Abstract
Many symbioses involve multiple partners in complex, multi-level associations, yet little is known concerning patterns of nutrient transfer in multi-level marine mutualisms. We used the anemonefish symbiosis as a model system to create a balance sheet for nitrogen production and transfer within a three-way symbiotic system. We quantified diel patterns in excretion of ammonia by anemonefish and subsequent absorption by host sea anemones and zooxanthellae under laboratory conditions. Rates of ammonia excretion by the anemonefish Amphiprion bicinctus varied from a high of 1.84 μmole g−1 h−1 at 2 h after feeding, to a basal rate of 0.50 μmole g−1 h−1 at 24–36 h since the last meal. Conversely, host sea anemones Entacmaea quadricolor absorbed ammonia at a rate of 0.10 μmole g−1 h−1 during the daytime in ammonia-enriched seawater, but during the night reduced their absorption rate to near zero, indicating that ammonia uptake was driven by zooxanthella photosynthesis. When incubated together, net ammonia excretion was virturally zero, indicating that host anemones absorbed most of the ammonia produced by resident fish. Adult anemonefish weighed about 11 g under laboratory conditions, but on the coral reef may reach up to 64 g, resulting in a maximal potential ammonia load of >200 μmole h−1 produced by two adult fish during daylight hours. In contrast, host sea anemones weighed about 47 g in the laboratory, but under field conditions, large individuals may reach 680 g, so their maximal ammonia clearance rates may reach about 70 μmole h−1 during the daytime. As such, the ammonia load produced by adult anemonefish far exceeds the clearance rate of host anemones and zooxanthellae. Ammonia transfer likely occurs mainly during the daytime, when anemonefish consume zooplankton and excrete rapidly, and in turn the zooxanthellae are photosynthetically active and drive rapid ammonia uptake. We conclude that zooplanktivorous fishes that form mutualisms with coral reef cnidarians may serve as an important link between open water and benthic ecosystems, through the transfer of large quantities of nutrients to zooxanthellate hosts, thus enhancing coral reef productivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many symbioses consist of multi-level associations among members of at least three species belonging to widely-separated taxonomic groups. For example, a four-way association occurs among some cleaner shrimps that remove parasites from client fishes on coral reefs, and also shelter in sea anemones that host endosymbiotic microalgae (zooxanthellae) (Nizinski 1989; Bunkley-Williams and Williams 1998). These sea anemones provide the basis for a network of complex interactions among mutualistic partners that may impact multiple trophic levels on coral reefs (Becker and Grutter 2004; Arvedlund et al. 2006). Most of the evolutionary theory and empirial evidence on mutualisms, however, focusses on two-species systems, or on only two partners within multi-level associations (Doebeli and Knowlton 1998; Herre et al. 1999; Stachowicz 2001; but see Bruno et al. 2003). Symbiotic organisms often utilize the metabolic byproducts of their partners, thus enhancing the efficiency of nutrient and energy transfer both within and among ecosystems (Meyer and Schultz 1985a, b; Ellis and Midgley 1996; Stachowicz 2001; Davy et al. 2002; Vanni 2002). In aquatic environments where dissolved nutrients become easily dispersed, nutrient exchange among symbiotic partners may be common, especially in oligotrophic ecosystems such as coral reefs where levels of dissolved nutrients limit the growth of marine plants (Muscatine and Porter 1977; Crossland 1983; Falkowski et al. 1993; Hoegh-Guldberg and Williamson 1999). However, evidence on the patterns and ecological impacts of nutrient exchange within complex, multilevel symbioses in the marine environment is extremely rare. One of the few documented examples is the association among cnidarians, zooxanthellae, and a variety of invertebrate ectosymbionts. Some shrimps, barnacles, and mussels form obligate associations with host corals and sea anemones, and excrete ammonium and phosphate metabolic byproducts that the host potentially absorbs and can utilize for its own growth and that of its zooxanthellae (Fautin et al. 1995; Achituv and Mizrachi 1996; Spotte 1996; Mokady et al. 1998).
One of the most conspicuous symbioses on coral reefs is the association between groups of zooplanktivorous damselfishes and large cnidarians such as branching corals and giant sea anemones. These fishes shelter among the branches or tentacles of the host cnidarians (Allen 1991; Fautin and Allen 1997; Randall and Fautin 2002) and are thereby protected from predation by large reef piscivores such as groupers and sharks, greatly enhancing fish diversity and abundance on coral reefs. As vertebrates, these fishes metabolize more rapidly than do their invertebrate hosts and so produce relatively large quantities of metabolic waste products, mostly in the form of dissolved ammonia and particulate organic matter (Meyer and Schultz 1985a; Bray et al. 1986; Schaus et al. 1997). The main nitrogenous excretion of marine fish is ammonia (75–85%), which rapidly binds to hydrogen ions to form ammonium ions in aqueous solution (e.g., Jobling 1981; Durbin and Durbin 1981; Meyer and Schultz 1985a). This form of nitrogen is readily assimilated by corals and other cnidarians (Muscatine and D’Elia 1978). As such, mutualistic fishes may serve as major contributors to the fitness of cnidarian hosts and zooxanthellae through their metabolic byproducts, but almost nothing is known about the process of nutrient transfer among these partners or impacts on benthic reef productivity. Because most damselfish symbiotic with cnidarians are zooplanktivores (Allen and Randall 1980; Allen 1991; Fautin and Allen 1997), they import nutrients to the coral reef from the plankton, and thus may serve as an important link between open ocean and benthic ecosystems via nutrient transfer to their symbiotic hosts, thereby greatly enhancing reef productivity.
The excretions of free-living, non-symbiotic fishes also serve as an important source of nutrients on coral reefs. For example, large schools of migrating grunts (Haemulon spp.) that feed during the night and rest over colonies of the stony coral Porites furcata during the day enhance growth rates of the coral (Meyer and Schultz 1985a, b). Fish excretions also are important in freshwater ecosystems, where they may affect the flux of nutrients between benthic and pelagic habitats (Vanni 2002; Glaholt and Vanni 2005).
About ten species of giant sea anemones serve as symbiotic hosts to >25 species of obligate anemonefishes, and also harbor zooxanthellae within their tissues, forming a three-way mutualism (Fautin and Allen 1997). Until recently, most of the benefits in this multi-level symbiosis were assumed to accrue to the fish, mainly in form of protection from predation (Fautin 1991; Fautin and Allen 1997). However, the anemone host and zooxanthellae also benefit by being protected from specialized predators that consume cnidarians (Fricke 1979; Godwin and Fautin 1992; Porat and Chadwick-Furman 2004). Protection of the sea anemone host may be restricted to the adults of some fish species that exhibit high levels of territorial behavior and are large enough to aggressively drive off potential predators (Godwin and Fautin 1992; Fautin and Allen 1997; Randall and Fautin 2002). Recent studies also have demonstrated physiological benefits to the host, in which the anemonefish release ammonia and the host sea anemones absorb it (Cleveland et al. 2003, 2006), resulting in enhanced growth of the host when inhabited by fish (Holbrook and Schmidt 2005; Porat and Chadwick-Furman 2005; Roopin 2007). However, the percent contribution of anemonefish ammonia to the inorganic nutrient budget of host sea anemones and their zooxanthellae remains unknown, as well as temporal and spatial variation in the process of nutrient transfer.
Here, we use the anemonefish mutualism as a model system to quantify patterns of nutrient transfer in a three-way symbiosis. Specifically, we determine the extent of inorganic nutrient contribution by obligate fish partners to the nitrogen balance of sea anemone hosts and their zooxanthellae. This laboratory-based study complements related research on patterns of ammonia flux in this system, and the ecophysiological impacts on members of this mutualism under natural conditions on coral reefs (Roopin 2007).
Methods
Study organisms and maintenance
Bulb-tip anemones Entacmaea quadricolor (Rüppell and Leuckart, 1828) were transported to Auburn University from the Waikiki Aquarium (Hawaii, USA) where they had been cultured and propagated via clonal replication from individuals collected in Palau in 1985. Two-band anemonefish Amphiprion bicinctus Rüppell, 1828 were acquired from Oceans Reefs and Aquariums (ORA), an aquaculture facility at Harbor Branch Oceanographic Institution at Fort Pierce, Florida, USA. Members of these species co-occur on coral reefs in the Red Sea, where we have conducted previous studies on this mutualism (Porat and Chadwick-Furman 2004, 2005; Chadwick and Arvedlund 2005). The cultured fish and anemones were distributed haphazardly among 12 identical closed-system aquaria and were maintained under the conditions described below for at least 2 month prior to experiments.
Each aquarium system circulated ∼160 L of artificial sea water (Reef Crystals, Aquarium Systems, Inc., OH, USA) between an upper aquarium and a lower sump that each contained 80 L of sea water and measured 71 cm × 33 cm × 35 cm. Water flowed into the upper aquarium from the sump alternately through two pipe outlets using a SCWD-wave maker (3iQ Ventures LLC, Manhattan Beach, CA, USA). Each system contained a protein skimmer, live rock, and macroalgal cultures in the sump as mechanical and biological filters, and a layer of fine sand in both sump and aquarium. All systems were maintained at a salinity of 34–35 ppt and a temperature of 26°C on a 12 h light:12 h dark photoperiod. Over each aquarium was suspended a 6-bulb TEK-LIGHT™T5 high output fluorescent light, with a combination of 3 39 W T5 Midday 6,000 K and 3 39 W T5 Pure Actinic Giesemann PowerChrome fluorescent bulbs, which provided a constant irradiance of about 200 μmol quanta s−1 m−2 of photosynthetically-active radiation (PAR) at the bottom of the aquarium to 800 μmol quanta s−1 m−2 at the water surface, as measured with a QSL-2101 Scalar PAR Sensor (Biospherical Instruments, San Diego, CA, USA). This level of irradiance was equivalent to that at about 10–30 m depth on coral reefs in the Red Sea (Stambler 2005), where these organisms naturally occur (Chadwick and Arvedlund 2005). All anemones were fed weekly to satiation with small pieces of fish or shrimp, and the anemonefish were fed daily to satiation with a combination of dry pellets (Formula One, AquaPet Americas, UT, USA) and frozen foods (Cyclop-Eeze Copepods, Argent Laboratories, Redmond, Washington, USA, and Mysis Shrimp and Spirulina Brine Shrimp, Hikari Sales, Hayward, CA, USA). Feeding behavior was monitored carefully to ensure that all animals ingested the proffered foods. Both the fish and anemones grew substantially under these culture conditions prior to experiments.
Before the start of experiments, the wet mass of each fish was determined. The fish ranged in wet mass from 5.3 to 25.0 g (X ± SE = 11.3 ± 1.0 g, N = 9 fish). The relationship between wet mass and tentacle crown diameter (TCD) of the sea anemones was determined in a separate study as y = 0.20 x 2.10, where y = wet mass in grams and x = tentacle crown diameter in cm (Godinot and Chadwick unpublished data). Thus, sea anemone sizes were determined by photographing each while fully expanded in its home aquarium, then measuring TCD from the digital photographs using Image Tool for Windows version 3.00 (UTHSCSA). The TCD of the anemones ranged from 8.2 to 16.9 cm (X ± SE = 13.2 ± 0.4 cm), and their estimated wet mass ranged from 16.5 to 75.3 g (47.0 ± 3.0 g, N = 30 anemones).
Ammonia measurements
We determined the resting ammonia (defined as the combination of NH3 gas and NH4 + ion) flux of anemones and anemonefish in both light and dark under controlled laboratory conditions. Rates of ammonia excretion/uptake were measured by placing each organism in a separate 1–2 L experimental vessel (glass beaker, Fisher Scientific) filled with 500–1,000 ml of seawater depending on animal size. Experiments were conducted in a water bath at a constant temperature of 26°C under 400 W Radium Metal Halide Lamps (Ocean Light 250, Aqua Medic), which provided about 500 μmol quanta s−1 m−2 of photosynthetically-active radiation (PAR), similar to that experienced by anemones in the holding aquaria (see above).
Ammonia in seawater can be eliminated by air bubbles or bacterial activity, and this loss can underestimate the final dissolved ammonia concentration in measured samples. Therefore, all experimental trials contained a control vessel with no animals, and either just seawater or ammonia-spiked seawater, depending on the experiment. All experimental and control vessels were aerated continuously using an airstone attached to a pump set at a low level, which kept the water aerated and stirred, but did not cause excessive evaporation. Preliminary trials were conducted with incubation times of 40 min to 6 h, and an incubation time of 100 min was observed to provide the most constant, repeatable excretion rates. Thus, the total incubation time of the animals in each experiment was set at 100 min, and 5 ml water samples were withdrawn for ammonia analysis every 20 min using a Rainin pipette (adapted from Wilkerson and Muscatine 1984; Spotte 1996). Ammonia concentration in the experimental vessels was determined using the indophenol blue method (Solarzano 1969), scaled down ten-fold due to the small incubation volumes, on a GENESYS 5 Spectrophotometer (Thermo Electron Spectronic, MA, USA). This modified procedure was tested repeatedly against a standard curve of 0–25 μM NH4Cl; results produced using the two procedures did not differ significantly [Student’s t test, t (10) = −0.256, P = 0.802]. Average ammonia flux (excretion or uptake) in each incubation was computed as the difference between the final and initial amounts of ammonia in the total volume of each experimental vessel. The amount of ammonia generated or consumed per hour per gram of animal wet mass was calculated from the ammonia concentration, water volume in the experimental vessel, and the wet mass of each fish or anemone tested (μmole g−1 h−1). Changes in the water volume due to sampling, and the displacement of water due to animal volume, were incorporated into all calculations.
Fish excretion rates
Ammonia excretion rates were quantified for nine fish, each examined during four time periods (2, 6, 24, and 36 h post-feeding). In order to avoid stressing the fish, only one excretion trial was performed per day on each fish; within 3 weeks, ammonia assessments of all fish were completed. The above time periods were selected because A. bicinctus anemonefish are diurnal zooplanktivores (Fautin and Allen 1997), so in the field, the shortest time periods (2 and 6 h post-feeding) reflected excretion rates during the day. In contrast, ∼8–20 h post-feeding occurs at night in these fish, when the sea anemone hosts decrease their uptake rates significantly (see Results), so this time period was not examined. The time periods of 24 and 36 h post-feeding revealed basal excretion rates when the fish were starved. Anemonefish were placed in experimental vessels and were allowed to acclimate for 20 min prior to performing two complete changes of water, to ensure that initial ammonia levels were close to zero when measurements began. Thus, the 20-min acclimation period was prior to and separate from the 100 min of incubation time for excretion rates. The fish appeared calm and swam normally while excretion rates were monitored, and did not appear to be stressed or breathing rapidly (according to opercular opening rates).
Changes in the concentration of ammonia also were measured in a separate experiment in vessels that each contained an anemonefish and a sea anemone (N = 9 pairs total), and these were compared with changes in ammonia levels produced by the same anemonefish in the absence of an anemone. Fish and anemones were paired at random with respect to body size. It was predicted that ammonia levels would be lower in this separate experiment with both fish and sea anemone together than in those with fish alone, because the anemones were expected to absorb the dissolved ammonia (after Porat and Chadwick-Furman 2005). Food was withheld from fish 24 h prior to this experiment, to bring the fish close to their basal excretion rate. In each trial, an anemonefish was placed with its host anemone in a 2 L experimental vessel containing 1 L of seawater, and the ammonia level was quantified following the procedures described above.
Sea anemone uptake rates
Rates of ammonia uptake were quantified for a total of 30 anemones: ten during the daytime in seawater with no ammonia added, ten during the daytime in seawater enriched with each of two concentrations of NH4Cl (10 and 20 μM), and ten during the night at each of two times after the onset of darkness in seawater enriched with 10 μM of NH4Cl. Between the daytime tests at each of the two enriched ammonia concentrations, the anemones were maintained under their normal feeding regime (see above) in their original tanks for at least 3 weeks to ensure no effects of the previous testing concentration. Uptake was measured as the reduction in ammonia concentration in the water in the experimental vessels. At the end of each experimental trial, the anemones were removed from the experimental vessels and returned to their original aquaria. This procedure did not appear to greatly affect the anemones: some contracted their tissues when transferred to the experimental vessels, but all re-expanded and attached their basal disks to the glass within the first 5–10 min of the experiment. Because the anemones were maintained under constant irradiance throughout the light period (12 h) each day, it was assumed that any “time of day” effect would be minor, and thus all daytime experiments were conducted at midday.
Statistical analyses
All statistical analyses were conducted using SPSS 15.0 for Windows. Normality and homogeneity of variances were tested using the Shapiro–Wilk statistical test for N < 50. Variation in fish ammonia excretion rate versus time after feeding was examined using a one-way repeated-measures analyses of variance (ANOVA) with time as the repeated variable, followed by post hoc pairwise comparisons among groups. Differences were considered significant at P < 0.05. The ammonia uptake rates of sea anemones were examined in stages, with different groups of individual anemones available for testing during each stage. Thus, one group of anemones was tested repeatedly during the daytime at two levels of ammonia enrichment (10 and 20 μM), one group was repeatedly tested at two times during the night at a single level of ammonia enrichment (10 μM), and one group was tested in zero ammonia (negative control). Due to our use in stages of different groups of anemones, a combination of paired and independent sample t tests was used to compare ammonia flux among the treatments. Variation in the level of ammonia buildup in vessels with anemonefish in the presence versus absence of anemones was determined using a paired t test. The Bonferroni correction was applied to the significance level when more than 1 t test was used. All results are presented as mean ± 1 SE unless otherwise indicated.
Results
Fish excretion rates
The ammonia concentrations within control vessels that contained no animals (N = 5) remained at their initially-set values of 0–20 μM, depending on the type of experiment being performed (see Methods). The ammonia concentrations in these control vessels varied by <0.5% throughout each experiment, and did not differ significantly between the start and end of each experiment [paired t test, t (5) = 0.37, P = 0.71]. Thus, changes in ammonia concentration due to uptake by microbes or other causes were considered to be negligible throughout each experiment, and adjustments were not made to the data. Similar results were obtained by Haines and Wheeler (1978) and Bray et al. (1986).
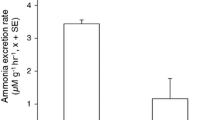
Large fish produced more ammonia than did small ones, but the mass-specific excretion rate did not vary significantly with fish size (r 2 = 0.379, P = 0.78, N = 9). The rate of ammonia excretion was highest immediately after feeding (1.84 ± 0.1 μmole g−1 h−1 at 2 h post-feeding) and decreased significantly thereafter [repeated measures ANOVA, F (3,24) = 66.9, P < 0.001, Eta2 = 0.893, Fig. 1]. By 6 h after feeding, the rate had dropped to 1.07 ± 0.08 μmole g−1 h−1, and by 24 h the ammonia excretion rate appeared to have reached a baseline value of 0.60 ± 0.08 μmole g−1 h−1. By 24 h post-feeding, starvation had caused a reduction in excretion to approximately one-third of the maximal, fed rate. After 36 h, excretion rate decreased again to 0.42 ± 0.04 μmole g−1 h−1, but was not significantly different from that at 24 h (multiple comparisons test, P = 0.31). An exponential fit of the data yielded a release rate coefficient (λ) of 0.0399 h−1 (r 2 = 0.94). The mean of excretion rate at 24 and 36 h after feeding was determined as the basal excretion rate (0.50 μmole g−1 h−1), and accounted for 27.6% of the maximum excretion rate measured.
Sea anemone uptake rates
The resting ammonia flux of sea anemones during the day in non-spiked seawater (negative control) was essentially zero (−0.003 ± 0.001 μmole g−1 h−1, N = 10), indicating that ammonia uptake by endosymbiotic zooxanthellae appeared to mask ammonia excretion by host anemones during the daytime. The rate of ammonia uptake by sea anemones during the daytime in seawater spiked with 20 μM ammonia [0.103 ± 0.012 μmole g−1 h−1) was significantly faster than that in 10 μM (0.045 ± 0.006 μmole g−1 h−1, paired t test, t (9) = −7.166, P < 0.001, Fig. 2]. As expected, anemones did not show net excretion in unspiked seawater, and their rate of ammonia uptake was significantly slower than that of anemones incubated in either 10 μM ammonia [t test, t (18) = −7.503, P < 0.001] or 20 μM ammonia [t test, t (18) = −8.527, P < 0.001, Fig. 2].
Variation in ammonia uptake with external seawater ammonia concentration by giant sea anemones Entacmaea quadricolor during the daytime. Photosynthetically active radiation (PAR) was supplied at ∼500 μmol quanta s−1 m−2, similar to that at 15 m depth at noon on the coral reef. Values are shown as mean ± SE (N = 10). Uptake rates with different letter values were significantly different at P < 0.05. See text for details
The sea anemones absorbed ammonia at a significantly slower rate in the dark (nighttime conditions) than when exposed to light [daytime conditions, t test, t (18) = −3.654, P < 0.002]. During the night, uptake rates remained consistently low and did not differ significantly between 1 and 4 h after the onset of darkness [0.015 ± 0.008 vs. 0.008 ± 0.007 μmole g−1 h−1, respectively, paired t test, t (9) = 0.719, P = 0.49, Fig. 3]. However, the mean uptake rate at 4 h after darkness was about half that at 1 h, indicating that rates may have continued to decline, but this decrease was not detectable here due to the variability among individuals.
Variation in ammonia uptake rate with light conditions in giant sea anemones Entacmaea quadricolor incubated in 10 μM NH4Cl-spiked seawater. For daytime measurements, photosynthetically active radiation (PAR) was supplied at ∼500 μE m−2 s−1, similar to that at 15 m depth at noon on the coral reef. Values are shown as mean ± SE (N = 10). Uptake rates with different letter values were significantly different at P < 0.05. See text for details
Ammonia load versus clearance
The net ammonia buildup in vessels containing fish alone (40.86 ± 7.12 μM) was about 5× higher than in vessels containing fish-anemone pairs [8.05 ± 0.77 μM, paired t test, t (8) = −4.855, P < 0.001, Fig. 4]. In all cases, the ammonia load created by the anemonefish built up more rapidly than the clearance rate of the sea anemones, leading to an ever-increasing enrichment of ammonia in the experimental vessels. The anemones absorbed most (>80%) of the ammonia released by the fish, at an average rate of about 5.3 μmole h−1.
Ammonia buildup in experimental vessels with anemonefish Amphiprion bicinctus alone versus when incubated together with giant sea anemones Entacmaea quadricolor. The same fish were measured with and without their host anemones (paired replicates). Values are shown as mean ± SE (N = 9). Error bars for the open circles were smaller than the symbols
Discussion
We demonstrate here that substantial nutrient transfer occurs in this three-way symbiosis, and that the rate at which ammonia is generated by anemonefish appears to greatly exceed the rate at which it is cleared by host sea anemones and their zooxanthellae, thereby providing a major source of nitrogen to the microalgae in this association. The adult anemonefish examined here generated large amounts of ammonia during the day when they consumed zooplankton, and host sea anemones and their zooxanthellae rapidly absorbed it. In contrast, during the night, the fish did not feed and the host zooxanthellae did not photosynthesize, resulting in minimal rates of dissolved nutrient transfer. Thus, the daily peak of metabolic activity coincides in all three members of this symbiosis, allowing maximal absorption of nutrients by host anemones when fish excretions also are at their highest.
Our laboratory measurements here allow us to estimate the amount of ammonia generated by anemonefish in the field. The mean length of the anemonefish A. bicinctus inhabiting the host anemone E. quadricolor in the Red Sea is about 5–9 cm depending on location, and maximum length varies from 11 to 13 cm (Chadwick and Arvedlund 2005). Thus, mean fish mass in the field is about 3–20 g, and maximal adult mass ranges from 38 to 64 g, based on the exponential relationship between length and wet mass in this species (see Methods). Because a mated pair of anemonefish usually inhabits each host anemone (Fautin and Allen 1997; Chadwick and Arvedlund 2005), each fish pair produces a potential mean ammonia load of 10–74 μmole h−1, with a maximal load of 140–236 μmole h−1, based on their daytime rates of ammonia excretion quantified here. Sea anemones in the wild also may be occupied by 2–4 smaller anemonefishes in addition to the mated adult pair (Fautin and Allen 1997). This group occupancy of hosts can increase further the ammonia load produced by the fish associated with each anemone. Many factors will alter how much of this excreted ammonia becomes available to sea anemone hosts under field conditions, including dilution by water currents and the shapes of coral reef crevices occupied by the anemones. In addition, the amount of time that fish spend separated from their anemone hosts while they feed in the water column may vary among anemonefish species, and will affect the rate of nutrient transfer.
In contrast to the potentially rapid ammonia production rate of anemonefishes, the ammonia clearance rate of host sea anemones appears to be relatively slow. The mean body size of the host anemone E. quadricolor on coral reefs in the northern Red Sea ranges from 17 to 30 cm tentacle crown diameter depending on location, and maximum size reaches 40–48 cm diameter (Chadwick and Arvedlund 2005). Thus, the mean wet mass of these sea anemones in the field is approximately 77–253 g, and maximum wet mass may reach 463–679 g, based on the relationship between anemone diameter and wet mass (see Methods). Given the body sizes of these organisms on coral reefs, individuals of this sea anemone potentally can absorb ammonia from seawater at mean clearance rates of about 8–25 μmole h−1, and maximum rates of 46–68 μmole h−1 for the largest observed individuals during the daytime. Because, we obtained these clearance rates from anemones incubated in a relatively high concentration of 20 μM external ammonia, we over-estimate here the actual clearance rates of sea anemones under field conditions. Another factor that can affect this estimate is the unknown relationship between anemone body size and clearance rate. We conclude based on the available data that the ammonia load produced by resident anemonefish likely outstrips the clearance rate of host sea anemones, unless the anemone is much larger than average and the associated fish are less abundant and smaller than average. Both the abundance and size of resident anemonefishes correlate positively with the size of host anemones E. quadricolor in the field (Hattori 2005; Kobayashi and Hattori 2006; Chadwick unpublished data). This pattern narrows the supply/demand relationship for ammonia transfer in this system, and indicates that supply may keep up with demand in many cases.
Anemonefish may excrete ammonia at a slower rate in the field than under laboratory conditions, due to variation in the abundance of their zooplankton prey and other factors. After a full day of feeding on coral reefs at Eilat, the ammonia load produced by anemonefish weighing 7.4–36.8 g wet mass (X ± SE = 20.1 ± 4.8, N = 5) was about 12.6 μmole h−1 per fish (mass-specific rate of 0.63 μmole g−1 h−1), thus a pair of fish could produce >25 μmole h−1 (Roopin 2007). As such, even with naturally lower rates of ammonia load under field than laboratory conditions, these fish appear to supply a large proportion of the ammonia needs of host anemones in their natural habitat. This ammonia contribution may enrich the host anemone’s immediate environment above low background ammonia levels in the Red Sea of 0.05–0.5 μM (Israel National Monitoring Program, May 2006, http://www.iui-eilat.ac.il/NMP/indexE.htm). Porat and Chadwick-Furman (2005) reported a mean excretion rate of 0.61 μmole g−1 h−1 by large adult A. bicinctus anemonefish in the field, similar to the field rate shown above, and also to that of 24-h starved fish in our laboratory measurements reported here. Variation in rates of feeding and metabolism by the anemonefish likely contributed to differences in excretion rates observed between these field and laboratory measurements.
Our observation that mass-specific excretion rate did not vary significantly with fish body size contrasts with past studies on larger fishes, in which mass-specific excretion rates decreased with body size (Jobting 1981; Meyer and Schultz 1985a; Schaus et al. 1997; Hall et al. 2007). This difference may be due in part to the small number of individuals and narrow mass range of the fish examined here, which may have prevented our detection of a trend in mass-specific excretion rate. However, we also did not observe this trend in our field measurements on anemonefish (Porat and Chadwick-Furman 2005; Roopin 2007). Thus, anemonefishes and other damselfishes that inhabit cnidarians may be too small to detect variation with body size in their mass-specific excretion rates, a pattern also known for fish metabolic rates (Enders et al. 2006).
Rates of ammonia excretion by A. bicinctus here are within the range of those known for other marine fishes and invertebrates (Table 1), and are very similar to those of other reef fish that contribute nutrients to the growth and survival of benthic organisms (Meyer and Schultz 1985a; Bray et al. 1986). Thus, the rapid rates of fish excretion reported here may be used as a model to infer the importance and impacts of symbiont excretions on the growth and productivity of other benthic host organisms.
The temporal pattern of ammonia excretion exhibited here by A. bicinctus is characteristic of diurnal fishes (Brett and Zala 1975; Jobling 1981; Meyer and Schultz 1985a; Bray et al. 1986). Although excretion rates of the anemonefish A. bicinctus fall within the range reported by Meyer and Schultz (1985a, b) for schools of resting grunts (Haemulon spp.), their actual contribution to benthic productivity may be different because anemonefish and grunts differ in their behavior. Anemonefish are obligate, close associates with their hosts, unlike juvenile grunts, which migrate to and from the reef. Anemonefish directly contact the tissues of host anemones for many hours during the day and all hours at night, while grunts rest near coral colonies only during the day. Also, in contrast to the corals that grunt schools rest over, some host sea anemones occur in crevices or holes on the reef (Fautin and Allen 1997; M. Roopin personal observation) that potentially reduce the dilution rate of ammonia supplements (see Bray et al. 1986) and make them more readily available to the anemones. Finally, ammonia excretion rates for grunts were calculated on a dry weight basis, while in the present study A. bicinctus ammonia excretion rates were calculated using wet weight, essentially underestimating the actual excretion rate of anemonefish compared to grunts. Conversion of anemonefish ammonia excretion rates to a dry weight basis [Y (wet weight) = 3.04 X (dry weight), Godinot and Chadwick unpublished data] ndicates that they excrete ammonia at a more rapid mass-specific rate than do the much larger grunts.
Mobile invertebrate associates such as the the spotted anemone shrimp (Periclimenes yucatanicus), which is a common ectosymbiont of giant sea anemones in the Caribbean Sea, also supply their hosts with supplemental nitrogen through ammonia excretions (Spotte 1996). These organisms excrete ammonia at a higher rate (2.36 μmole g−1 h−1) than do anemonefish, but their average mass (0.14 ± 0.02 g wet weight, Spotte 1996) is about 100-fold lower, so their total daily contribution of ammonia to their host is much smaller. Also, some anemoneshrimps may consume the tentacles of host anemones as food (Fautin et al. 1995), so their nitrogen contribution may be at least partly recycled from the host itself. Anemonefish, in contrast, are net importers of nitrogen from the water column because they forage for zooplankton during the day, ranging up to several meter from their sea anemone host (Fricke 1979; Fautin and Allen 1997; Meroz and Fishelson 1997; Porat and Chadwick-Furman 2004). In this way, anemonefish serve as a link in nutrient transport between planktonic and benthic ecosystems. This cross-ecosystem nutrient transfer extends the ecological importance of anemonefish from benefactors in a specific symbiotic interaction to mediators that affect nutrient cycling at a higher level, a pattern that also may apply to many damselfish residing in branching stony corals.
The capacity of the sea anemones examined here to absorb ammonia was directly related to exposure to light, suggesting that this process is driven by the symbiotic algal partner, as known for other zooxanthellate cnidarians (reviewed by Miller and Yellowlees 1989). Our results also indicate that in E. quadricolor, ammonia uptake increases with external ammonia concentration. Supply of inorganic nutrients by anemonefish may be sufficient to create advantageous nutritional conditions under which sea anemones can sustain high zooxanthella densities. In this way, anemonefish may indirectly enhance the ammonia uptake dynamics of their hosts by augmenting zooxanthella densities, resulting in higher levels of energy translocated to the host from the zooxanthellae and increased growth rates of the host tissues (Meyer and Schultz 1985b; Spotte 1996; Mokady et al. 1998; Porat and Chadwick-Furman 2005). The connection among algal densities in host tissues, ammonia uptake dynamics, and retention capabilities has not been examined in detail, and may be a potentially important factor in elucidating the dynamics of cnidarian–algal–ectosymbiont interactions.
Inhibition of ammonia uptake rates by darkness also has been reported for other cnidarian species, but in contrast to our results here, an extended period of dark incubation often is required to hinder absorption (Muscatine and D’Elia 1978; Wilkerson and Muscatine 1984). In free-living algae, a high rate of nutrient uptake in the dark is an indicator of nutrient deficiency (Syrett 1981). Thus, the immediate decrease of ammonia uptake in response to dark by the anemones examined here may indicate a sufficient nutrient supply in anemones that contain resident fish (Roopin 2007). During the night, anemonefish continue to supply a smaller but considerable amount of ammonia that is more available to the host than during the day, because the fish sleep among the host tentacles. So, although in this study anemones absorbed ammonia at a very slow rate in the dark, ammonia-starved anemones likely increase their dark ammonia uptake rate, and can absorb fish-excreted ammonia throughout the night.
Our results show that anemonefish can rapidly enrich an area the size of a typical anemone hole (about 1 L, see Methods) with >10 times the ambient ammonia concentration in the Red Sea. In response, the sea anemones can clear 80% of this enrichment at a rate of 5.3 μmole h−1. These results join a growing body of evidence that fish and invertebrates living in symbiotic associations with zooxanthellate cnidarians provide substantial nutritional benefits to the host holobiont. We conclude that the contributions of anemonefishes to the nutritional budget of host sea anemones and their zooxanthellae, and by inference, those of other zooplanktonic ectosymbionts on cnidarian hosts, are ecologically important as a corridor connecting trophic levels within the reef, and between the reef and the surrounding oceanic ecosystem. This nutrient transfer between ecosystems via mutualistic association may be a major contributor to the high productivity observed on coral reefs. Recent studies with labeled nitrogen suggest that nutrient transfer may be a two-way process, in that ectosymbiotic fish may assimilate nutrients produced by host anemone metabolism (Cleveland et al. 2003, 2006). Further studies are needed to quantify how these processes vary spatially and temporally, especially the potentially large-scale effects on coral reef productivity of the copious nutrients contributed by zooplanktivorous fishes to host sea anemones and stony corals.
References
Achituv Y, Mizrahi L (1996) Recycling of ammonium within a hydrocoral (Millepora dichotoma)—zooxanthellae— cirripede (Savignium milleporum) symbiotic association. Bull Mar Sci 58:856–860
Allen GR (1991) Damselfishes of the World. Mergus Publishers, Melle, Germany, 271 pp
Allen GR, Randall JE (1980) A review of the damselfishes (Teleostei: Pomacentridae) of the Red Sea. Israel J Zool 29:1–98
Arvedlund M, Iwao K, Brolund TM, Takemura A (2006) Juvenile Thalassoma amblycephalum Bleeker (Labridae, Teleostei) dwelling among the tentacles of sea anemones: a cleanerfish with an unusual client? J Exp Mar Biol Ecol 329:161–173
Becker JH, Grutter AS (2004) Cleaner shrimp do clean. Coral Reefs 23:515–520
Bray RN, Purcell LJ, Miller AC (1986) Ammonium excretion in a temperate-reef community by a planktivorous fish, Chromis punctipinnis (Pomacentridae), and potential uptake by young giant kelp, Macrocystis pyrifera (Laminariales). Mar Biol 90:327–334
Bray RN, Miller AC, Johnson S, Krause PR, Robertson DL, Westcott AM (1988) Ammonium excretion by macroinvertebrates and fishes on a subtidal rocky reef in southern California. Mar Biol 100:21–30
Brett JR, Zala CA (1975) Daily pattern of nitrogen excretion and oxygen consumption of sockeye salmon (Oncorhynchus nerka) under controlled condition. J Fish Res Bd Can 32:2479–2486
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125
Bunkley-Williams L, Williams EH (1998) Ability of Pederson cleaner shrimp to remove juveniles of the parasitic cymothoid isopod, Anilocra haemuli, from the host. Crustaceana 71:862–869
Chadwick NE, Arvedlund M (2005) Abundance of giant sea anemones and patterns of association with anemonefish in the northen Red Sea. J Mar Biol Assoc UK 85:1287–1292
Cleveland A, Verde EA, Lee R (2003) Investigating the physiological basis for the clownfish/host anemone symbiosis: do resident fish provide their host with nitrogen? Abstract, 4th internat symbiosis soc conference, Halifax, Nova Scotia
Cleveland A, Verde EA, Lee R (2006) Nutritional exchange in clownfish/host anemone symbiosis: stable isotope analysis demonstrates nutrient exchange is bi-directional. Abstract, 5th internat symbiosis congress, Vienna
Crossland CJ (1983) Dissolved nutrients in coral reef waters. In: Bames DJ (ed) Perspectives on coral reefs. Australian Institute of Marine Science, Townsville, pp 56–68
Danilo TD, Yap HT (2000) Ammonium and phosphate excretion in three common echinoderms from Philippine coral reefs. J Exp Mar Ecol Biol 251:227–238
Davy KS, Trautman DA, Borowitzka MA, Hinde R (2002) Ammonium excretion by a symbiotic sponge supplies the nitrogen requirements of its rhodophyte partner. J Exp Biol 205:3505–3511
Doebeli M, Knowlton N (1998) The evolution of interspecific mutualisms. Proc Natl Acad Sci USA 95:8676–8680
Durbin EG, Durbln AG (1981) Assimilation efficiency and nitrogen excretion of a filter-feeding planktivore, the Atlantic menhaden, Brevoortia tyrannus (Pisces: Clupeidae). Fish Bull US 79:601–616
Ellis AG, Midgley JJ (1996) A new plant–animal mutualism involving a plant with sticky leaves and a resident hemipteran. Oecologia 106:478–481
Enders EC, Boisclair D, Boily P, Magnan P (2006) Effect of body mass and water temperature on the standard metabolic rate of juvenile yellow perch, Perca flavescens (Mitchill). Environ Biol Fish 76:399–407
Falkowski PG, Dubinsky Z, Muscatine L, McCloskey L (1993) Population control in symbiotic corals: ammonium ions and organic materials maintain the density of zooxanthellae. Bioscience 43:606–611
Fautin DG (1991) The anemonefish symbiosis: what is known and what is not. Symbiosis 10:23–46
Fautin DG, Allen GR (1997) Anemone fishes and their host sea anemones: a guide for aquarists and divers, revised edition.Western Australian Museum, Perth, 160 pp
Fautin DG, Guo CC, Hwang JS (1995) Costs and benefits of the symbiosis between the anemoneshrimp Periclimenes brevicarpalis and its host Entacmaea quadricolor. Mar Ecol Prog Ser 129:77–84
Fricke HW (1979) Mating system, resource defense and sex change in the anemonefish Amphiprion akallopisos. Zeits Tierpsychol 50:313–326
Glaholt SP Jr, Vanni MJ (2005) Ecological responses to simulated benthic-derived nutrient subsidies mediated by omnivorous fish. Freshw Biol 50:1864–1881
Godwin J, Fautin DG (1992) Defense of host actinians by anemonefishes. Copeia 3:902–908
Haertel-Borer SS, Allen DM, Dame RF (2004) Fishes and shrimps are significant sources of dissolved inorganic nutrients in intertidal salt marsh creeks. J Exp Mar Biol Ecol 311:79–99
Haines KC, Wheeler PA (1978) Ammonium and nitrate uptake by marine macrophytes Hypnea musciformis (Rhodophyta) and Macrocystis pyrifera (Phaeophyta). J Phycol 14:319–324
Hall RO, Koch BJ, Marshall MC, Taylor BW, Tronstad LM (2007) How body size mediates the role of animals in nutrient cycling in aquatic ecosystems. In: Hildrew AG, Raffaelli DG, Edmonds-Brown R (eds) Body size: the structure and function of aquatic ecosystems. Cambridge University Press, Cambridge, pp 286–305
Hattori A (2005) High mobility of the protandrous anemonefish Amphiprion frenatus: nonrandom pair formation in limited shelter space. Ichthyol Res 52:57–63
Herre EA, Knowlton N, Mueller UG, Rehner SA (1999) The evolution of mutualism: exploring the paths between conflict and cooperation. Trends Ecol Evol 14:49–53
Hoegh-Guldberg GO, Williamson J (1999) Availability of two forms of dissolved nitrogen to the coral Pocillopora damicornis and its symbiotic zooxanthellae. Mar Biol 133:561–570
Holbrook SJ, Schmitt RJ (2005) Growth, reproduction and survival of a tropical sea anemone (Actiniaria): benefits of hosting anemonefish. Coral Reefs 24:67–73
Jobling M (1981) The influence of feeding on the metabolic rates of fishes: a short review. J Fish Biol 18:385–400
Kobayashi M, Hattori A (2006) Spacing pattern and body size composition of the protandrous anemonefish Amphiprion frenatus inhabiting colonial host anemones. Ichthyol Res 53:1–6
Meroz A, Fishelson L (1997) Juvenile production of Amphiprion bicinctus (Pomacentridae, Teleostei) and rehabilitation of impoverished habitats. Mar Ecol Prog Ser 151:295–297
Meyer JL, Schultz ET (1985a) Migrating haemulid fishes as a source of nutrients and organic matter on coral reefs. Limnol Oceanogr 30:146–156
Meyer JL, Schultz ET (1985b) Tissue condition and growth rate of corals associated with schooling fish. Limnol Oceanogr 30:157–166
Miller DJ, Yellowlees D (1989) Inorganic nitrogen uptake by symbiotic marine cnidarians: a critical review. Proc R Soc Lond Ser B 237:109–125
Mokady O, Loya Y, Lazar B (1998) Ammonium contribution from boring bivalves to their coral host—a mutualistic symbiosis? Mar Ecol Prog Ser 169:295–301
Muscatine L, D’Elia CF (1978) The uptake, retention, and release of ammonium by reef corals. Limnol Oceanogr 23:725–734
Muscatine L, Porter JW (1977) Reef corals mutualistic symbioses adapted to nutrient-poor environments. BioScience 27:454–460
Nizinski MS (1989) Ecological distribution, demography and behavioral observations on Periclimenes anthophilus, an atypical symbiotic cleaner shrimp. Bull Mar Sci 45:174–188
Porat D, Chadwick-Furman NE (2004) Effects of anemonefish on giant sea anemones: expansion behavior, growth, and survival. Hydrobiologia 530/531:513–520
Porat D, Chadwick-Furman NE (2005) Effects of anemonefish on giant sea anemones: ammonium uptake, zooxanthella content and tissue regeneration. Mar Freshw Behav Physiol 38:43–51
Randall JE, Fautin DG (2002) Fishes other than anemonefishes that associate with sea anemones. Coral Reefs 21:188–190
Roopin M (2007) Symbiotic benefits to sea anemones from the metabolic byproducts of anemonefish. M.Sc thesis, Department of Biological Sciences, Auburn University, 157 pp
Schaus MH, Vanni MJ, Wissing TE, Bremigan MT, Garvey JE, Stein RA (1997) Nitrogen and phosphorus excretion by detrivorous gizzard shad in a reservoir ecosytem. Limnol Oceanogr 42:1386–1397
Solorzano L (1969) Determination of ammonium in natural waters by the phenolhypochlorite method. Limnol Oceanogr 14:799–801
Spotte S (1996) Supply of regenerated nitrogen to sea anemones by their symbiotic shrimp. J Exp Mar Biol Ecol 198:27–36
Stachowicz JJ (2001) Mutualism, facilitation, and the structure of ecological communities. BioScience 51:235–246
Stambler N (2005) Bio-optical properties of the northern Red Sea and the Gulf of Eilat (Aqaba) during winter 1999. J Sea Res 54:186–203
Syrett PJ (1981) Nitrogen metabolism of microalgae. Can Bull Fish Aquat Sci 210:182–210
Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst 33:341–370
Whitledge TE (1982) Regeneration of nitrogen by the nekton and its significance in the northwest Africa upwelling ecosystem. Fish Bull 80:327–335
Wilkerson FP, Muscatine L (1984) Uptake and assimilation of dissolved inorganic nitrogen by a symbiotic sea anemone. Proc R Soc Lond Ser B 221:71–86
Acknowledgments
We thank the many undergraduate students and technicians who assisted in aquarium tank maintenance and animal care during this study. This study was submitted in partial fulfillment of the M.Sc. degree to MR at Auburn University. Funding was provided by start-up funds to NEC from Auburn University and by NSF IBN 02-30005 to RPH. Experiments performed in this study comply with the current laws of USA. This is publication #32 of the Marine Biology Program at Auburn University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.A. Poulet.
Rights and permissions
About this article
Cite this article
Roopin, M., Henry, R.P. & Chadwick, N.E. Nutrient transfer in a marine mutualism: patterns of ammonia excretion by anemonefish and uptake by giant sea anemones. Mar Biol 154, 547–556 (2008). https://doi.org/10.1007/s00227-008-0948-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-0948-5