. Mar Ecol Prog Ser 236:301–306
Pautzke CG (1979) Phytoplankton primary production below Arctic Ocean pack ice: an ecosystem analysis. PhD Oceanography, University of Washington
Quinones RA, Platt T, Rodríguez J (2003) Patterns of biomass-size spectra from oligotrophic waters of the Northwest Atlantic. Prog Oceanogr 57:405–427
Raskoff KA, Purcell JE, Hopcroft RR (2004) Gelatinous zooplankton of the Arctic Ocean: in situ observations under the ice. Polar Biol DOI 10.1007/s00300-004-0377
Richter C (1994) Regional and seasonal variability in the vertical distribution of mesozooplankton in the Greenland Sea. Rep Polar Res 154:1–87
Roff JC, Hopcroft RR (1986) High precision microcomputer based measuring system for ecological research. Can J Fish Aquat Sci 43:2044–2048
Sabatini M, Kiørboe T (1994) Egg production, growth and development of the cyclopoid Oithona similis. J Plankton Res 16:1329–1351
Shiga N (1976) Maturity stages and relative growth of Oikopleura labradoriensis Lohmann (Tunicata, appendicualria). Bull Plankton Soc Jpn 29:119–128
Sirenko BI (2001) List of species of free-living invertebrates of Eurasian Arctic Seas and adjacent deep waters. Russian Academy of Sciences, St. Petersburg
Smith SL, Schnack-Scheil SB (1990) Polar zooplankton polar oceanography part B: chemistry biology and geology. Academic, San Diego, pp 527–598
Sturm M, Perovich DK, Serreze MC (2003) Meltdown in the North. Sci Am 289:60–67
Thibault D, Head EJH, Wheeler PA (1999) Mesozooplankton in the Arctic Ocean in summer. Deep Sea Res 46:1391–1415
Unstad KH, Tande KS (1991) Depth distribution of Calanus finmarchicus and C. glacialis to environmental conditions in the Barents Sea. In: Sakshaug E, Hopkins CCE, Øslashritsland NA (eds) Proceedings of the Pro Mare symposium on polar marine ecology. Polar Res 10:409–420
Welch HE, Siferd TD, Bruecker P (1996) Population densities, growth, and respiration of the chaetognath Parasagitta elegans in the Canadian High Arctic. Can J Fish Aquat Sci 53:520–527
Yamaguchi A, Ikeda T (2000) Vertical distribution, life cycle and body allometry of two oceanic calanoid copepods (Pleuromamma scutullata and Heterorhabdus tanneri) in the Oyashio region, western North Pacific Ocean. J Plankton Res 22:29–46
Acknowledgements
We wish to thank F. McLaughlin and K. Crane for accommodating our sampling needs during an otherwise over-committed JWACS/NOAA-OE cruise on the CCGS Louis St. Laurent. S. Toews was invaluable in providing sampling support. This research was supported by the Ocean Exploration Office of the National Oceanic and Atmospheric Administration (NOAA) under award NA16RP2625.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hopcroft, R.R., Clarke, C., Nelson, R.J. et al. Zooplankton communities of the Arctic’s Canada Basin: the contribution by smaller taxa. Polar Biol 28, 198–206 (2005). https://doi.org/10.1007/s00300-004-0680-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-004-0680-7