Abstract
The zoanthid genus Isaurus (Anthozoa: Hexacorallia) is known from both the Indo-Pacific and Atlantic Oceans, but phylogenetic studies examining Isaurus using molecular markers have not yet been conducted. Here, two genes of markers [mitochondrial cytochrome oxidase subunit I (COI) and mitochondrial 16S ribosomal DNA (mt 16S rDNA)] from Isaurus specimens collected from southern Japan (n = 19) and western Australia (n = 3) were sequenced in order to investigate the molecular phylogenetic position of Isaurus within the order Zoantharia and the family Zoanthidae. Additionally, obtained sequences and morphological data (polyp size, mesentery numbers, mesogleal thickness) were utilized to examine Isaurus species diversity and morphological variation. By comparing our obtained sequences with the few previously acquired sequences of genera Isaurus as well as with Zoanthus, Acrozoanthus (both family Zoanthidae), and Palythoa spp. (family Spenophidae) sequences, the phylogenetic position of Isaurus as sister to Zoanthus within the Family Zoanthidae was suggested. Based on genetic data, Isaurus is most closely related to the genus Zoanthus. Despite considerable morphological variation (in particular, polyp length, mesentery numbers, external coloration) between collected Isaurus specimens, all specimens examined are apparently conspecific or very closely related based on molecular data and observed morphological variation within colonies. Additionally, obtained internal transcribed spacer of ribosomal DNA (ITS-rDNA) sequences from symbiotic zooxanthellae (Symbiodinium spp.) from all Isaurus specimens were shown to be subclade C1-related Symbiodinium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The order Zoantharia (Anthozoa: Hexacorallia) currently consists of five recognized families, including two mainly zooxanthellate families (possessing symbiotic zooxanthellae of the dinoflagellate genus Symbiodinium); the sand-encrusted Sphenopidae, and the non-encrusted Zoanthidae. Species from both families are often seen in sub-tropical and tropical shallow coral reef habitats worldwide. Zoanthidae is currently organized into three genera: Zoanthus, Acrozoanthus, and Isaurus.
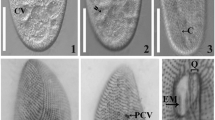
Isaurus spp. are known from both the Atlantic and Indo-Pacific, and are generally found in intertidal or shallow subtropical and tropical waters. Currently, three species are recognized; I. tuberculatus Gray from Hawaii, Fiji, Australia, East Africa, and the Caribbean (pan-tropical distribution); I. maculatus Muirhead and Ryland described from Fiji; and I. cliftoni Gray from western Australia (see Muirhead and Ryland 1985), although 22 species have been historically listed in the literature (Fautin 2006). In Japan, Isaurus spp. are believed to be very rare, and have been reported from only a handful of locations (Uchida 2001; Uchida and Iwase personal communication; see also Fig. 1). The observed rarity of Isaurus may be partially due to its cryptic appearance (see Fig. 2) and/or its preference for habitats on rocky shores facing the open ocean that are often difficult to access.
Various Isaurus morphotypes nominally identified as Isaurus tuberculatus a specimen IOtsH6 in situ at Hozaki site (depth = 4.0 m), b two polyps from different colonies at Oshirgai-Matsubai (IOtsOM5 left and IOtsOM6 right) showing polyp size and color variation in a tank at BIK. c Colony with Isaurus sp. B morphology from Oshirigai-Matsubai (IOtsOM4) in a tank at BIK. All scale bars = 1 cm. All images originally from Reimer (2007). Note in in situ images the recumbent, non-erect polyps characteristic of Isaurus
The status of Isaurus as separate from Zoanthus is somewhat confused when examining past literature. Many researchers placed nominal Isaurus spp. samples in Zoanthus (discussed in Muirhead and Ryland 1985), and even in more recent literature the extreme similarity in external morphology between Zoanthus praelongus and I. maculatus has been noted (Muirhead and Ryland 1985). Isaurus has been defined to be different from Zoanthus by having recumbent, non-erect polyps (although Z. praelongus shares this characteristic with Isaurus) and the presence of tubercules on the polyps (although I. cliftoni does not have tubercules), but otherwise shares many morphological characters [e.g. not sand-encrusted, zooxanthellate, colonial, generally “liberae” polyps (see Pax 1910)] with Zoanthus, making phylogenetic placement of this genus as separate to Zoanthus open to speculation.
Recent genetic studies investigating the genera Zoanthus (Reimer et al. 2006a) and Palythoa (Reimer et al. 2006b, 2007b) have demonstrated that relatedness in many zoanthids is difficult to judge based solely on morphology. This is largely due to zoanthids often being very morphologically plastic with regards to their external morphology (polyp and colony shape, etc.) (Karlson 1982; Larson and Larson 1982). DNA sequencing and analyses have often resulted in taxonomic revision of species (Reimer et al. 2006b) and genera (Reimer et al. 2006c).
However, until now only three single genetic sequences from Isaurus spp. have been deposited in GenBank; one mitochondrial (mt) 16S ribosomal DNA (mt 16S rDNA) sequence from I. tuberculatus (AF398919—Burnett unpublished), and two sequences [mt 16S rDNA (AY995945) and 12S ribosomal DNA (AY995922)] from Sinniger et al. (2005), who examined a single unidentified Isaurus sp. sample of origin unknown acquired from the aquarium trade. These sequences suggest the specimens examined are closely related to the genus Zoanthus. However, due to the limited number of Isaurus, Zoanthus, and Palythoa sequences examined in Sinniger et al. (2005), as well the overall lack of research conducted on Isaurus, questions remain about the phylogenetic position of Isaurus within Zoanthidea.
Another method that may aid in characterizing zooxanthellate zoanthid species is by identifying their symbiotic dinoflagellates from the genus Symbiodinium (order Suessiales). This is primarily achieved through sequencing of the internal transcribed spacer of ribosomal DNA (ITS-rDNA) of Symbiodinium. While some zooxanthellate zoanthid species are known to host more than one type of Symbiodinium within individuals (LaJeunesse 2002), the majority of zooxanthellate zoanthids examined thus far host one type/subclade of Symbiodinium per colony/individual (see Reimer et al. 2006d, e). Additionally, particular zoanthid species often specifically associate with certain Symbiodinium types (Reimer et al. 2006d, 2007a). Thus, identification of Symbiodinium may often help in facilitating host zoanthid species identification, although symbiont data alone should not be used for any identification. However, no data on Symbiodinium distribution patterns in Isaurus spp. have been reported as of date.
In this study, two molecular markers [mitochondrial cytochrome oxidase subunit I (COI) and mt 16S rDNA] previously shown to be able to discern between zoanthid genera and species (see Reimer et al. 2004, 2006b, c; Sinniger et al. 2005) were utilized to confirm the position of the genus Isaurus within the family Zoanthidae, and to investigate potential species diversity within Isaurus specimens in Japan. In addition, identification of Symbiodinium spp. from all collected samples was performed using ITS-rDNA to aid in potential Isaurus species identification.
Materials and methods
Sampling
Samples of Isaurus spp. (n = 19) representing a variety of morphotypes were collected from several sites in Kochi and Kagoshima, Japan (Fig. 1) between August 2004 and October 2006 (Table 1), and stored in 100% ethanol at −20°C at the Biological Institute on Kuroshio (BIK) until further utilization. Despite large variation in polyps (external coloration, tubercule shape, polyp dimensions), most samples were nominally classified as Isaurus tuberculatus sensu Muirhead and Ryland (1985) (Fig. 2a, b). Uchida (2001) lists specimens from Tatsukushi, Japan as I. assymmetricus, but I. assymmetricus was included in I. tuberculatus (along with three other putative species) by Muirhead and Ryland (1985). Photographs of I. assymmetricus from Tatsukushi are morphogically similar to I. tuberculatus, and thus we have nominally classified samples from Japan collected here as I. tuberculatus following Muirhead and Ryland (1985). Two colonies (IOtsOM4, IOtsNM1) were observed to be morphologically different [having smaller polyps, different polyp coloration (see Fig. 2), smaller tubercules, larger (thousands of polyps) colony sizes] from the majority of samples, and were nominally classified as Isaurus sp. B (Fig. 2c). Additionally, a sample preserved in formalin (thus no genetic examinations were possible) received from Dr. H. Uchida from the Danjo Islands (sample IND1; see Table 1) (collected 26 October 1983 by F. Iwase) also matched the external morphology of Isaurus sp. B. As samples were collected in situ photographs were taken to assist in identification and for collection of morphological data (oral disk/polyp diameter, color, polyp form). Unlike Zoanthus and Palythoa spp., during collection Isaurus polyps in situ were observed to always be closed, and no tentacle count data were obtained, although it is believed to be close to the mesentery number (data shown in Results). Five western Australian zoanthid samples with external morphology consistent with either Isaurus (n = 3) or Zoanthus praelongus (n = 2) [see Muirhead and Ryland (1985) for a discussion on identification problems between these two groups] loaned from the Western Australian Museum (WAM) were also included in subsequent DNA analyses (Tables 1, 2). Sample nomenclature is explained in Tables 1 and 2.
Morphological analyses
Digital in situ photographs of all collected Isaurus specimens were examined, and the following morphological data were collected: polyp and coenenchyme form, polyp external dimensions and color. Internal morphological examinations followed Ono et al. (2005), with specimens first fixed in formalin and then fixed with Bouin’s fluid and embedded in paraffin. Samples were cross-sectioned into 8-μm thick sections, stained with Azan, and observed under the microscope. Data were collected on polyp diameter (maximum and minimum), mesogleal thickness (maximum and minimum), and number of mesenteries, as well as maximum tubercule dimensions (when available).
DNA extraction, PCR amplification, and sequencing
The DNA was extracted from samples weighing 5–20 mg using a spin-column Dneasy Animal Extraction protocol (Qiagen, Santa Clarita, CA, USA). PCR amplification using the genomic DNA as a template was performed using HotStarTaq DNA polymerase (QIAGEN, Tokyo, Japan) according to the manufacturer’s instructions. Mitochondrial (mt) 16S rDNA was amplified following procedures outlined in Sinniger et al. (2005). COI was amplified following procedures outlined in Reimer et al. (2004). The ITS-rDNA region of Symbiodinium was amplified following procedures outlined in Reimer et al. (2006e). The amplified products were visualized by 1.5% agarose gel electrophoresis.
Phylogenetic analyses
New sequences obtained in the present study were deposited in GenBank (accession numbers EF452239-EF452292) (Table 1). By using CLUSTAL X version 1.8 (Thompson et al. 1997), the nucleotide sequences of mt 16S rDNA from samples were aligned with previously published mt 16S rDNA sequences from Isaurus (AF398919—Burnett unpublished; AY995945—Sinniger et al. 2005), as well as Palythoa (AB 219218, AB219223, AB219225; Reimer et al. 2006c), Zoanthus (AB219187, AB219191, AB219192—Reimer et al. 2006b; AB235407—Reimer et al. 2006a) and Acrozoanthus (AY995946, AY995947—Sinniger et al. 2005) sequences, with a newly obtained sequence from Parazoanthus gracilis sensu Uchida 2001 as the outgroup (Table 2). Obtained COI sequences were aligned with previously published Palythoa (AB219199, AB219214, AB219217; Reimer et al. 2006c) and Zoanthus (AB214166, AB214175, AB214177; Reimer et al. 2004 supplemental), with a previously obtained sequence [AB214178 (Reimer et al. 2004 supplemental)] from Parazoanthus gracilis sensu Uchida 2001 as the outgroup (Table 2). Obtained Symbiodinium ITS-rDNA sequences (Table 1) were aligned with previously obtained clade C1/C3 and C1/C3-related Symbiodinium sequences [AY186567 (Rodriguez-Lanetty; Hoegh-Guldberg 2003); AF195144 (Baillie et al. 2000); AY237296, DQ072720, DQ068036 (Bui et al. unpublished); DQ335255, DQ335271, DQ335284, DQ335319, DQ335325, DQ335366, DQ335367. DQ335376, DQ335396 (Reimer et al. 2007a)], with Symbiodinium subclade C15/C91 and related sequences [AF195157 (Baillie et al. 2000); AJ291514, AJ291519 (Pawlowski et al. 2001); AJ311944 (Pochon et al. 2001), AB190278, AB190279, AB190284 (Reimer et al. 2006e)] as the outgroup (Table 2). The alignments were inspected by eye and manually edited. All ambiguous sites of the alignments were removed from the dataset for phylogenetic analyses. Consequently, three alignment datasets were generated: (1) 790 sites of 24 sequences (Isaurus mt 16S rDNA); (2) 302 sites of 27 sequences (Isaurus COI); and (3) 422 sites of 31 sequences (Symbiodinium ITS-rDNA). The alignment data are available on request from the corresponding author.
The alignments of mt 16S rDNA, COI and ITS-rDNA were tested for optimal fit of various nucleotide substitution models using the MODELTEST version 3.06 (Posada and Crandall 1998). The base frequencies, proportion of invariable sites and a gamma distribution were estimated from the datasets. For the mt 16S rDNA and ITS-rDNA datasets, the TN model (Tamura and Nei 1993) incorporating variable sites (TN + I) was selected by MODELTEST. For the COI dataset, the Hasegawa, Kishino and Yano (HKY) model (Hasegawa et al. 1985) incorporating a discrete gamma distribution (four categories) (HKY + Γ) was selected by MODELTEST. The maximum likelihood (ML) analyses with PhyML (Guindon and Gascuel 2003) of these datasets were independently performed using an input tree generated by BIONJ (Gascuel 1997) with the models selected by MODELTEST. PhyML bootstrap trees (500 replicates) were constructed using the same parameters as the individual ML trees.
ML distances of the three datasets were calculated under the optimal models described above with PAUP* Version 4.0 (Swofford 1998). Distance trees were constructed using the neighbour-joining (NJ) method (Saitou and Nei 1987). The ML distance bootstrap analyses with 1,000 replicates were also performed.
Bayesian trees were reconstructed by using the program MrBayes 3.1.2 (Ronquist and Huelsenbeck 2003) under the general time reversible (GTR) model (Rodriguez et al. 1990) of nucleotide substitution for the mt DNA 16S rDNA dataset, and under HKY for the COI and ITS-rDNA datasets [all models selected by MrModeltest (Nylander 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University)]. One cold and three heated Markov chain Monte Carlo (MCMC) chains with default-chain temperatures were run for 1,000,000 generations, sampling log-likelihoods (InLs), and trees at 100-generation intervals (10,000 InLs and trees were saved during MCMC). The likelihood plot for mt 16S rDNA, COI and ITS-rDNA datasets suggested that MCMC reached the stationary phase after the first 40,000, 30,000 and 30,000 generations, respectively. Thus, the remaining 960,000, 970,000 and 970,000 trees of mt 16S rDNA, COI and ITS-rDNA were used to obtain posterior probabilities and branch-length estimates, respectively.
Results
Morphological analyses
Morphological data acquired from cross-sections (shown in Fig. S1) are shown in Table 3. Collected Isaurus specimens were shown to have large amounts of variation not only in external coloration and polyp length (0.7–4.0 cm) (see Table 1), but also in number of mesenteries (36–42), mesogleal thickness (130–920 μm), and polyp diameter (3,200–4,400 μm). Putative Isaurus sp. B specimens had morphological dimensions and counts both greater and lesser than putative Isaurus tuberculatus specimens (Table 3), excepting polyp length.
Genetic analyses
mt 16S rDNA
The mt 16S rDNA tree (Fig. 3) showed all obtained Isaurus sequences from Japan forming a highly supported [ML bootstrap support = 99%, NJ bootstrap support = 97%, posterior probability of Bayesian inference (PP) = 0.89] monophyly together with AY995945 and WAMZ40075. In fact, these sequences were identical over the 790 bp length of the alignment analyzed. Additionally, truncated mt 16S rDNA sequences from other Isaurus samples not included in the alignment (IOtsOS1, IOtsOM2, IOtsH2) also were identical over their entire length to the sequences in the monophyletic group of Isaurus. The mt 16S rDNA sequence from an Isaurus tuberculatus specimen (Burnett unpublished) from a location unspecified (AF398919) differed from the other Isaurus samples by 1 bp over its 464 bp length, but was not included in the alignment as the sequence was substantially shorter than our alignment.
Maximum likelihood tree of mitochondrial 16S ribosomal DNA (mt 16S rDNA) sequences. Values at branches represent ML and NJ bootstrap probabilities, respectively (>50%). Bayesian posterior probabilities of >95% are represented by thick branches. For sample name abbreviations see Tables 1 and 2. Sample names with Accession Numbers are from previous studies (see Table 2). “tuber” (=Isaurus tuberculatus) and “B” (=Isaurus sp. B) in parentheses after sample names show the morphological identity/type of each specimen
The Isaurus monophyly was sister to a moderately supported (ML = 76%, NJ = 77%, PP = 0.73) Zoanthus clade. The two Acrozoanthus sequences radiated within the Zoanthus clade, sister to the Zoanthus vietnamensis/Z. kuroshio clade. Samples WAMZ40080 and WAMZ40082 were also within the Zoanthus radiation, basal to both the Acrozoanthus and Zoanthus vietnamensis/Z. kuroshio clades and to Z. sansibaricus. Palythoa sequences formed a well-supported clade (ML = 91%, NJ = 87%, PP = 0.73) separate from the Zoanthus and Isaurus clades. The Isaurus and Zoanthus/Acrozoanthus clades formed one large clade (=Family Zoanthidae) with high support (ML = 99%, NJ = 100%, PP = 1.00).
COI
The COI tree (Fig. 4) showed all collected Isaurus specimens from Japan and western Australia forming a highly supported (ML = 99%, NJ = 96%, PP = 1.00) monophyly together with AB247631, separate from the Zoanthus sequences. The Isaurus monophyly was derived from Zoanthus sequences, which did not form a monophyly. However, together the Zoanthus and Isaurus sequences (=Family Zoanthidae) formed a highly supported monophyly (ML = 99%, NJ = 99%, PP = 1.00). Palythoa sequences formed a poorly supported clade (ML = <50%, NJ = <50%, PP = <0.50) separate from Zoanthidae.
Maximum likelihood tree of mitochondrial cytochrome oxidase subunit I (COI) sequences. Values at branches represent ML and NJ bootstrap probabilities, respectively (>50%). Bayesian posterior probabilities of >95% are represented by thick branches. For sample name abbreviations see Tables 1 and 2. Sample names with Accession Numbers are from previous studies (see Table 2). “tuber” (=Isaurus tuberculatus) and “B” (=Isaurus sp. B) in parentheses after sample names show the morphological identity/type of each specimen
Symbiodinium ITS-rDNA
Based on phylogenetic analyses, no ITS-rDNA sequences from Isaurus specimens belonged to any Symbiodinium clade other than clade C. All Symbiodinium ITS-rDNA sequences from collected Isaurus specimens from Japan were within the very highly supported (ML = 100%, NJ = 100%, PP = 1.00) clade consisting of types C1 and C3 (sensu LaJeunesse 2004) and related types (Fig. 5). While most sequences from Isaurus were identical to sequences previously noted as Symbiodinium types C1/C3 sensu LaJeunesse (2004) (DQ068036, DQ072720, AF195144—see Reimer et al. 2007a), five obtained ITS-rDNA sequences had small differences (SymIOtsH5, SymITaM1, SymIOtsOM4, SymIOtsB1, SymIOtsH6), and radiate from the basal C1/C3 branch. These sequences are, however, still clearly related to types C1/C3. Additionally, shorter ITS-rDNA sequences from other Isaurus samples not included in the alignment (IOtsH1, IOtsH2, IOtsH3) also were clearly within the C1/C3 related radiation based on our phylogenetic analyses (data not shown).
Maximum likelihood tree of internal transcribed spacer ribosomal DNA (ITS-rDNA) sequences from Symbiodinium. Values at branches represent ML and NJ bootstrap probabilities, respectively (>50%). Bayesian posterior probabilities of >95% are represented by thick branches. For sample name abbreviations see Tables 1 and 2. Sample names with Accession Numbers are from previous studies (see Table 2 )
No Symbiodinium ITS-rDNA sequences from Isaurus specimens were seen to cluster within a separate and moderately well supported (ML = 70%, NJ = 64%, PP = 0.94) clade of Symbiodinium sequences from Zoanthus sansibaricus (Reimer et al. 2006e, 2007a) derived from C1/C3 types.
Discussion
Species diversity of examined Isaurus specimens
Cnidarian mitochondrial DNA is known to evolve at a very slow rate when compared to most other groups of animals (Shearer et al. 2002), and thus it is possible that the two mitochondrial genetic markers used here are not sensitive enough to distinguish between Isaurus spp. However, as shown in Table 4, sequence divergence rates for our Isaurus spp. specimens are much lower than observed previously between other congeneric zoanthids within Zoanthidae and Sphenopidae. COI sequences have been shown to be unable to distinguish between Palythoa tuberculosa and P. mutuki (see Fig. 4 and Reimer et al. 2006c), but mt 16S rDNA did delineate (see Fig. 3; Table 4) between these two closely related species (Reimer et al. 2006c). Similarly, while COI sequences did not differ between Zoanthus kuroshio and Z. vietnamensis sensu Uchida (2001), mt 16S rDNA did show a low level of variation (Table 4) between these two putative species groups (Reimer et al. 2006a), and thus far there have been no cases where the combined mt 16S rDNA and COI sequences have been unable to distinguish between any examined Zoanthidae or Sphenopidae congeners. If the specimens nominally identified as Isaurus sp. B (based on external morphology) are in fact a separate species from I. tuberculatus, then based on our phylogenetic results these two groups must be very closely related. Further studies on these specimens using genetic markers that are faster evolving than mitochondrial DNA (such at nuclear ITS-rDNA) may help reconfirm the results seen in this study, Unfortunately, previously reported zoanthid-specific ITS-rDNA primers (Reimer et al. 2007b, c) did not reliably work on Isaurus specimens in this study. Preliminary nuclear 18S rDNA and 5.8S rDNA data indicate specimens of both I. tuberculatus and putative I. sp. B (sequences n = 2 each, data not shown) form a monophyly separate from Zoanthus and Palythoa spp., but due to the unreliability of the primer sets used and small number of sequences obtained, further investigation is needed on this subject before phylogenetic conclusions based on these markers can be confirmed.
However, our phylogenetic results combined with morphological data suggest that despite wide variation in some morphological characteristics (see Fig. 2; Tables 1, 3) that all Isaurus spp. specimens examined in this study were conspecific (I. tuberculatus). Furthermore, morphological data showing putative Isaurus sp. B specimens having morphological characteristics within the range of I. tuberculatus (excepting polyp length, see Tables 1, 3) make the case for conspecificity even stronger. These results are very similar to both Larson and Larson (1982) and Muirhead and Ryland (1985), who noted the large amounts of not only intraspecific variation in Isaurus, but also intra-colony variation of polyps due to differing habitats and apparent degrees of exposure. As seen in Fig. 2b, we also observed large amounts of intra-colony polyp variation, with large amounts of variation in polyp coloration and size even in small colonies.
The Isaurus specimens examined here from western Australia (WAMZ40074, WAMZ40075, WAMZ40081) also had identical mt 16S rDNA and COI sequences to the Japanese Isaurus specimens, supporting the hypothesis that I. tuberculatus is distributed over a wide range (Muirhead and Ryland 1985). Furthermore, the results here may lend support to the assertion made by Muirhead and Ryland (1985) that I. tuberculatus consists of several previously presumed species, as zoanthid species were traditionally identified and described by their locality and morphology, which has shown to be highly variable.
Placement of Isaurus within Zoanthidae
Our phylogenetic results suggest the placement of Isaurus within the family Zoanthidae as a separate monophyly from Zoanthus. While COI analyses show Isaurus as derived from non-monophyletic Zoanthus, the COI sequences here are much shorter than mt 16S rDNA sequences. As discussed above, mt 16S rDNA has been shown previously with both Palythoa spp. and Zoanthus spp. to be a slightly more accurate marker than COI (both at the species and higher-taxa levels), and it is likely that the mt 16S rDNA tree topology is more reflective than the COI tree of the phylogeny of Zoanthidae.
Preserved specimens of Z. praelongus obtained from western Australia (WAMZ40080, WAMZ40082) were very difficult to morphologically distinguish from Isaurus specimens (see Muirhead and Ryland 1985); both Z. praelongus and Isaurus spp. have recumbent, ‘liberae’ (see Pax 1910) polyps and are of similar size and external morphology. However, the mt 16S rDNA and COI results clearly showed Z. praelongus samples within the Zoanthus radiation, to the exclusion of Isaurus sequences (Figs. 3, 4). Levels of sequence divergence between Zoanthus spp. and Isaurus spp. as well as the topology of both the COI and mt 16S rDNA trees support the separation of these two groups into separate genera.
One further result of note from the mt 16S rDNA data is that Acrozoanthus specimens were located within the Zoanthus clade, most closely related to the Z. kuroshio/Z. vietnamensis group (Fig. 3). Clearly more research utilizing more samples and genetic markers is needed to confirm whether Acrozoanthus is congeneric with Zoanthus.
Regardless of the placement of Acrozoanthus, the results here clearly demonstrate the monophyly of the Family Zoanthidae, further supporting the observations made in Sinniger et al. (2005) on the validity of this family.
Symbiodinium spp. in Isaurus
Based on our Symbiodinium ITS-rDNA phylogeny (Fig. 5), Japanese Isaurus specimens examined in this study apparently specifically associate with Symbiodinium of types C1/C3 sensu LaJeunesse (2004) or very closely related Symbiodinium types. These results are very similar to data seen in Palythoa spp. in Japan, which also apparently specifically associate with C1/C3. C1/C3 has been theorized to be both a host-generalist (LaJeunesse 2004) as it associates with many different host species, and also an environmental generalist (Reimer et al. 2006d), as it is found over a wide environmental range.
Such a specific association with one or a few closely related types of Symbiodinium does not conclusively demonstrate conspecificity of the specimens examined, particularly since C1/C3 is very common in the Indo-Pacific (LaJeunesse 2004). Therefore, further data on Symbiodinium distribution patterns from the other two Isaurus species (I. maculatus and I. cliftoni) are needed. Previous examples of specific associations seen in zooxanthellate zoanthids include the Z. kuroshio/Z. vietnamensis group that associates with Symbiodinium of C15 and related types (Reimer et al. 2006e); and Z. sansibaricus that harbors either C1/C3-derived Symbiodinium unique to Zoanthus or A1 Symbiodinium (Reimer et al. 2006e, 2007a).
How rare are Isaurus spp.?
In the past, little research has been conducted on Isaurus spp., in part due to difficulty in finding specimens (F. Iwase, personal observation). In Japan it has been believed until now that Isaurus spp. are quite rare. Our field studies here show that this may not be the case even though local frequency of colonies appears to be very low. Several (14) new colonies of Isaurus spp. were found in the Tatsukushi-Otsuki region during the course of this study, as well as one colony on Yakushima, where no previous records for Isaurus spp. exist (Fig. 1). All specimens collected in this study were found at locations not easily accessible; on or near rocky shorelines in areas that experience high and consistent levels of current and/or waves. As well, I. tuberculatus specimens in this study usually had coloration that closely resembles the rock and seaweed surrounding the colony (see Fig. 2), providing camouflage and making the colonies very difficult to spot unless specifically searched for. Based on these observations, it may be that Isaurus spp. distribution worldwide is wider than currently known.
Conclusions
The molecular and morphological data strongly suggest all Isaurus specimens examined in this study are conspecific I. tuberculatus, although it remains to be conclusively (i.e. hybridization experiment results, etc.) shown if putative Isaurus sp. B specimens are in fact I. tuberculatus. Our results support the hypotheses suggested by Larson and Larson (1982) and Muirhead and Ryland (1985) that I. tuberculatus has considerable intraspecific morphological variation (in particular external coloration). Even if the nominal Isaurus sp. B specimens are in fact a different species from I. tuberculatus specimens (although data here do not suggest this), we observed high levels of morphological variation (in particular coloration and polyp size) between both different I. tuberculatus specimens and within individual colonies. Despite the apparent low species diversity of Isaurus specimens here, our results also highlight the morphologically variable and cryptic nature of Isaurus spp., and suggest that despite relatively low frequency that this genus may be more widespread than previously believed. In the future, utilization of faster genetic markers (such as ITS-rDNA from Isaurus) combined with more Symbiodinium distribution data and ecological studies (reproduction characteristics, etc.) will help further increase our understanding of this enigmatic zoanthid genus.
References
Baillie BK, Belda-Baillie CA, Maruyama T (2000) Conspecificity and Indo-Pacific distribution of Symbiodinium genoptypes (Dinophyceae) from giant clams. J Phycol 36:1153–1161
Fautin DG (2006) Hexacorallians of the World. http://geoportal.kgs.ku.edu/hexacoral/anemone2/index.cfm
Gascuel O (1997) BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol 14:685–695
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Hasegawa M, Kishino H, Yano T (1985) Dating of the human–ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 22:160–174
Karlson RH (1982) Reproductive patterns in Zoanthus spp. from Discovery Bay, Jamaica. In: Proceedings of the 4th international coral reef symposium, Manila, vol 2, pp 699–704
LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400
LaJeunesse TC (2004) “Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene–Pliocene transition. Mol Biol Evol 22:570–581
Larson KS, Larson RJ (1982) On the ecology of Isaurus duchassaingi (Andres) (Cnidaria: Zoanthidea) from South Water Cay, Belize. In: Rutzler K, MacIntyre IG (eds) The Atlantic barrier ecosystems at Carrie Bow Cay, Belize, I: structure and communities. Smithsonian Contributions to the Marine Science 12, Washington, DC, pp 475–488
Muirhead A, Ryland JS (1985) A review of the genus Isaurus Gray 1828 (Zoanthidea), including new records from Fiji. J Nat His 19:323–335
Ono S, Reimer JD, Tsukahara J (2005) Reproduction of Zoanthus sansibaricus in the infra-littoral zone at Taisho Lava Field, Sakurajima, Kagoshima, Japan. Zool Sci 22:247–255
Pawlowski J, Holzmann M, Fahrni JF, Pochon X, Lee JJ (2001) Molecular identification of algal endosymbionts in large miliolid Foraminifera: 2. Dinoflagellates. J Eukaryot Microbiol 48:368–373
Pax F (1910) Studien an westindischen Actinien. Zool Jahrb Suppl 11:157–330
Pochon X, Pawlowski J Zaninetti L Rowan R (2001) High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in sorotid foraminiferans. Mar Biol 139:1069–1078
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics (Oxf) 14:817–818
Reimer JD (2007) Preliminary survey of zooxanthellate zoanthid diversity (Hexacorallia: Zoantharia) from southern Shikoku, Japan. Kuroshio Biosphere 3:1–16 + 7 pls
Reimer JD, Ono S, Takishita K, Fujiwara Y Tsukahara J (2004) Reconsidering Zoanthus spp. diversity: molecular evidence of conspecifity within four previously presumed species. Zool Sci 21:517–525
Reimer JD, Ono S, Iwama A, Tsukahara J, Maruyama T (2006a) High levels of morphological variation despite close genetic relatedness between Zoanthus aff. vietnamensis and Zoanthus kuroshio (Anthozoa: Hexacorallia). Zool Sci 23:755–761
Reimer JD, Ono S, Iwama A, Tsukahara J, Takishita K, Maruyama T (2006b) Morphological and molecular revision of Zoanthus (Anthozoa: Hexacorallia) from southwestern Japan with description of two new species. Zool Sci 23:261–275
Reimer JD, Ono S, Takishita K, Tsukahara J, Maruyama T (2006c) Molecular evidence suggesting species in the zoanthid genera Palythoa and Protopalythoa (Anthozoa: Hexacorallia) are congeneric. Zool Sci 23:87–94
Reimer JD, Takishita K, Maruyama T (2006d) Molecular identification of symbiotic dinoflagellates (Symbiodinium spp.) from Palythoa spp. (Anthozoa: Hexacorallia) in Japan. Coral Reefs 25:521–527
Reimer JD, Takishita K, Ono S, Maruyama T, Tsukahara J (2006e) Latitudinal and intracolony ITS-rDNA sequence variation in the symbiotic dinoflagellate genus Symbiodinium (Dinophyceae) in Zoanthus sansibaricus (Anthozoa: Hexacorallia). Phycol Res 54:122–132
Reimer JD, Ono S, Tsukahara J, Takishita K, Maruyama T (2007a) Non-seasonal clade-specificity and subclade microvariation in symbiotic dinoflagellates (Symbiodinium spp.) in Zoanthus sansibaricus (Anthozoa: Hexacorallia) at Kagoshima Bay, Japan. Phycol Res 55:58–65
Reimer JD, Takishita K, Ono S, Maruyama T (2007b) Diversity and evolution in the zoanthid genus Palythoa (Cnidaria: Hexacorallia) utilizing nuclear ITS-rDNA. Coral Reefs 26:399–410
Reimer JD, Takishita K, Ono S, Tsukahara J, Maruyama T (2007c) Molecular evidence suggesting intraspecific hybridization in Zoanthus (Anthozoa: Hexacorallia). Zool Sci 24:346–359
Rodriguez F, Oliver JL, Marin A, Medina JR (1990) The general stochiatic model of nucleotide substitution. J Theor Biol 142:485–501
Rodriguez-Lanetty M, Hoegh-Guldberg O (2003) Symbiont diversity within the scleractinian coral Plesiastrea versipora, across the northwestern Pacific. Mar Biol 143:501–509
Ronquist F, Huelsenbeck JP (2003) Bayesian phylogenetic inference under mixed models. Bioinformatics (Oxf) 19:1572–1574
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Shearer TL, van Oppen MJH, Romano SL, Worheide G (2002) Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Mol Ecol 11:2475–2487
Sinniger F, Montoya-Burgess JI, Chevaldonne P, Pawlowski J (2005) Phylogeny of the order Zoantharia (Anthozoa, Hexacorallia) based on mitochondrial ribosomal genes. Mar Biol 147:1121–1128
Swofford DL (1998) PAUP*. V Phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, MA, USA
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Uchida H (2001) Sea anemones in Japanese Waters. TBS Britannica, Tokyo (in Japanese). pp 118–124
Acknowledgments
JDR would like to thank Koki Tanaka (BIK), Mai Miyamoto and Miho Watanabe (both Tokyo University of Marine Science and Technology), Dr. Kensuke Yanagi (Coastal Branch of Natural History Museum and Institute, Chiba), Hiroko Haraguchi (Kochi University), Yuichi Nagata and Mika Reimer for assistance in sampling. As well, Kazutoshi Nishimoto and Atsushi Nishimoto (both Tatsukushi Port) helped with guidance during zoanthid surveys. Dr. Jane Fromont and Dr. Mark Salotti (WAM) kindly loaned Z. praelongus and Isaurus samples, with an introduction from Max Rees (Australian Institute of Marine Science). Australian samples were collected by WAM as part of a SRFME funded project on the Central West Coast of Australia. Additionally, Dr. Hiroomi Uchida (Kushimoto Marine Park, Wakayama) and Frederic Sinniger (University of Geneva) kindly loaned us other samples or information utilized in this study. At JAMSTEC, Masaru Kawato and Hirokazu Kuwahara helped with sequencing. Dr. Kiyotaka Takishita (JAMSTEC) and comments from three anonymous reviewers greatly improved the manuscript. All experiments described within comply with the current laws of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Nishida.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reimer, J.D., Ono, S., Tsukahara, J. et al. Molecular characterization of the zoanthid genus Isaurus (Anthozoa: Hexacorallia) and associated zooxanthellae (Symbiodinium spp.) from Japan. Mar Biol 153, 351–363 (2008). https://doi.org/10.1007/s00227-007-0811-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0811-0