Abstract
The anemone–anemonefish association is the quintessential symbol of a symbiotic mutualism from the Indo-Pacific waters. Both historical field documentation and extant scientific research advocate that these interactions are fundamental at the level of nutrient exchanges and evolutionary driving forces (natural selection) to facilitate this mutualism. Through the use of 15N and 13C stable isotope tracers, complementary laboratory- and field-based experiments were implemented in the Philippines to investigate the possibility of nutrient transmission from the host anemone, Heteractis crispa and/or endosymbiotic zooxanthellae, to two species of exosymbiotic anemonefishes (Amphiprion clarkii and A. perideraion). Mass spectrometry analyses suggest that 15N and 13C concentrations were significantly higher in tissues of the anemonefishes (intestines, liver, gills, and gonads), anemone host, and zooxanthellae compared with controls. We interpret the presence of 15N and 13C in the anemonefish tissues as direct empirical evidence for the transmission of nitrogen and/or carbon from host anemone and endosymbiotic zooxanthellae to resident anemonefish. These “translocations” and resultant recycling of elements within this classical tripartite relationship highlight the fundamental role of nutrient dynamics in this synergistic symbiosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Throughout the Indo-West Pacific Region, there are 28 species of anemonefishes within the genera Amphiprion and Premnas that form an obligate symbiosis with 10 species from several families of tropical sea anemones (Fautin and Allen 1997). Balamurugan et al. (2014) provide evidence that a combination of visual, tactile, and biochemical signals is utilized by the anemonefish to distinguish its specific host anemone species. These anemone lineages include the Actiniidae (Entacmaea and Macrodactyla), Stichodactylidae (Heteractis and Stichodactyla), and Thalassianthidae (Cryptodendrum) (Fautin and Allen 1997). These anemone hosts may be highly specific regarding the number of anemonefish species that are accommodated, or they may be generalists and house a wider number of anemonefish species. Examples of anemone specialists are Cryptodendrum adhaesivum (Klunzinger) and Heteractis malu (Haddon and Shackleton) which only forms an association with one anemonefish, Amphiprion clarkii (Fautin and Allen 1997). By contrast, the anemone Heteractis crispa (Ehrenberg) is the host for 14 anemonefish species in the genus Amphiprion (Fautin and Allen 1997) which is the most diverse assemblage of fish species for any given anemone host. Two anemonefishes hosted by H. crispa, A. clarkii (Bleeker) and A. perideraion (Bleeker) are commonly found at Okinawa, Japan (Hattori 2000) and Dumaguete, Philippines (Cleveland et al. 2011).
In the absence of an appropriate anemone host, other surrogate cnidarians or molluscs may be utilized by anemonefishes while in captivity (Arvedlund and Takemura 2005). Likewise, Arvedlund and Takemura (2005) observed a single adult A. clarkii closely allied with Lobophytum, a soft coral, over the course of almost 2 years. This association of A. clarkii and surrogate “anemone” in the field was thought to be triggered by the absence of appropriate anemone hosts due to the widespread 1998 global bleaching event (Arvedlund and Takemura 2005). Alternatively, there are fishes other than anemonefishes that form “associations” with anemones both in tropical (blennies, butterfly fishes, cardinalfishes, damselfishes, hawkfishes and wrasses) and in temperate (greenling) waters (Elliott 1992; Randall and Fautin 2002; Fautin and Allen 1997).
The term “connexion” was utilized by Collingwood (1868) to describe his initial observations between sea anemones and anemonefishes from “Pulo Pappan” (currently known as Pulau Papan), Malaysia. De Crespigny (1869) used the term “friendship” to describe the interactions between Premnas biaculeatus and Actinia crassicornis (i.e., Entacmaea quadricolor). Currently, this fish–cnidarian alliance has been characterized as being mutualistic in nature where both partners positively benefit from each other (Mariscal 1970; Fautin and Allen 1997). Many of these anemonefishes actively defend their host anemones from vertebrate predators such as chaetodontid fishes (Fautin 1991; Godwin and Fautin 1992; Fautin and Allen 1997; Porat and Chadwick-Furman 2004; Holbrook and Schmitt 2005) and turtles (Godwin and Fautin 1992), help mix any stagnant water, and improve water motion (Liberman et al. 1995; Szczebak et al. 2013) to remove sediments and anemone waste products within the tentacles (Fautin and Allen 1997; Goldshmid et al. 2004; Stewart et al. 2006).
The host anemone reciprocates the favor(s) by providing the anemonefishes and the developing fish eggs with a protective habitat from their predators (Verwey 1930; Mariscal 1970; Fautin and Allen 1997) such as Emydocephalus annulatus, the turtle-headed sea snake (Goiran et al. 2013). The anemone’s ectodermal layer contains high densities of nematocysts or toxins (see reviews by Anderluh et al. 2011; Frazao et al. 2012) which presumably affords protection from predators. Recently, Goiran and Shine (2014) described the mouth gaping and body recoiling behavior of the sea snake, Hydrophis major, soon after inadvertently tongue-flicking E. quadricolor. As such, Nedosyko et al. (2014) suggested that the host anemone’s chemical toxicity may play a fundamental role in both the formation and preservation of this fish–anemone association.
The anemonefishes may also promote anemone health by removing parasites or increasing water motion among the tentacles of the anemone to remove waste products (Fautin and Allen 1997). Szczebak et al. (2013) showed that direct contact between A. bicinctus and E. quadricolor increased the metabolic rates of the host anemone during the night; such enhanced respiration is most likely from improved water motion through the anemone tentacles induced by increased swimming behavior by the anemonefish (Szczebak et al. 2013).
Several studies have documented, utilizing indirect measurements of nutrient transfer, the key role resident anemonefishes may play in either host anemone (Porat and Chadwick-Furman 2004, 2005; Holbrook and Schmitt 2005; Roopin et al. 2008; Roopin and Chadwick 2009) or coral growth from nearby fish (Liberman et al. 1995; Meyer et al. 1983; Meyer and Schultz 1985). There is also strong evidence that the anemone host (E. quadricolor) and intracellular zooxanthellae obtain nitrogen resources such as ammonium (Porat and Chadwick-Furman 2005; Roopin et al. 2008, 2011; Roopin and Chadwick 2009) from excreted urine and feces products from the anemonefish (A. bicinctus). Cleveland et al. (2011) used both 15N and 13C isotopes as tracers to demonstrate that two anemonefish species (A. clarkii and A. perideraion) directly provide nutrients to both the anemone host (H. crispa) and zooxanthellae symbionts.
Although some specific anemonefishes (if not all) do provide inorganic nutrients to their anemone hosts and zooxanthellae, the question of whether the host anemone and endosymbiotic zooxanthellae can reciprocate this nutrient exchange to anemonefish remains unresolved. Accordingly, the primary purpose of this study was to ascertain whether the anemone holobiont is capable of “back-translocating” organic or inorganic nutrients in the form or carbon or nitrogen compounds to the resident anemonefishes. We hypothesized that both anemone host and intracellular zooxanthellae provide a predictable and substantial source of carbon and/or nitrogen to their anemonefish symbionts. We specifically asked these questions:
-
1.
Are any C- and N-containing products obtained by the anemone host (via feeding) directly transferred to resident anemonefishes?
-
2.
Are any C-containing products obtained by zooxanthellae (via photosynthesis) directly transferred to resident anemonefishes?
Both field- and laboratory-based H. crispa were exposed to 15N and/or 13C, and both fish and anemone tissues were analyzed for the presence of 15N and/or 13C. The presence of any isotope above background in the fish internal tissues was interpreted as direct transfer of C and/or N from host anemone holobiont to the resident anemonefish.
Methods
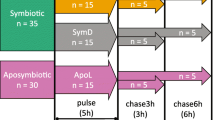
The experiments described occurred between May and August, in 2007 and 2010. Laboratory-based experiments (2007) were conducted at the Silliman University Marine Laboratory (SUML), Dumaguete, Negros Oriental, Philippines, and field-based experiments (2010) were conducted in situ on patch reefs in front of the laboratory. Table 1 displays both anemonefish sample sizes and biometric information regarding weights and lengths used in this study. In order to use similar-sized anemones as Cleveland et al. (2011), we selected anemones with a tentacular diameter of 20 ± 1 cm since this was the mean (± SE) tentacular diameter from their study.
Collection and maintenance
Heteractis crispa were collected as described by Cleveland et al. (2011). The rock or coral rubble on which the anemone was attached was removed as were any materials attached to the anemone’s verrucae. The anemones, which were arbitrarily sorted into isotopic (N = 81) and control (N = 24) treatment groups, were placed into two independent concrete raceways (4.6 × 1.2 × 0.6 m; volume = 3312 L) with ambient flow-through seawater (~900 L h−1) and irradiance levels. The anemones were maintained in this system for 2 days prior to labeling with isotopes.
Isogro-enriched anemone food
Fresh raw shrimp were purchased at a local seafood market and the exoskeletons were removed. The shrimp were placed into a food processor and converted into a thick paste. Unlabeled shrimp paste (control) was removed and placed into 50-mL centrifuge tubes and refrigerated at 4 °C. A ratio of 6 g of 13C- and 15N-double-labeled amino acid mixture (Isogro, Sigma-Aldrich) per 100 g of the shrimp paste was thoroughly mixed together until a uniform brown color was obtained; the Isogro-labeled shrimp paste for each anemone feeding session was stored in 50-mL centrifuge tubes and refrigerated at 4 °C. Both control and labeled shrimp paste were initially fed to anemones within 24 h of formulation and all shrimp food was used within 4 days of preparation.
NaH13CO3-enriched seawater
In order to isotopically label only the zooxanthellae, the seawater carbon in the form of H12CO3 −1 was completely replaced with isotopic H13CO3 −1. The method of Weis (1993) was used to exchange soluble 12CO2 for 13CO2 with the following modifications (for scaling to larger volumes). A 6-L volume of unfiltered natural seawater was placed in a Plexiglas container equipped with a magnetic stir bar to continuously mix the seawater. The pH of the initial seawater was noted (pH = 8.18–8.23) and continuously measured with a Mettler-Toledo (Model 120) portable pH meter calibrated to both pH 4.0 and 7.0. Between 1.2 and 1.3 mL of 12 M HCl was added (in 250-µL increments) until a pH of 4.0–4.4 was achieved to convert all dissolved HCO3 −1 into gaseous CO2. An aeration stone was lowered to the bottom of the container and purified N2 gas was vigorously bubbled into the seawater for 10 min in order to displace all gaseous CO2. A 600-µL aliquot of 10 M NaOH was added (in 100-µL increments) until a stable pH of ≈ 9.6 was achieved. Approximately 2.6 g of NaH13CO3 stable isotope (Sigma-Aldrich) was added to the seawater and allowed to dissolve completely, and the final pH was adjusted to ≈ 8.2 with dropwise additions of either 10 M NaOH or 12 M HCl. This artificial H13CO3 −1-labeled seawater was transferred into 20-L bottled water containers that were resealed, covered with two layers of thick black plastic bags (to prevent phytoplankton uptake of 13CO2 via photosynthesis), and stored for 2 days until utilized. A total volume of 100 L of H13CO3 −1-labeled seawater was formulated. Control seawater (total volume of 60 L) was also synthesized the same way using non-enriched NaH12CO3.
Isogro feeding
Both Isogro-labeled anemones (N = 44) and control (N = 10) anemones were fed twice daily for 3 days. Prior to each feeding session at 0800 and 1600, the control and Isogro-labeled shrimp pastes were “thawed” and formed into smaller amounts with disposable wooden chopsticks. The shrimp paste was placed directly on the oral disk near the coelenteron and was ingested by the anemone over the course of several minutes. Subsequent, all anemones were monitored during the 3 day feeding period and for another 2 days after the feeding period was finished for the release of undigested shrimp waste (a pink-tinted buoyant ball) that was expelled from the coelenteron. After all artificial anemone food was consumed and expelled, the anemones were not fed for the duration of the experiment in the laboratory or in the field although the anemones were capable of capturing zooplankton from the surrounding seawater. Prior observations (unpub data) documented that these shrimp balls are expelled within 8–12 h of feeding anemones, and Shick (1991) reported anemone egesta detected after 3.5–8 h post-feeding; consequently, 1.5 days of extra clearing time “insured” that anemonefishes were not able to directly feed on these labeled egesta.
H13CO3 −1 seawater incubations
Either 13C isotope-labeled or control seawater was placed in three (two for isotopic seawater, one for control seawater) separate white plastic basins (diameter = 0.6 m, height = 0.16 m, volume = 45.2 L). Arbitrarily chosen anemones, completely different from anemones fed Isogro label, were selected for 13C incubation (N = 37) or for controls (N = 14). Prior to placement in the white basins, anemones were agitated to expel internal coelenteronic seawater by gently squeezing and massaging them so that anemones would “re-inflate” with the experimental seawater in each basin. Both temperature (°C) and light intensity (µmol photons m−2 s−1) were monitored at 30-min intervals throughout the day using thermometers and a LI-COR Spherical Quantum Sensor (UWQ 6695) attached to an LI-1400 data logger.
The three basins containing the anemones (maximum of 10 per basin) were directly exposed to either overcast sunlight (<1000 µmol photons m−2 s−1) or covered with neutral density screens during full sunlight (>1000 µmol photons m−2 s−1) from 0800 to 1800 on the same day. Water temperature was never allowed to reach ≥2 °C above ambient seawater temperature via the removal of warm experimental water and the addition of control or isotopic bicarbonate seawater. After the exposures were completed, the anemones were again agitated to induce contraction and eliminate as much of the experimental seawater from the coelenteron as possible. The anemones (both 13C-labeled and controls) were placed in separate outdoor seawater raceways for 2 days to allow them to clear any isotopic seawater within their coelenterons prior to deploying in aquaria (2007) or in the field (2010).
Aquarium experiments
In order to determine how soon the anemonefishes acquired isotopic signatures during 2007, Isogro-labeled (N = 21) and control (N = 4) anemones were placed individually into outdoor yet shaded 38 L flow-through aquaria (flushing rate ~82 L h−1) as described by Cleveland et al. (2011). In a temporally separate experiment, H13CO3 −1-labeled (N = 17) and control (N = 8) anemones were placed individually into 38 L aquaria as described above (Cleveland et al. 2011). For the duration of all aquaria experiments, the anemones were fully inflated during both night and day time suggesting that they were healthy and not stressed due to the ambient environmental conditions. Previously captured adult fish (Cleveland et al. 2011), one per anemone of either A. clarkii (N = 25) or A. perideraion (N = 25), were arbitrarily assigned to each aquarium. Individual fish were fed twice a day with unlabeled fish food (Cleveland et al. 2011) until the end of both isotope exposure experiments which lasted for 14 days.
Field experiments
During 2010, Isogro (N = 23) or H13CO3 −1(N = 20)-labeled and control (combined N = 12) anemones were transplanted to the reef (adjacent to other anemones with anemonefishes) at depths ranging from 3.2 to 9.1 m depending on the tidal cycle. All anemones were covered with a bamboo cage (averages: diameter = 0.46 m, height = 0.62 m, volume = 104.4 L) with an average mesh size of 2.4 cm to allow seawater exchange and light penetration. Anemones were allowed to attach to coral rubble and left undisturbed for 24 h prior to addition of anemonefishes. The bamboo cages were secured to the sediment by weights and rebar forced into the sand or coral rubble; cages were scrubbed every other day once filamentous algae started growing on them. Previously captured adult fishes (Cleveland et al. 2011) of either A. clarkii (N = 29) or A. perideraion (N = 26) were arbitrarily assigned to each cage (one fish per anemone) and introduced to the anemone 24 h after the anemones were transplanted on the reef. At the end of the experiments (14 days for Isogro and 28 days for H13CO3 −1), the anemonefishes were recaptured and processed for isotopic tissue content as described below. The field Isogro experiment only lasted 14 days; it was terminated early due to storm surge rocking the cages and consequently allowing fish to escape.
All newly recruited larval fishes (Amphiprion (N = 4) and Dascyllus (N = 4)) were captured from Isogro-labeled anemones with an aquarium dip net, the fish measured for both length (mm) and weight (g), and processed as described below. Similarly, shrimp (Periclimenes (N = 10)) were captured from Isogro-labeled anemones by a “slurp” gun (50-mL syringe outfitted with 10-cm clear tubing) and processed as described below.
Anemone tissue processing
At the end of each isotopic experiment during 2007, individual anemones were processed to obtain the three anemone fractions (intact, animal, and zooxanthellae) and subsequently analyzed as described by Cleveland et al. (2011). At the end of each isotopic experiment during 2010, an arbitrarily chosen tentacle was excised from individual anemones and placed in the center of a Whatman GF/C filter (2.4 cm), folded, and inserted into a microfuge tube. Only a tentacle was obtained in order to verify tracer uptake by the anemone and to avoid unnecessary killing of anemones. All tentacle filters were dried at 63 °C for 24–48 h and stored at SUML; additional drying at 90 °C for 6–7 days occurred at Maine Maritime Academy (MMA). Stable isotope analyses were performed at Washington State University (WSU) as described below.
Fish/shrimp tissue processing
At the end of each isotopic experiment, each anemonefish was dispatched and the intestine, liver, gill, muscle, fin, and gonadal tissues (when visible) were removed and inserted into individual microfuge tubes. Individual larval fish and were dispatched and inserted into microfuge tubes; the shrimp recruits were dispatched, placed onto GF/C filters and into microfuge tubes. All tissues or samples were dried at 63 °C for 24–48 h and stored at SUML. After additional drying at 90 °C for 6–7 days at MMA, the tissues were powdered using an aluminum mortar and motorized aluminum pestle. Stable isotope analyses were performed at WSU as described below.
Stable isotope analysis
Dried animal/algal tissue or GF/C filter were added to tin capsules and combusted in a Costech (Valencia, USA) elemental analyzer. The resulting N2 and CO2 gases were separated by gas chromatography and admitted into the inlet of a GV Instruments (Manchester, UK) Isoprime isotope ratio mass spectrometer (IRMS) for determination of 13C/12C and 15N/14N ratios. Typical precision of analyses was ±0.2 ‰ for δ13C and ±0.5 ‰ for δ15N where δ (‰) = [(Rsample × R −1standard ) – 1] × 1000 and R = 13C/12C or R = 15N/14N. The standard for δ13C was Peedee belemnite (PDB), and the standard for δ15N was atmospheric nitrogen. Delta (δ) values correlate with 13C and 15N content of samples with higher δ values corresponding to higher 13C and 15N content. Egg albumin was used as a daily internal reference material.
Egesta and tentacle consumption
Additional H. crispa (N = 10) were collected (2007) and maintained as described above. When freshly extruded egesta was observed exiting the coelenteron of each anemone, the egesta were immediately collected, partitioned into smaller amounts, and single portions offered to aquaria-housed A. clarkii (N = 13) and A. perideraion (N = 12) prior to the regular morning feeding session by dropping the egesta fragment at the surface of the water. Prior to the next morning’s feeding cycle, two tentacles were removed using surgical tweezers and scissors from H. crispa and provided to the same fish which were fed egesta by dropping the tentacles at the surface of the water. These paired feeding experiments took place for 24 h (two morning feedings), and each fish was observed for behavior involving sampling or ingesting the anemone egesta or tentacles.
During the summer of 2013, a survey of whether anemonefishes would ingest freshly cut tentacles in the field was conducted. In the field, using SCUBA, multiple tentacles were cut from anemones (N = 123) with surgical scissors and released into the water column where resident anemonefishes (N = 123+) had access to them. If anemonefishes clearly swallowed and retained the tentacles, they were scored as having eaten the tentacles; if they swallowed the tentacles and subsequently spit them out, they were scored as not having eaten tentacles. Both the anemone and anemonefish species were recorded, and colored tape was tied near the anemone to prevent resampling of that particular association.
Statistical analyses
If data sets met assumptions of normality and homogeneity of variances (Barlett’s test), parametric statistical analysis were conducted (Sokal and Rohlf 2012; Zar 2009): These analyses were Student’s t test, analysis of variance (one-, two-, three-way ANOVA), and post hoc Tukey HSD. In cases where data sets were non-normal or heterogeneous, the data were log-transformed prior to statistical analysis. All analyses were performed using Statistica (StatSoft, Inc). From year to year (between 2007 and 2010), δ15N or δ13C data sets for either control or experimental categories (for either fish or anemone tissues) that were not significantly different (P > 0.05) were subsequently pooled. The data were graphed using SlideWrite Plus (Advanced Graphics Software). Data are reported as average ± standard error (SE).
Supplementary data
Calculated average (±SE) δ values, sample sizes (N), and δ percent changes between experimental and control tissues are provided as electronic supplementary material (ESM). The “Atom % value” was calculated from the Rsample term and the percent change in 13C or 15N in the experimental tissue, compared to the control tissue, was calculated using the following equation:
Results
Incorporation of 15N and 13C by anemones
The average values (Figs. 1, 2, 3, 4 5), along with the main and interaction effects of the three primary variables (Fish, Tissue, Treatment), show that both isotopes 15N and 13C were successfully incorporated at significantly higher quantities (see Treatment, two- and three-way ANOVA, F ≥ 238, P < 0.001; ESM Table 3) by all three Heteractis crispa fractions in 2007 and tentacles in 2010 than controls. Within Isogro experiments during 2007, the percent change for 15N within anemone tissue fractions (Fig. 1) ranged between 87 and 351 times higher than for control tissues. For the 2010 tentacle snips (Fig. 4), values were 205–206 times higher for tentacles than for controls. Although not as great in magnitude, the 13C signature in anemone tissues from Isogro experiments (Fig. 2) was still 5–15 times higher than corresponding anemone control tissues in 2007 and 26–27 times higher for tentacles during 2010 (Fig. 4). Similarly, within 13C bicarbonate experiments (2007), the percent change for 13C within labeled anemone tissue fractions (Fig. 3) ranged from 36 to 122 times higher than for control anemones and 19–20 times higher for labeled tentacles during 2010 (Fig. 5).
δ15N of intact, animal, and zooxanthellae (zoox) fractions of the anemone host Heteractis crispa fed a 15N–13C Isogro-labeled or control shrimp diet and exposed to anemonefish during 2007. Anemones and fish were maintained together for 14 days in 38 L aquaria with running seawater. A comparison of a experimental versus control A. clarkii, b experimental versus control A. perideraion and c experimental A. clarkii versus A. perideraion. Histogram bars that share letters are not significantly different (Tukey HSD, P > 0.05). Asterisks denote comparison between experimental versus control anemone fraction (Tukey HSD, ***P < 0.001) and ns not significant (Tukey HSD, P > 0.05). Data are mean ± SE. For values, sample sizes (N), and percent changes between experimental and control tissues, please refer to the appropriate column in ESM Table 1
δ13C of intact, animal, and zooxanthellae (zoox) fractions of the anemone host Heteractis crispa fed a 15N–13C Isogro-labeled or control shrimp diet and exposed to anemonefish during 2007. Anemones and fish were maintained together for 14 days in 38 L aquaria with running seawater. A comparison of a experimental versus control A. clarkii, b experimental versus control A. perideraion and c experimental A. clarkii versus A. perideraion. Histogram bars that share letters are not significantly different (Tukey HSD, P > 0.05). Asterisks denote comparison between experimental versus control anemone fraction (Tukey HSD, ***P < 0.001) and ns not significant (Tukey HSD, P > 0.05). Data are mean ± SE. For values, sample sizes (N), and percent changes between experimental and control tissues, please refer to the appropriate column in ESM Table 1
δ13C of intact, animal, and zooxanthellae (zoox) fractions of the anemone host Heteractis crispa incubated with 13C bicarbonate-labeled or control seawater and exposed to anemonefish during 2007. Anemones and fish were maintained together for 14 days in 38 L aquaria with running seawater. A comparison of a experimental versus control A. clarkii, b experimental versus control A. perideraion and c experimental A. clarkii versus A. perideraion. Histogram bars that share letters are not significantly different (Tukey HSD, P > 0.05). Asterisks denote comparison between experimental versus control anemone fraction (Tukey HSD, ***P < 0.001) and ns not significant (Tukey HSD, P > 0.05). Data are mean ± SE. For values, sample sizes (N), and percent changes between experimental and control tissues, please refer to the appropriate column in ESM Table 2
δ15N (a) and δ13C (b) of tentacles from the anemone host Heteractis crispa fed a 15N–13C Isogro-labeled or control shrimp diet and exposed to anemonefish during 2010. Anemones and fish were maintained together in the field for 14 days in 104 L bamboo cages. Histogram bars that share letters are not significantly different (Tukey HSD, P > 0.05). Asterisks denote comparison between experimental versus control anemone tentacle (Tukey HSD, ***P < 0.001). Data are mean ± SE. For values, sample sizes (N), and percent changes between experimental and control tissues, please refer to the appropriate column in ESM Table 1
δ13C of tentacles from the anemone host Heteractis crispa incubated with 13C bicarbonate-labeled or control seawater and exposed to anemonefish during 2010. Anemones and fish were maintained together in the field for 28 days in 104 L bamboo cages. Histogram bars that share letters are not significantly different (Tukey HSD, P > 0.05). Asterisks denote comparison between experimental versus control anemone tentacle (Tukey HSD, ***P < 0.001). Data are mean ± SE. For values, sample sizes (N), and percent changes between experimental and control tissues, please refer to the appropriate column in ESM Table 2
In the 2007 aquarium trials, all anemone tissue fractions (intact, animal, zooxanthellae) exposed to Isogro were significantly enhanced with 15N (Fig. 1a, b) compared with controls (Tukey HSN, P < 0.001) and between tissue types (Tukey HSN, P < 0.001). The 15N levels were significantly lower in intact and animal tissue fractions compared to the zooxanthellae fraction (Tukey HSN, P < 0.001); however, intact and animal tissue fractions were not significantly different (Tukey HSN, P > 0.05) in 15N levels from each other. The pattern of 15N values within the anemone tissue fractions was not affected by the fish species placed with the anemones (Tukey HSN, P > 0.05, Fig. 1c).
For anemones exposed to 13C from Isogro in 2007, all tissue fractions were also significantly elevated with 13C (Fig. 2a, b) compared to controls (Tukey HSN, P < 0.001). Intact and animal tissue fractions were 2.3–2.9 times higher in 13C levels (Tukey HSN, P < 0.001) than the zooxanthellae fraction, although intact and animal fractions did not differ significantly (Tukey HSN, P > 0.05; Fig. 2c) from each other. This pattern was true regardless of which anemonefish species was present in the aquaria.
Tissue fractions in anemones exposed to 13C bicarbonate during 2007 (Fig. 3a, b) were significantly higher (Tukey HSN, P < 0.001) in 13C than in controls, indicating significant uptake of labeled carbon as demonstrated by the Isogro experiments. However, the pattern of 13C enhancement in the bicarbonate experiment showed the reverse pattern of 13C enhancement in the Isogro experiment. Here, 13C levels were 2.4–2.9 times lower in intact and animal fractions (Tukey HSN, P < 0.001, Fig. 3a, b) than in the zooxanthellae fraction. Again, fish species did not have a significant effect on 13C enhancement patterns (Tukey HSN, P > 0.05, Fig. 3c) as patterns were similar between anemones housed with different species.
Both 15N (Fig. 4a) and 13C (Fig. 4b) enhancement of the H. crispa tentacles (regardless of anemonefish species) during the 2010 field studies were significantly higher (Tukey HSN, P < 0.001) than their respective controls; for 15N and 13C, this represents a 205–206 and 26–27 % change, respectively. For both 15N and 13C, no significant difference (Tukey HSN, P > 0.05) was present between anemones harboring either anemonefish species. The 13C enhancement of anemone tissue via 13C bicarbonate exposure (Fig. 5) closely mirrored the 13C Isogro experiments (Fig. 4b); irrespective of anemonefish species, the tentacles were significantly higher (Tukey HSN, P < 0.001) than their respective controls and represent a 19–20 % change. Regardless of anemonefish species, tentacle 13C levels were not significantly different (Tukey HSN, P > 0.05) from each other.
Laboratory uptake of 15N and 13C by anemonefishes
The main and interaction effects of the three primary variables (Fish, Tissue, Treatment) show that both 15N and 13C were successfully incorporated at significantly higher quantities (see Treatment, three-way ANOVA, F ≥ 67, P < 0.001, ESM Table 3) by experimental fish tissues in both laboratory (2007) and field (2010) experiments compared to control fish tissues.
In the aquarium-based Isogro experiments (2007), the 15N levels in most tissues of anemonefishes were significantly higher (Tukey HSN, P < 0.01) than controls; percent change ranged from 0.5 to 9.1 when compared to control tissues (Fig. 6a, b). The exception to this pattern occurred in muscle tissues of both species where 15N levels did not differ between experimental and control experiments. This was also seen with the eggs of A. clarkii and testes of A. perideraion. The highest 15N concentration enhancement in the anemonefishes occurred in the following order: intestine > liver > remaining tissues. Regardless of fish species (Fig. 6c), there were no significant differences (Tukey HSN, P > 0.05) in 15N values within the same anemonefish tissue types.
δ15N of specific fish tissues from anemonefish exposed to the anemone host Heteractis crispa fed a 15N–13C Isogro-labeled or control shrimp diet during 2007. Anemonefish and anemones were maintained together for 14 days in 38 L aquaria with running seawater. A comparison of a experimental versus control A. clarkii, b experimental versus control A. perideraion and c experimental A. clarkii versus A. perideraion. Histogram bars that share letters are not significantly different (Tukey HSD, P > 0.05). Asterisks denote comparison between experimental versus control fish tissue (Tukey HSD, **P < 0.01; ***P < 0.001) and ns not significant (Tukey HSD, P > 0.05). Data are mean ± SE. For values, sample sizes (N), and percent changes between experimental and control tissues, please refer to the appropriate column in ESM Table 5
In contrast to the 15N signal, significant 13C increases (Tukey HSN, P < 0.001) within fish tissues from exposure to Isogro-fed anemones only occurred within the intestines (and liver of A. perideraion) compared to control fish tissues (Fig. 7a, b). However, a pattern of consistently higher 13C signals in experimental fish exposed to enhanced anemones suggests 13C uptake. The 13C within the fish tissues was similar (Tukey HSN, P > 0.05) between the anemonefish species (Fig. 7c) for all tissue types.
δ13C of specific fish tissues from anemonefish exposed to the anemone host Heteractis crispa fed a 15N–13C Isogro-labeled or control shrimp diet during 2007. Anemonefish and anemones were maintained together for 14 days in 38 L aquaria with running seawater. A comparison of a experimental versus control A. clarkii, b experimental versus control A. perideraion and c experimental A. clarkii versus A. perideraion. Histogram bars that share letters are not significantly different (Tukey HSD, P > 0.05). Asterisks denote comparison between experimental versus control fish tissue (Tukey HSD, *P < 0.05; ***P < 0.001) and ns not significant (Tukey HSD, P > 0.05). Data are mean ± SE. For values, sample sizes (N), and percent changes between experimental and control tissues, please refer to the appropriate column in ESM Table 6
In the 2007 aquarium bicarbonate exposure studies, the 13C enhancement of anemonefish intestine, liver, and gills (Fig. 8a, b) were significantly higher (Tukey HSN, P < 0.001) than their respective controls; in addition, the 13C in eggs and fins of A. perideraion (Fig. 8b) were also significantly higher (Tukey HSN, P < 0.05) than controls. For A. clarkii (Fig. 8a) the highest 13C increase occurred in the intestine whereas in A. perideraion (Fig. 8b), both intestine and liver showed the highest levels of 13C assimilation. These elevated intestine and liver tissue values of A. perideraion were significantly higher than those seen in A. clarkii (Tukey HSN, P < 0.05, Fig. 8c), but significant differences between species for the other tissue types were not seen (Tukey HSN, P > 0.05).
δ13C of specific fish tissues from anemonefish exposed to the anemone host Heteractis crispa incubated with 13C bicarbonate-labeled or control seawater during 2007. Anemonefish and anemones were maintained together for 14 days in 38 L aquaria with running seawater. A comparison of a experimental versus control A. clarkii, b experimental versus control A. perideraion and c experimental A. clarkii versus A. perideraion. Histogram bars that share letters are not significantly different (Tukey HSD, P > 0.05). Asterisks denote comparison between experimental versus control fish tissue (Tukey HSD, *P < 0.05; **P < 0.01; ***P < 0.001) and ns not significant (Tukey HSD, P > 0.05). Data are mean ± SE. For values, sample sizes (N), and percent changes between experimental and control tissues, please refer to the appropriate column in ESM Table 7
Field uptake of 15N and 13C by anemonefishes and shrimp
With respect to 15N enhancement via Isogro-fed anemones during the 2010 field experiments, the 15N in intestine, liver, and gills of A. clarkii were significantly elevated over controls (Tukey HSN, P < 0.01), but muscle, gonad, and fin tissue were not (Tukey HSN, P > 0.05) although the pattern of elevated levels was conserved (Fig. 9a). All tissue types in A. perideraion were significantly higher (Tukey HSN, P < 0.001) than the controls (Fig. 9b). Regardless of anemonefish species, both the intestine and liver displayed the highest increase in 15N followed by the gills, gonads, and fins. Muscle tissue consistently showed the lowest increase for 15N; in all cases, A. perideraion consistently had significantly higher (Tukey HSN, P < 0.01) 15N concentrations than A. clarkii (Fig. 9c).
δ15N of specific fish tissues from anemonefish exposed to the anemone host Heteractis crispa fed a 15N–13C Isogro-labeled or control shrimp diet during 2010. Anemonefish and anemones were maintained together in the field for 14 days in 104 L bamboo cages. A comparison of a experimental versus control A. clarkii, b experimental versus control A. perideraion and c experimental A. clarkii versus A. perideraion. Histogram bars that share letters are not significantly different (Tukey HSD, P > 0.05). Asterisks denote comparison between experimental versus control fish tissue (Tukey HSD, **P < 0.01; ***P < 0.001) and ns not significant (Tukey HSD, P > 0.05). Data are mean ± SE. For values, sample sizes (N), and percent changes between experimental and control tissues, please refer to the appropriate column in ESM Table 5
The pattern of 13C levels in fish tissues of anemonefish exposed to anemones fed Isogro reflected the same pattern seen for 15N. The 13C levels were significantly higher (Tukey HSN, P < 0.001) for both intestine and liver tissues of both fish species over controls (Fig. 10a, b). Additionally, A. perideraion showed significantly greater intestine and liver tissue 13C over A. clarkii (Tukey HSN, P < 0.01, Fig. 10c). Amphiprion perideraion exposed to treated anemones also demonstrated significant 13C levels in the gill, egg, and fin tissue (Tukey HSN, P < 0.05) relative to controls; these values were not significantly higher than those seen for A. clarkii even though those values were not different from controls for A. clarkii.
δ13C of specific fish tissues from anemonefish exposed to the anemone host Heteractis crispa fed a 15N–13C Isogro-labeled or control shrimp diet during 2010. Anemonefish and anemones were maintained together in the field for 14 days in 104 L bamboo cages. A comparison of a experimental versus control A. clarkii, b experimental versus control A. perideraion and c experimental A. clarkii versus A. perideraion. Histogram bars that share letters are not significantly different (Tukey HSD, P > 0.05). Asterisks denote comparison between experimental versus control fish tissue (Tukey HSD, *P < 0.05; **P < 0.01; ***P < 0.001) and ns not significant (Tukey HSD, P > 0.05). Data are mean ± SE. For values, sample sizes (N), and percent changes between experimental and control tissues, please refer to the appropriate column in ESM Table 6
The 13C level in tissues of field maintained (2010) anemonefishes (Fig. 11a, b), exposed to anemones incubated in 13C bicarbonate, were significantly higher (Tukey HSN, P < 0.001) than controls for all tissues except for muscle and gonadal tissue in A. clarkii. Regardless of anemonefish species, the intestine (followed by liver) consistently showed the highest amounts of 13C than the rest of the tissues. There were no significant differences (Tukey HSN, P > 0.05) between the two anemonefish species when comparing the 13C enhancement levels within tissue types (Fig. 11c).
δ13C of specific fish tissues from anemonefish exposed to the anemone host Heteractis crispa incubated with 13C bicarbonate-labeled or control seawater during 2010. Anemonefish and anemones were maintained together in the field for 28 days in 104 L bamboo cages. A comparison of a experimental versus control A. clarkii, b experimental versus control A. perideraion and c experimental A. clarkii versus A. perideraion. Histogram bars that share letters are not significantly different (Tukey HSD, P > 0.05). Asterisks denote comparison between experimental versus control fish tissue (Tukey HSD, ***P < 0.001) and ns not significant (Tukey HSD, P > 0.05). Data are mean ± SE. For values, sample sizes (N), and percent changes between experimental and control tissues, please refer to the appropriate column in ESM Table 7
Similar sized, newly recruited fish larvae of Amphiprion sp. (Fig. 12a) and Dascyllus sp. (Fig. 12b) displayed total 13C levels that were significantly elevated (t test, P < 0.05) compared to respective fish controls regardless of the source of isotope (13C-bicarb or 13C-Isogro). Likewise, the 15N levels of the anemonefishes were also significantly higher (t test, P < 0.001) than the control fishes. Similar to the juvenile fish data, the 13C and 15N levels in the whole Periclimenes sp. shrimp were also significantly higher (t test, P < 0.001; Fig. 12c) than for control shrimp.
δ13C and δ15N levels of recently settled larval a Amphiprion sp. and b Dascyllus sp. and c adult Periclimenes sp. shrimp exposed to field-transplanted anemone host, Heteractis crispa, incubated or fed with stable isotopes without during 2010. Asterisks denote comparison between experimental versus control whole organism tissue (t test, *P < 0.05; **P < 0.01; ***P < 0.001). Data are mean ± SE
Egesta and tentacle ingestion
Regardless of species, all anemonefishes (combined N = 25) in aquaria immediately consumed, without any hesitation, the anemone egesta when it was offered to them. By contrast, none of these same anemonefishes ever ingested any of the tentacles offered to them; each fish sampled the tentacle by swallowing it, but the anemonefish subsequently spit it out. However, unlike aquaria fish, most of the wild anemonefish that were offered freshly cut tentacles of their host anemone did ingest tentacles (Table 2).
Discussion
Both anemonefish species (Amphiprion clarkii and A. perideraion) demonstrated elevated concentrations of 15N and/or 13C in various tissues of experimental fishes relative to controls in both laboratory and in situ environments. These data provide the first direct evidence of isotopic nutrient transfer from intracellular zooxanthellae (13C via photosynthesis) and host anemones (13C and 15N via “predation”) to the resident anemonefishes. This evidence, combined with the data from Cleveland et al. (2011), which document the transfer of 15N and 13C stable isotopes from resident anemonefishes to anemone host and intracellular zooxanthellae, unequivocally confirms the dynamic transfer and cycling of C and N in this unique tripartite symbiotic system.
The observed enhancement of isotopes from anemone tissue fractions (intact, animal, zooxanthellae) is dependent on the element; these elevated enhancement levels provide confirmation that both techniques for incorporation of 15N and 13C into the anemone and zooxanthellae were highly effective. During the 2007 laboratory experiments where anemones were fed double-labeled Isogro, the isolated zooxanthellae fraction displayed the greatest accumulation of 15N. This mirrors the results obtained by Cleveland et al. (2011) for 15N uptake by anemones hosting Isogro-fed anemonefishes and agrees with Roberts et al. (1999) who showed that zooxanthellae from the anemone Anemonia viridis are highly enhanced (17×) with 15N relative to host (intact and animal) tissues. The significantly higher (~3×) enhancement of the zooxanthellae fraction with 15N supports the idea that the algal symbionts are the primary sink for nitrogen in this association.
Within the cnidarian holobiont, this is not surprising as several studies have documented this transfer of 15N to zooxanthellae in the temperate anemone Anemonia viridis (Roberts et al. 1999), temperate coral Oculina arbuscula (Piniak et al. 2003; Piniak and Lipschultz 2004), tropical anemone Aiptasia pallida (Piniak et al. 2003) and tropical coral Oculina diffusa (Piniak and Lipschultz 2004). The prominent 15N signature in zooxanthellae supports more pronounced uptake of nitrogen over carbon, suggesting that zooxanthellae may be nitrogen limited (Cook et al. 1992, 1994; Marubini and Davies 1996; Davy et al. 2012). Since the Isogro fed to anemones is comprised of amino acids/peptides (65 %), salts (30 %), water (3 %), and glucose (2 %) with an elemental isotopic purity of 98 % for 15N and 99 % for 13C (Sigma-Aldrich), the 15N that was initially absorbed by the host anemone, and preferentially acquired by the zooxanthellae, originated from the amino acid/peptide portion. Consequently, the elevated concentrations of 15N within the zooxanthellae portion (this study) is initially acquired by the host anemone by oral ingestion and subsequently transferred to the endosymbiotic algae. Such a process has been documented by Piniak et al. (2003) and Piniak and Lipschultz (2004). A review of the nutrient processing and trafficking of nitrogen (and phosphorus) within symbiotic cnidarians is provided by Davy et al. (2012). Recently, Kopp et al. (2015) exposed the coral Pocillopora damicornis to seawater containing 15N-nitrate and subsequently detected the 15N tracer in the zooxanthellae within 30 min. Although 15N-nitrate dissolved in seawater was not utilized in our isotope exposure experiments, we would expect a similar time frame would occur in the zooxanthellae within anemones hosting anemonefish.
In contrast to the pattern of elevated concentrations of 15N in the zooxanthellae fraction, 13C was lowest in this same fraction relative to the animal and intact anemone fractions. As such, carbon does not seem to be a limiting element probably due to the high host anemone respiration rates and the abundance of extracellular HCO3 −1 and/or intracellular HCO3 −1 (Goiran et al. 1996; Al-Moghrabi et al. 1996; Furla et al. 1998a, b; Furla et al. 2000a) available via host anemone carbonic anhydrase conversion to CO2 (Weis et al. 1989; Weis 1991; Furla et al. 2000b). The 13C enhancement of the zooxanthellae fraction in the 2007 laboratory 13C-bicarbonate experiments validates 13CO2 fixation via the photosynthetic pathway; subsequently, the fixed 13C is translocated to the host anemone tissues as demonstrated by the prominent 13C concentration within both the intact and animal fractions of the association. In Stylophora pistillata (Tremblay et al. 2012) and P. damicornis (Kopp et al. 2015), 13C-labeled algal photosynthate accumulates in coral tissues within 15 min of exposure to the stable isotope and suggests a fairly rapid rate of translocation. If such a short time frame is also true for anemones hosting anemonefishes, the availability of algal-derived carbon products for anemonefish consumption and assimilation could essentially be “immediate.” Davy et al. (2012) provide a comprehensive review of carbon fluxes via metabolic exchange in various algal-cnidarian symbioses.
Our data show the original experimental source of the nitrogen and/or carbon, whether initially from the anemone host by oral ingestion (15N and 13C) or the intracellular zooxanthellae via photosynthesis (13C), is the definitive source of the substantially higher amounts of 15N and 13C in specific tissues of both species of resident anemonefishes (A. clarkii and A. perideraion) of H. crispa. These results ultimately show that both anemone host and intracellular zooxanthellae play a unique and previously unreported role in anemonefish nutrition and point to a tightly coupled interplay of nutritional resources within this multilateral symbiosis. If the anemone host can also synthesize and release essential amino acids, as is the case with scleractinian corals (Fitzgerald and Szmant 1997), and if anemonefishes can acquire these nutrients, they may potentially be obtaining a rather substantive nutritional windfall. The most parsimonious explanation for the transfer of 15N and 13C from the anemone holobiont to the anemonefishes is ingestion of various anemone or algal products: egested mucous balls of undigested food, gametes, expelled zooxanthellae, external mucous coat, and perhaps anemone tentacles themselves. This assertion is supported by the highest concentrations of 15N and 13C in both intestine and liver tissues. Four lines of evidence provide strong verification of the anemonefish oral uptake hypothesis.
First, ingestion by anemonefishes of undigested material/organic waste released from the coelenteron and mouth of the anemone host has been observed in both field and in laboratory studies (Verwey 1930; Moser 1931; Gohar 1934; Gohar 1948; Eibl-Eibesfeldt 1960; Koenig 1960 as cited by Mariscal 1970). These egested products, when viewed under light microscopy, consist of abundant mucus, both unfired and fired nematocysts, zooxanthellae, and other unidentifiable substances (Verde, per obs). Steele (1975, 1976) documented expulsion of both fecal and zooxanthellae pellets by laboratory-maintained Aiptasia tagetes; likewise Hill and Scott (2012) reported that mucous-bound, freshly expelled zooxanthellae from thermally and high-light stressed E. quadricolor were photosynthetically viable. In addition, bacterial biomass may be another component of anemone egesta since Herndl and Velimirov (1986) showed higher densities of bacteria in the coelenteron than in surrounding seawater and also higher clearance rates of the coelenteron when bacterial loads were elevated.
Anemonefishes in our 2007 laboratory experiments readily ingested freshly released egesta from laboratory acclimated H. crispa. Egesta offered to both A. clarkii and A. perideraion housed with H. crispa, in all cases regardless of anemonefish species, was immediately consumed and retained by anemonefishes. In contrast, when cut tentacles of H. crispa were offered to these same fishes, they initially swallowed them but immediately rejected them. This selective ingestion of freshly egested material versus fresh tentacle by anemonefishes is intriguing as both items are essentially the same (mucus, zooxanthellae, both fired and unfired nematocysts) and points to the complex evolution of this symbiosis. Perhaps the nematocysts in egesta are heavily coated with mucus or proteinaceous material and unable to fire, whereas in intact tentacles, the nematocysts are better able to fire within the mouth of anemonefish.
During the 2010 field experiments, the intestine and liver tissues of A. perideraion consistently displayed significantly higher amounts of both 15N and 13C than A. clarkii. We suggest these results may simply represent the amount of time each species of adult anemonefish spends in close proximity to its host anemone or simply due to its smaller size. Amphiprion perideraion spends ~83 % of its time within 25 cm of H. crispa; in contrast, A. clarkii only spends ~38 % if its time within 25 cm of H. crispa and more time higher in the water column feeding (unpubl obs). As well, female A. clarkii associates with an average of ~5 anemones compared to female A. perideraion which associate with ~3 anemones (unpubl data). Given that A. perideraion are consistently in closer proximity to fewer host anemones, the probability of these fishes observing and consequently procuring and ingesting anemone egesta or gametes (see below) is greater. Alternatively, since A. perideraion are inherently smaller than A. clarkii, ingestion of the same amount of isotope would raise their tissue isotope levels more than in a larger fish.
Buston (pers comm) observed field A. percula feeding on egesta from the anemone H. magnifica on several occasions in Madang, Papua New Guinea (PNG); this egesta is clearly important to these anemonefish because it is one of only two contexts in which there is aggressive engagement between A. percula fishes within the same anemone—the other being forcible eviction from H. magnifica by the dominant female anemonefish. Likewise, this egesta-eating behavior was also observed in A. ocellaris within H. magnifica on several occasions by Verde (pers obs) in the Philippines in 2013. As well, both A. bicinctus and A. ocellaris have been observed consuming egesta pellets extruded from E. quadricolor and H. crispa in captivity (Chadwick, pers comm) and these mucous-encased boluses of undigestible food material are expelled from the anemone ~24 h after their weekly feeding session (Chadwick, pers comm).
Amphiprion akindynos, when ingesting the chlorophyte alga Enteromorpha flexuosa, displayed between 15 and 79 % digestion of total dry matter, demonstrates that some anemonefish are capable of digesting algae (Galetto and Bellwood 1994). Mariscal (1970) determined that the primary contents within the stomachs of A. akallopisus were zooxanthellae and that within the hindgut, the zooxanthellae showed advanced degradation, consistent with the interpretation that zooxanthellae are being digested. If correct, anemonefishes may acquire substantial amounts of metabolites during the digestion of zooxanthellae as they pass along the fish’s alimentary canal.
Second, some anemonefishes may benefit by feeding on reproductive products released into the water during host anemone-spawning events. To date, only Scott and Franscisco (2006) have documented anemonefishes directly consuming host anemone reproductive products; they observed resident A. clarkii directly consuming eggs of spawning Stichodactyla haddoni. Photographs by Scott and Francisco (2006) clearly show anemone eggs being released into the water column via the greatly extended anemone mouth; all three resident A. clarkii (female, male and sub-male) readily ingested the newly released eggs and there were no overtly aggressive behavioral displays on the part of the female toward either male A. clarkii (Francisco, pers comm).
While the evidence for anemonefishes feeding on host anemone reproductive products remains minimal, several studies of closely related pomacentrids have documented fishes feeding on coral eggs and larvae. Pomacentrus moluccensis and Abudefduf whitleyi (Pratchett et al. 2001) and P. amboinensis (McCormick 2003) have been seen preferentially feeding on coral eggs or larvae (coral propagules). If anemonefish predation on host anemone eggs turns out to be a significant source of energy to the anemonefishes, it may represent a significant cost to the host anemones. Such a negative interaction does not fit the classic distinction of a truly mutualistic association and offers intriguing questions for future research.
Third, anemonefishes may be foraging on the mucous film and/or parasites and bacteria associated with host anemone tentacles. Several female Premnas biaculeatus at Madang, PNG, were observed aggressively biting and tugging on Entacmaea quadricolor tentacles while causing no apparent physical damage to them (Verde, per obs). These mouthing and tugging episodes (Fig. 13a, b) may result in anemone mucus and cells being ingested by the anemonefishes as mentioned by Mariscal (1970). Benson and Muscatine (1974) have documented many fish species that actively consumed scleractinian, alcyonacean, and tridacnian mucus that may contain high-energy organic molecules such as waxes, triglycerides, phospholipids, sterols (Benson and Muscatine 1974), and proteins (Daumas et al. 1981). Such consumption of energy-rich cnidarian glycoprotein mucus (Wild et al. 2004; Bythell and Wild 2011), and associated bacterial assemblage by marine organisms, is clearly advantageous. Using 14CO2 tracers via zooxanthellate photosynthesis, both Rinkevich et al. (1991) and Simon-Blecher et al. (1999) demonstrated that the crabs Trapezia cymodoce and Crytochirus coralliodytes, respectively, obtain 14C by oral consumption of coral hosts’ tissue or products. Similarly, 32P tracer studies by Gorlick (1980) provide evidence for client fish mucus consumption by the Hawaiian cleaning wrasse, Labroides phthirophagus. Naumann et al. (2010) provide strong experimental evidence for the uptake of 15N labeled coral mucus as a food source by the epizoic acoelomorph Waminoa (initially described by Barneah et al. 2007) on various hard and soft coral species. It is also possible that external parasites on the anemones may be targeted for elimination as a form of grooming by the anemonefishes (Mariscal 1970; Fautin and Allen 1997), as is the case for many cleaner fishes (Arnal and Morand 2001).
Adult a Amphiprion chrysopterus showing tentacles of Entacmaea quadricolor within its mouth (photo by Howard Hall) and b A. ocellaris pulling a tentacle of Heteractis magnifica into its mouth (photo by Ulrika Kroon). A juvenile c A. melanopus observed biting off a tentacle from E. quadricolor and subsequently swallowing it (photo by Howard Hall)
An additional pathway for anemone nutrients that also requires further consideration involves the bacterial component associated with the anemone holobiont. Not only are there bacteria on the ectodermal and endodermal layers of the host cnidarian, but in the anemones Aiptasia pallida (Palincsar et al. 1989) and Metridium senile (Schuett et al. 2007), bacterial aggregates are actually located within the epidermal cells. These symbiotic bacterial communities may undergo either nitrogen fixation (Lesser et al. 2007; Fiore et al. 2010) and transformations (Fiore et al. 2010), sulfur recycling (Raina et al. 2009), or digestion within the coelenteron (Herndl and Velimirov 1986). Consequently, the bacterial component may provide the host cnidarian with a steady source of carbon, nitrogen, sulfur compounds, and even phosphorous and vitamin B12 (Agostini et al. 2009, 2012). Given the importance of the bacterial community to these cnidarian associations, it is highly plausible that both organic and inorganic nutrient interchange occurs among all components of this endosymbiont-exosymbiont-anemone symbiosis. Although bacterial-specific pathways of nutrient exchange were not specifically tested in this study or by Cleveland et al. (2011), future studies investigating the nuances and intricacies are in order (see Weis et al. 2008).
Fourth, anemonefishes may be feeding on host anemone tentacles. Direct biting of host anemones by resident anemonefishes has been observed by numerous investigators in both field and aquarium settings (Verwey 1930; Gohar 1934; Herre 1936; Mariscal 1970; Moyer and Bell 1976; Ross 1978). Nevertheless, we have not directly observed A. clarkii and A. perideraion naturally feeding on H. crispa tentacles during many hours of field work; however, in 2013 when tentacle snips were artificially provided, the anemonefishes ate them (see below).
Mariscal (1970) investigated the stomach contents of field A. akallopisus; microscopic analysis revealed both fired and unfired nematocysts and zooxanthellae from the host anemone, Radianthus ritteri (= H. magnifica), consistent with the notion that anemonefishes are ingesting anemone tentacles (although ingestion of anemone egesta cannot be ruled out). Hall (pers comm) observed a juvenile A. melanopus biting off a tentacle of E. quadricolor (Fig. 13c) which was subsequently consumed. We speculate that newly recruited (and highly defenseless) juvenile anemonefishes (and possibly Dascyllus) on anemones may be directly feeding on the host anemone products as a way of enhancing survival rates. Corroborating support for young fish feeding on their cnidarian hosts is provided by D’Ambra et al. (2015). Stable isotope analysis of fish and potential food sources showed that age-0 carangid Chloroscombrus chrysurus rely on its two pelagic scyphomedusae hosts, Aurelia sp. and Drymonema larsoni, as a principal source of food and that approximately 90 % of the fishes assimilated diet is contributed by the hosts (D’Ambra et al. 2015).
Cut anemone tentacle feeding experiments conducted in the field during 2013 suggest the potential for anemonefishes to feed on tentacles. If H. crispa is the host anemone, only an average of approximately 26 and 6 % of A. clarkii and A. perideraion, respectively, ingested the anemone tentacles in the field; this is higher than the 2007 aquarium-based anemonefishes which did not eat any tentacles at all. It may be that the anemonefishes in aquaria that are fed a high-quality food pellet learn to be more discriminatory and are “holding out” for better food than a mere tentacle whereas in the field, wild anemonefishes may be more opportunistic. In contrast to H. crispa, if E. quadricolor is the host anemone (regardless of the anemonefish species), the tentacles were readily consumed. Consequently, these results suggest that the consumption of host anemone tentacles can occur but may be highly dependent on the specific anemonefish–host anemone combination.
Collingwood (1868) used the term “quasi-parasitic fish” in the title of his paper to label his initial observations of anemonefishes and anemones in the field; given the biting/ingestion of tentacles (and consumption of anemone eggs), this may be a fairly accurate description of how some anemonefishes may obtain organic products from their host anemone. While these behaviors by anemonefishes would appear counter-intuitive in this mutualistic symbiosis, if the costs incurred by the host anemones by this “predation” are less than the benefits provided by resident anemonefishes (territorial defense, water circulation, nutrient transfer), the symbiotic association would develop and flourish. Bronstein (2001) discusses the ecology and evolution of mutualisms that are shaped by aggressive interactions like herbivory, predation, and parasitism when considering the overall costs of mutualistic collaborations.
Aside from the oral uptake of nutrients by anemonefish, another mechanism of nutrient acquisition from anemones potentially involves the movement of carbon and nitrogen through epithelial cells of post-settlement anemonefish larvae. Trans-epidermal uptake of nutrients from seawater is a common occurrence in invertebrates with soft tissues in direct contact with seawater (Ferguson 1982; Gomme 1982; DeFreese and Clark 1991; Ambariyanto and Hoegh-Guldberg 1999). The integument of fish (comprised of both the epidermis and dermis) is such that these epithelial cells are generally not sclerotized but composed of vascularized cells (Elliot 2000; Olsen 2000). This morphology of larval (and possibly juvenile and adult) anemonefishes may be a plausible conduit for organic nutrient uptake from seawater or direct contact with the tentacles of the anemone. Evidence for this hypothesis comes from epidermal wipes of mucus from adult anemonefishes over time showing a 2–4 times higher enhancement (compared to controls) in both 13C and 15N within 2 h after initial contact with stable isotope-augmented anemones (unpubl data). Alternatively, the elevated 13C and 15N on the epidermis and fins of adult fishes may simply be anemone mucous transferred from direct contact between anemonefish and anemone ectoderm.
Bearing in mind that certain pomacentrids (Stegastes planifrons and S. dorsopunicans) actively cultivate their food (Cleveland and Montgomery 2003) within territories which they aggressively defend (Robertson 1984; Cleveland 1999), this damselfish behavior could also be extended to pomacentrid anemonefishes. Since the anemone host and zooxanthellae serves as a food source for the anemonefishes, protection of a potential food resource may be another strategic reason to vigorously defend the anemone host (Godwin and Fautin 1992; Porat and Chadwick-Furman 2004) from anemone predators. Additionally, there are other fishes (Apogon, Starksia, Pterapogon, Thallasoma, and Cirrhitichthys) that show a facultative association with anemones (Randall and Fautin 2002); however, it is currently not known whether any of these fishes are able to benefit nutritionally from anemones.
There are other organisms that are also in a symbiotic association with anemones that do feed on anemone tentacles (Periclimenes; Fautin et al. 1995) or mucus and egesta (Allopetrolisthes; Valdivia and Stotz 2006). Such organisms include several porcellanid crab (Petrolisthes and Neopetrolisthes) and shrimp (Periclimenes) species that may benefit nutritionally from the anemones just as much, if not more, than the anemonefish since they are in direct contact with the anemone host virtually 100 % of the time. The data from field recruited Periclimenes shrimp onto anemones exposed to stable isotope provides evidence for this assertion since the average whole-body values were significantly higher than controls for both 13C (δ percent change = 7–11) and especially for 15N (δ percent change = 101). One could argue that these higher isotope values may simply reflect direct contact transference of isotopes between anemone ectoderm and shrimp exoskeleton and not necessarily organic products ingested by the shrimp. However, the magnitude of 13C for shrimp are much greater than the 13C values for the liver and intestines of anemonefishes; furthermore, the average 15N value of 1033 ‰ is an order of magnitude greater than any of the anemonefish liver and intestine 15N values. Consequently, these very prominent isotopic signals suggest that the shrimp are directly ingesting carbon and nitrogenous products from the anemone host.
Through the use of 13C and 15N, Cleveland et al. (2011) and this study provide definitive evidence that both carbon- and nitrogen-containing compounds are being cycled in a “closed-loop system” between the three primary associates of algal endosymbiont, anemone host, and anemonefish exosymbiont that underscores the interdependence of these organisms. However, both the quantity (magnitude) and quality (metabolite profiling) of these compounds are currently unknown and remain to be established. Stable isotope techniques (similar to the radioactive isotope study by Whitehead and Douglas 2003) as well as more contemporary techniques such as multi-isotope imaging mass spectrometry (MIMS, Davy et al. 2012) or the combination of both transmission electron microscopy (TEM) and nanoscale secondary ion mass spectrometry (NanoSIMS) ion microprobe imaging used by Kopp et al. (2015) should be considered in future studies.
It is clearly apparent that anemonefishes and their holobiont anemones are “co-dependent” on each other for carbon and nitrogen sources in an environment known for low nutrient levels; consequently, protecting both partners from human-induced stresses such as pollution, overharvesting, and climate change is critical to maintaining these strategic residents of coral reef ecosystems in order to preserve marine biodiversity as described by Roberts et al. (2002). The conclusions of this study are appropriate to the fundamental understanding of nutrient recycling in tropical reef ecosystems and highlight the intricate and dynamic collaborations in a marine ecosystem that encompasses more than 25 % of worldwide marine biodiversity. As such, the commercial collection of anemone hosts and resident anemonefishes in the Philippines (and probably elsewhere) should utilize more extensive conservation strategies and management policies (Shuman et al. 2005) to avoid overexploitation of either partner in this association. Such measures would not only help conserve these superb examples of symbiotic partnerships but also provide long-term income potential to local ornamental collectors (Madduppa et al. 2014) in a developing economy of a nation bordered by a tropical climate within the Pacific Ocean.
References
Agostini A, Suzuki Y, Higuchi T, Casareto BE, Nakano Y, Hidaka M, Badrun N (2009) Coral symbiotic complex: hypothesis through vitamin B12 for a new evaluation. J Coral Reef Studies 11:1–11. doi:10.3755/galaxea.11.1
Agostini A, Suzuki Y, Higuchi T, Casareto BE, Yoshinaga K, Nakano Y, Fujimura H (2012) Biological and chemical characteristics of the coral gastric activity. Coral Reefs 31:147–156. doi:10.1007/s00338-011-0831-6
Al-Moghrabi S, Goiran C, Allemand D, Speziale N, Jaubert J (1996) Inorganic carbon uptake for photosynthesis by the symbiotic coral/dinoflagellates association II. Mechanisms for bicarbonate uptake. J Exp Mar Biol Ecol 199:227–248. doi:10.1016/0022-0981(95)00202-2
Ambariyanto A, Hoegh-Guldberg O (1999) Net uptake of dissolved free amino acids by the giant clam Tridacna maxima: alternative sources of energy and nitrogen? Coral Reefs 18:91–96. doi:10.1007/s003380050161
Anderluh G, Sepcic K, Turk T, Macek P (2011) Cytolytic proteins from cnidarians: an overview. Acta Chim Slov 58:724–729
Arnal C, Morand S (2001) Importance of ectoparasites and mucus in cleaning interactions in the Mediterranean cleaner wrasse Symphodus melanocercus. Mar Biol 138:777–784. doi:10.1007/s002270000494
Arvedlund M, Takemura A (2005) Long-term observation in situ of the anemonefish Amphiprion clarkii (Bennett) in association with a soft coral. Coral Reefs 24:698. doi:10.1007/s00338-005-0007-3
Balamurugan J, Ajith Kumar TT, Kannan R, Pradeep HD (2014) Acclimation behaviour and bio-chemical changes during anemonefish (Amphiprion sebae) and sea anemone (Stichodactyla haddoni) symbiosis. Symbiosis 64:127–138. doi:10.1007/s13199-014-0310-2
Barneah O, Brickner I, Hooge M, Weis VM, LaJeunesse TC, Benayahu Y (2007) Three party symbiosis: acoelomorph worms, corals, and unicellular algal symbionts in Eilat (Red Sea). Mar Biol 151:1215–1223. doi:10.1007/s00227-006-0563-2
Benson AA, Muscatine L (1974) Wax in coral mucus: energy transfer from corals to reef fishes. Limnol Oceanogr 19:810–814. doi:10.4319/lo.1974.19.5.0810
Bronstein JL (2001) The costs of mutualism. Am Zool 41:825–839. doi:10.1093/icb/41.4.825
Bythell JC, Wild C (2011) Biology and ecology of coral mucus release. J Exp Mar Biol Ecol 408:88–93. doi:10.1016/j.jembe.2011.07.028
Cleveland A (1999) Energetic costs of agonistic behavior in two herbivorous damselfishes (Stegastes). Copeia 4:857–867. doi:10.2307/1447962
Cleveland A, Montgomery WL (2003) Gut characteristics and assimilation efficiencies in two species of herbivorous damselfishes (Pomacentridae: Stegastes dosopunicans and S. planifrons). Mar Biol 142:35–44. doi:10.1007/s00227-002-0916-4
Cleveland A, Verde EA, Lee RW (2011) Nutritional exchange in a tropical tripartite symbiosis: direct evidence for the transfer of nutrients from anemonefish to host anemone and zooxanthellae. Mar Biol 158:589–602. doi:10.1007/s00227-010-1583-5
Collingwood C (1868) Note on the existence of gigantic sea-anemones in the China Sea, containing within them quasi-parasitic fish. Ann Mag Nat Hist (J Nat Hist) 1:31–33. doi:10.1080/00222936808695633
Cook CB, Muller-Parker G, D’Elia CF (1992) Ammonium enhancement of dark carbon fixation and nitrogen limitation in symbiotic zooxanthellae: effects of feeding and starvation of the sea anemone Aiptasia pallida. Limnol Oceanogr 37:131–139. doi:10.4319/lo.1992.37.1.0131
Cook CB, Muller-Parker G, Orlandini CD (1994) Ammonium enhancement of dark carbon fixation and nitrogen limitation in zooxanthellae symbiotic with the reef corals Madracis mirabilis and Montastrea annularis. Mar Biol 118:157–165. doi:10.1007/BF00699230
D’Ambra I, Graham WM, Carmichael RH, Hernandez FJ Jr (2015) Fish rely on scyphozoan hosts as a primary food source: evidence from stable isotope analysis. Mar Biol 162:247–252. doi:10.1007/s00227-014-2569-5
Daumas R, Galois R, Thomassin BA (1981) Biochemical composition of soft and hard coral mucus on a New Caledonian lagoonal reef. In: Proceedings of the 4th international coral reef symposium, Manila, Philippines vol 2, pp 59–67
Davy SK, Allemand D, Weis VM (2012) Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol Mol Biol R 76:229–261. doi:10.1128/MMBR.05014-11
De Crespigny CC (1869) Notes on the friendship existing between the malacopterygian fish Premnas biaculeatus and the Actinia crassicornis. J Zool 37:248–249
DeFreese DE, Clark KB (1991) Transepidermal uptake of dissolved free amino acids from seawater by three sacoglossan opisthobranchs. J Mollus Stud 57:65–74. doi:10.1093/mollus/57.Supplement_Part_4.65
Eibl-Eibesfeldt I (1960) Beobachtungen und Versuche an Anemonenfishchen (Amphiprion) der Malediven und der Nicobaren. Z Tierpsychol 17(1):1–10
Elliot DG (2000) Integumentary system. In: Ostrander GK (ed) The laboratory fish. Academic Press, San Diego, pp 95–108, 271–306
Elliott J (1992) The role of sea anemones as refuges and feeding habitats for the temperate fish Oxylebius pictus. Environ Biol Fish 35:381–400. doi:10.1007/BF00004991
Fautin DG (1991) The anemonefish symbiosis: What is known and what is not. Symbiosis 10:23–46
Fautin DG, Allen GR (1997) Field guide to anemonefish and their host sea anemones. Western Australian Museum, Perth
Fautin DG, Guo C-C, Hwang J-S (1995) Costs and benefits of the symbiosis between the anemoneshrimp Periclimenes brevicarpalis and its host Entacmaea quadricolor. Mar Ecol Prog Ser 129:77–84. doi:10.3354/meps129077
Ferguson JC (1982) A comparative study of the net metabolic benefits derived from the uptake and release of free amino acids by marine invertebrates. Biol Bull 162:1–17
Fiore CL, Jarett JK, Olson NK, Lesser MP (2010) Nitrogen fixation and nitrogen transformations in marine symbioses. Trends Microbiol 18:455–463. doi:10.1016/j.tim/2010.07.001
Fitzgerald LM, Szmant AM (1997) Biosynthesis of ‘essential’ amino acids by scleractinian corals. Biochem J 322:213–221. doi:10.1042/bj3220213
Frazao B, Vasconcelos V, Antunes A (2012) Sea anemone (Cnidaria, Anthozoa, Actinaria) toxins: an overview. Mar Drugs 10:1812–1851. doi:10.3390/md10081812
Furla P, Benazet-Tambutte S, Jaubert J, Allemand D (1998a) Diffusional permeability of dissolved inorganic carbon through the isolated oral epithelial layers of the sea anemone, Anemonia viridis. J Exp Mar Biol Ecol 221:71–88. doi:10.1016/S0022-0981(97)00116-0
Furla P, Benazet-Tambutte S, Jaubert J, Allemand D (1998b) Functional polarity of the tentacle of the sea anemone Anemonia viridis: role in inorganic carbon acquisition. Am J Physiol Regul Integr Comp Physiol 274:R303–R310
Furla P, Galgani I, Durand I, Allemand D (2000a) Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J Exp Biol 203:3445–3457
Furla P, Orsenigo MN, Allemand D (2000b) Involvement of H+-ATPase and carbonic anhydrase in inorganic carbon absorption for endosymbiotic photosynthesis. Am J Physiol Regul Integr Comp Physiol 278:R870–R881
Galetto MJ, Bellwood DR (1994) Digestion of algae by Stegastes nigricans and Amphiprion akindynos (Pisces: Pomacentridae), with an evaluation of methods used in digestability studies. J Fish Biol 44:415–428. doi:10.1111/j.1095-8649.1994.tb01222.x
Godwin J, Fautin DG (1992) Defense of host actinians by anemonefishes. Copeia 1992:902–908. doi:10.2307/1446171
Gohar HAF (1934) Partnership between fish and anemones. Nature 134:291
Gohar HAF (1948) Commensalism between fish and anemone (with a description of the eggs of Amphiprion bicinctus Rüppell). Publs Mar Biol Stn Ghardaqa 6:35–44
Goiran C, Shine R (2014) Reaction of a sea snake (Hydrophis major) to contact with a sea anemone. Coral Reefs 33:793. doi:10.1007/s00338-014-1161-2
Goiran C, Al-Moghrabi S, Allemand D, Jaubert J (1996) Inorganic carbon uptake for photosynthesis by the symbiotic coral/dinoflagellates association I. photosynthetic performances of symbionts and dependence on sea water bicarbonate. J Exp Mar Biol Ecol 199:207–225. doi:10.1016/0022-0981(95)00201-4
Goiran C, Dubey S, Shine R (2013) Effects of season, sex and body size on the feeding ecology of turtle-headed sea snake (Emydocephalus annulatus) on Indo-Pacific inshore coral reefs. Coral Reefs 32:527–538. doi:10.1007/s00338-012-1008-7
Goldshmid R, Holzman R, Weihs D, Genin A (2004) Aeration of corals by sleep-swimming fish. Limnol Oceangr 49(5):1832–1839. doi:10.4319/lo.2004.49.5.1832
Gomme J (1982) Epidermal nutrient absorption in marine invertebrates: a comparative analysis. Am Zool 22:691–708. http://www.jstor.org/stable/3882589
Gorlick DL (1980) Ingestion of host fish surface mucus by the Hawaiian cleaning wrasse, Labroides phthirophagus (Labridae), and its effect on host species preference. Copeia 1980:863–868. doi:10.2307/1444466
Hattori A (2000) Social and mating systems of the protandrous anemonefish Amphiprion perideraion under the influence of a larger congener. Austral Ecol 25:187–192. doi:10.1046/j.1442-9993.2000.01035.x
Herndl GJ, Velimirov B (1986) Role of bacteria in the gastral cavity of anthozoa. Actes de Colloques 3:407–414. http://archimer.ifremer.fr/doc/1984/acte-989.pdf
Herre AWCT (1936) Some habits of Amphiprion in relation to sea anemones. Copeia 1936:167–168. doi:10.2307/1435828
Hill R, Scott A (2012) The influence of irradiance on the severity of thermal bleaching in sea anemones that host anemonefish. Coral Reefs 31:273–284. doi:10.1007/s00338-011-0848-x
Holbrook SJ, Schmitt RJ (2005) Growth, reproduction and survival of a tropical sea anemone (Actinaria): benefits of hosting anemonefish. Coral Reefs 24:67–73. doi:10.1007/s00338-004-0432-8
Koenig O (1960) Verhaltensuntersuchungen an Anemonenenfishen. Pyramide 8(2):52–56
Kopp C, Domart-Coulon I, Escrig S, Humbel BM, Hignette M, Meibom A (2015) Subcellular investigation of photosynthesis-driven carbon assimilation in the symbiotic reef coral Pocillopora damicornis. mBio 6(1):2–14. doi:10.1128/mBio.02299-14
Lesser MP, Falcon LI, Rodriguez-Roman A, Enriquez S, Hoegh-Guldberg O, Iglesias-Prieto R (2007) Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Mar Ecol Prog Ser 346:143–152. doi:10.3354/meps07008
Liberman T, Genin A, Loya Y (1995) Effects on growth and reproduction of the coral Stylophora pistillata by the mutualistic damselfish Dascyllus marginatus. Mar Biol 121:741–746. doi:10.1007/BF00349310
Madduppa HH, Juterzenka KV, Syakir M, Kochzius M (2014) Socio-economy of marine ornamental fishery and its impact on the population structure of the clown anemonefish Amphiprion ocellaris and its host anemones in Spermonde Archipelago, Indonesia. Ocean Coast Manage 100:41–50. doi:10.1016/j.ocecoaman.2014.07.013
Mariscal RN (1970) The nature of the symbiosis between Indo-Pacific anemone fishes and sea anemones. Mar Biol 6:58–65. doi:10.1007/BF00352608
Marubini F, Davies PS (1996) Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Mar Biol 127:319–328. doi:10.1007/BF00942117
McCormick MI (2003) Consumption of coral propagules after mass spawning enhances larval quality of damselfish through maternal effects. Oecologia 136:37–45. doi:10.1007/s00442-003-1247-y
Meyer JL, Schultz ET (1985) Tissue condition and growth rate of corals associated with schooling fish. Limnol Oceanogr 30:157–166. doi:10.4319/lo.1985.30.1.0157
Meyer JL, Schultz ET, Helfman GS (1983) Fish schools: an asset to corals. Science 220:1047–1049. doi:10.1126/science.220.4601.1047
Moser J (1931) Beobachtungen über die Symbiose von Amphiprion percula mit Aktinien. Sber Ges naturf Freunde Berl 1931(2):160–167
Moyer JT, Bell LJ (1976) Reproductive behavior of the anemonefish Amphiprion clarkii at Miyake-Jima, Japan. Jpn J Ichthyol 23:23–32
Naumann MS, Mayr C, Struck U, Wild C (2010) Coral mucus stable isotope composition and labeling: experimental evidence for mucus uptake by epizoic acoelomorph worms. Mar Biol 157:2521–2531. doi:10.1007/s00338-010-0612-7
Nedosyko AM, Young JE, Edwards JW, Burke da Silva K (2014) Searching for a toxic key to unlock the mystery of anemonefish and anemone symbiosis. PLoS ONE 9:e98449. doi:10.1371/journal.pone.0098449
Olsen KR (2000) Circulatory System. In: Ostrander GK (ed) The laboratory fish. Academic Press, San Diego, pp 161–171
Palincsar EE, Jones WR, Palincsar JS, Glogowski MA, Mastro JL (1989) Bacterial aggregates within the epidermis of the sea anemone Aiptasia pallida. Biol Bull 177:130–140. doi:10.2307/1541840
Piniak GA, Lipschultz F (2004) Effects of nutritional history on nitrogen assimilation in congeneric temperate and tropical scleractinian corals. Mar Biol 145:1085–1096. doi:10.1007/s00227-004-1410-y
Piniak GA, Lipschultz F, McClelland J (2003) Assimilation and partitioning of prey nitrogen within two anthozoans and their endosymbiotic zooxanthellae. Mar Ecol Prog Ser 262:125–136. doi:10.3354/meps262125
Porat D, Chadwick-Furman NE (2004) Effects of anemonefish on giant sea anemones: expansion behavior, growth, and survival. Hydrobiologia 530(531):513–520. doi:10.1007/s10750-004-2688-y
Porat D, Chadwick-Furman NE (2005) Effects of anemonefish on giant sea anemones: ammonium uptake, zooxanthellae content and tissue regeneration. Mar Fresh Beh Phys 38:43–51. doi:10.1080/10236240500057929
Pratchett MS, Gust N, Goby G, Klanten SO (2001) Consumption of coral propagules represents a significant trophic link between corals and reef fish. Coral Reefs 20:13–17. doi:10.1007/s003380000113
Raina J-B, Tapiolas D, Willis BL, Bourne DG (2009) Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Appl Environ Microbiol 75:3492–3501. doi:10.1128/AEM.02567-08
Randall JE, Fautin DG (2002) Fishes other than anemonefishes that associate with sea anemones. Coral Reefs 21:188–190. doi:10.1007/s00338-002-0234-9
Rinkevich B, Wolodarsky Z, Loya Y (1991) Coral-crab association: a compact domain of a multilevel trophic system. Hydrobiologia 216–217:279–284. doi:10.1007/BF00026475
Roberts JM, Davies PS, Fixter LM, Preston T (1999) Primary site and initial products of ammonium assimilation in the symbiotic sea anemone Anemonia viridis. Mar Biol 135:223–236. doi:10.1007/s002270050620
Roberts CM, McClean CJ, Veron JEN, Hawkins JP, Allen GR, McAllister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne C, Werner TB (2002) Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295:1280–1284. doi:10.1126/science.1067728
Robertson DR (1984) Cohabitation of competing territorial damselfishes on a Caribbean coral reef. Ecology 65:1121–1135. doi:10.2307/1938320
Roopin M, Chadwick NE (2009) Benefits to host sea anemones from ammonia contributions of resident anemonefish. J Exp Mar Biol Ecol 370:27–34. doi:10.1016/j.jembe.2008.11.006
Roopin M, Henry RP, Chadwick NE (2008) Nutrient transfer in a marine mutualism: patterns of ammonia excretion by anemonefish and uptake by giant sea anemones. Mar Biol 154:547–556. doi:10.1007/s00227-008-0948-5
Roopin M, Thornhill DJ, Santos SR, Chadwick NE (2011) Ammonia flux, physiological parameters, and Symbiodinium diversity in the anemonefish symbiosis on Red Sea coral reefs. Symbiosis 53:63–74. doi:10.1007/s13199-011-0110-x
Ross RM (1978) Behavior of the anemonefish Amphiprion melanopus on Guam. Copeia 1:103–107. doi:10.2307/1443829
Schuett CS, Doepke H, Grathoff A, Gedde M (2007) Bacterial aggregates in the tentacles of the sea anemone Metridium senile. Helgoland Mar Res 61:211–216. doi:10.1007/s10152-007-0069-4
Scott A, Francisco B (2006) Observations on the feeding behavior of resident anemonefish during host sea anemone spawning. Coral Reefs 25:451. doi:10.1007/s00338-006-0126-5
Shick JM (1991) A functional biology of sea anemones. Chapman & Hall, London
Shuman CS, Hodgson G, Ambrose RF (2005) Population impacts of collecting sea anemones and anemonefish for the marine aquarium trade in the Philippines. Coral Reefs 24:564–573. doi:10.1007/s00338-005-0027-z
Simon-Blecher N, Chemedanov A, Eden N, Achituv Y (1999) Pit structure and trophic relationship of the coral pit crab Cryptochirus coralliodytes. Mar Biol 134:711–717. doi:10.1007/s002270050587
Sokal RR, Rohlf FJ (2012) Biometry: the principles and practice of statistics in biological research, 4th edn. WH Freeman and Co, New York
Steele RD (1975) Stages in the life history of a symbiotic zooxanthellae in pellets extruded by its host Aiptasia tagetes (Duch. and Mich.)(Coelenterata, Anthozoa). Biol Bull 149:590–600. doi:10.2307/1540389
Steele RD (1976) Light intensity as a factor in the regulation of the density of symbiotic zooxanthellae in Aiptasia tagetes (Coelenterata, Anthozoa). J Zool 179:387–405. doi:10.1111/j.1469-7998.1976.tb02302.x
Stewart HL, Holbrook SJ, Schmitt RJ, Brooks AJ (2006) Symbiotic crabs maintain coral health by clearing sediments. Coral Reefs 25:609–615. doi:10.1007/s00338-006-0132-7
Szczebak JT, Henry RP, Al-Horani FA, Chadwick NE (2013) Anemonefish oxygenate their anemone hosts at night. J Exp Biol 216:970–976. doi:10.1242/jeb.075648
Tremblay P, Grover R, Maguer JF, Legendre L, Ferrier-Pages C (2012) Autotrophic carbon in coral tissue: a new 13C-based model of photosynthate translocation. J Exp Biol 215:1384–1393. doi:10.1242/jeb.065201
Valdivia N, Stotz W (2006) Feeding behavior of the porcellanid crab Allopetrolisthes spinifrons, symbiont of the sea anemone Phymactis papillosa. J Crustacean Biol 26:308–315. doi:10.1651/C-2607.1
Verwey J (1930) Coral reef studies I. The symbiosis between damselfishes and sea anemones in Batavia Bay. Treubia 12:305–366
Weis VM (1991) The induction of carbonic anhydrase in symbiotic sea anemone Aiptasia pulchella. Biol Bull 180:496–504. doi:10.2307/1542351
Weis VM (1993) Effect of dissolved inorganic carbon concentration on the photosynthesis of the symbiotic sea anemone Aiptasia pulchella Carlgren: role of carbonic anhydrase. J Exp Mar Biol Ecol 174:209–225. doi:10.1016/0022-0981(93)90018-J
Weis VM, Smith GJ, Muscatine L (1989) A “CO2 supply” mechanism in zooxanthellate cnidarians: role of carbonic anhydrase. Mar Biol 100:195–202. doi:10.1007/BF00391958
Weis VM, Davy SK, Hoegh-Guldberg O, Rodriguez-Lanetty M, Pringle JR (2008) Cell biology in model systems as the key to understanding corals. Trends Ecol Evol 23:369–376. doi:10.1016/j.tree.2004.03.004
Whitehead LF, Douglas AE (2003) Metabolite comparisons and the identity of nutrients translocated from symbiotic algae to animal host. J Exp Biol 206:3149–3157. doi:10.1242/jeb.00539
Wild C, Huettel M, Klueter A, Kremb SG, Rasheed MYM, Jørgensen BB (2004) Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 428:66–70. doi:10.1038/nature02344
Zar JH (2009) Biostatitical Analysis, 5th edn. Pearson, London
Acknowledgments
We are thankful of A. Duncanson, E. Cochrane, C. Blair, and D. Dallis for laboratory and field support and H. Calumpong and the scientific staff at Silliman University Marine Laboratory for logistical and laboratory support. This study was undertaken within the waters of Dumaguete, Philippines, and complied with all relevant local, regional, and national regulations. We thank L.R. McCloskey, N. Chadwick, and an anonymous reviewer for their time and constructive comments because it ultimately crystalized the manuscript’s arguments. Financial support was provided by the Corning School of Ocean Studies and Maine Maritime Academy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: C. Harrod.
Reviewed by N. Chadwick and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alan Verde, E., Cleveland, A. & Lee, R.W. Nutritional exchange in a tropical tripartite symbiosis II: direct evidence for the transfer of nutrients from host anemone and zooxanthellae to anemonefish. Mar Biol 162, 2409–2429 (2015). https://doi.org/10.1007/s00227-015-2768-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2768-8