Abstract
In highly dynamic and unpredictable environments such as the Southern Ocean, species that have evolved behaviors that reduce the effects of intra-specific competition may have a selective advantage. This is particularly true when juveniles face disadvantages when foraging due to morphological or physiological limitation, which is the case for many marine mammals. We tracked the at-sea movements of 48 juvenile southern elephant seals (Mirounga leonina) between the ages of 1 and 4 years from the population at Macquarie Island using locations derived from recorded light levels. There were significant differences in the total amount of the Southern Ocean covered by the different age-groups. The younger seals used a smaller area than the older seals. On average, the younger individuals also made more trips to sea than the older seals and did not travel as far on each trip. Females spent more time at sea than males and there were no significant differences between the total areas used by male and females. In summary, younger seals remained closer to the island at all times, and they spent more time in more northerly regions that older seals. These differences in behavior created temporal and spatial segregation between juveniles of different ages. Therefore, we suggest that these temporal and spatial separations help to avoid intra-specific competition for resources on land, space on beaches, and at-sea foraging areas. Such modifications of haul-out timing and behavior enable them to exploit a patchy and unpredictable environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For nearly half a century, niche theory has provided a framework for explaining competition within ecological communities and the mechanisms by which they function (Pianka 1981; Bolnick et al. 2003). A major force in driving community structure is derived from inter-specific competition for resources (Schoener 1986); more recently, intra-specific competition has been identified as a significant component in the evolution of niche width (Polis 1984). For many species, groups of individuals classed according to age, sex and morphology exhibit significant variation in foraging behavior and diet specialization (Bolnick et al. 2003), or even through individual variation, and all contribute to the definition of the species’ or population’s niche width. An important component in the evolution of population dynamics is phenotypic variation within a population that occurs between age and sex classes (Schoener 1986). Ontogenetic niche shifts (Woodward and Hildrew 2002) have been proposed for many species as the major component of total niche width attributed to age/size structure (Warren 1996; Williams and Martinez 2000; Bolnick et al. 2003).
Resource partitioning may function through interference competition, or exclusion from resources or habitat as an evolutionary trait of sexual selection (Polis 1984). Although this usually increases a surviving individual’s fitness, the resulting increase in competition may restrict juvenile recruitment during times of resource limitation. Therefore, ontogenetic shifts in morphology, habitat use and foraging behavior may promote population stability more effectively over evolutionary time (Polis 1984). The reduction of intra-specific competition through resource partitioning has been observed for many species over a range of spatial scales, especially when resources are limited and environmental predictability is low (Perry 1996; Kato et al. 2000; Wikelski and Wrege 2000; Bowen et al. 2002; Pearson et al. 2002; Bradshaw et al. 2003). For example, size-specific resource partitioning in little brown bats (Myotis lucifugus) correlates to shifts in habitat use and diet with ontogenetic development (Adams 1996).
The distribution of biological resources within the Southern Ocean is highly variable, unpredictable and patchy (Constable et al. 2003). This variability and pronounced patchy distribution may require marine species to develop intra-specific niche specialization to maximize the probability of securing resources for growth and reproduction. Although some studies have documented ontogenetic and morphometric shifts in the diving behavior of marine vertebrates, most have focused on developmental physiology and behavioral aspects (Burns 1999; Baechler et al. 2002) rather than the ecological or evolutionary function of these shifts. One recent study has shown that, on a local scale, marine iguanas (Amblyrhynchus cristatus) have developed ontogenetic foraging niches that increase probability of survival in an unpredictable environment (Wikelski and Wrege 2000).
Southern elephant seals (Mirounga leonina) are wide-ranging, deep-diving predators within the Southern Ocean ecosystem that spend more than 80% of their annual cycle at sea and are large consumers of fish and squid (Bradshaw et al. 2003; Hindell et al. 2003). The population of M. leonina at Macquarie Island has been declining for reasons that are still unclear (Hindell et al. 1994), though is most likely due to changes in food availability and distribution (McMahon et al. 2004). Recently, it has been shown that juvenile (1–4 years) survival is the most important factor affecting the population rate of change (McMahon et al. 2003). Thus, it appears that the potential for ontogenetic shifts in foraging behavior and diet through changes in morphology and physiology might have important implications for the ecological dynamics of this population in particular (Field et al. 2004; McMahon et al. 2004). Until recently, knowledge of juvenile southern elephant seals was restricted to studies of individuals ashore (Le Boeuf and Laws 1994; Wheatley 2001). The annual cycle of juveniles is unusual in that in addition to the annual molt (November–January) it incorporates a facultative mid-year time ashore (April–August), the purpose of which is unclear; the possibilities include physiological restrictions, parasite reduction and social stimulation (Ling and Bryden 1981; Neumann 1999). Another function could be that the mid-year haul-out may have evolved as a by-product of intra-specific resource partitioning through ontogenetic shifts in foraging ability. The foraging ecology of southern and northern (M. angustirostris) elephant seals has been studied extensively (Slip 1997; Slip et al. 1994; Le Boeuf 1994; Stewart 1997; Hindell et al. 1999) but only two studies have described the at-sea movements of non-naïve (>1 year-old) southern juveniles (van den Hoff et al. 2002; Field et al. 2004). For both species, there are profound physiological and behavior changes between juveniles and adults (Le Boeuf et al. 2000).
In this paper, we examine the foraging patterns of juvenile southern elephant seals and test the hypotheses that as juveniles mature: (1) the different age groups will use different regions of the Southern Ocean through differences in the haul-out patterns, durations of time spent at sea, distances traveled, and the total area used by individuals grouped according to age and sex; and (2) individuals demonstrate fidelity to foraging areas that reinforce spatial separation. Observed patterns of foraging are discussed in light of the possible evolutionary mechanisms responsible for ontogenetic resource partitioning that may have occurred in a species demonstrating some of the greatest horizontal and vertical movements of any mammal.
Materials and methods
The southern elephant seal population at Macquarie Island (158° 57′E, 54° 30′S) has been the focus of a long-term mark-recapture demographic study since 1993 (McMahon et al. 2003). We studied juvenile seals, of known age, between 1 and 4 years old, and having no breeding experience. Seals from 1 to 2 years are referred to as “yearlings” and after that, as 2- or 3-year-olds.
We used temperature–light loggers (LL; R. Hansworth, Kingston, TAS, Australia) and temperature–depth-recorders (TDRs; Wildlife Computers, Redmond, USA) to provide location data for the juvenile seals. The LL units were 60×45×25 mm in size and had an 8-Mbit FLASH memory for storage of data. Light and temperature data were collected every 45 s. The temperature readings had a resolution of ±0.2°C and a range of −12 to +31°C. The TDRs used included Mk3, Mk5, Mk6 and Mk7 models and measured temperature and light at the same sampling interval as the LLs. All units weighed less than 350 g, which represented <0.5% of the departure mass of the smallest seal in the study (78 kg).
Forty-eight juvenile seals were equipped with TDRs or LLs between 1999 and 2001 (16 in 1999–2000 and 32 in 2000–2001) encompassing 83 individual foraging trips. Seals were caught as they were about to leave the island at the end their annual molt. During captures all restraint and disturbance to seals were kept to a minimum. Seals were caught and anesthetized intravenously using prescribed doses (Field et al. 2002) of tiletamine and zolazepam (Telazol, Forte Dodge, Castle Hill, NSW, Australia).
Once anaesthetized, the LLs and TDRs were attached by gluing them to the hair on the dorsal surface of the seals between the shoulder blades using epoxy (Araldite 268, Ciba Geigy). Over the study period, the beaches on and near the northern isthmus of the island were searched daily for marked individuals returning ashore to calculate the haul-out patterns of these age groups and for individuals with data-logging units. The units were retrieved either by capturing the seals when the seals returned to shore, or by collecting the units from the beach after they were shed during the molt. LL and TDR data also contributed to the separation of the land and sea phases for the calculation of haul-out patterns.
Estimation of location from light levels
At-sea locations were derived using geo-location software (Multi-trace, Jensen Software, Germany) giving two locations per day. These data were filtered to exclude positions that would have exceeded the maximum distance that could have been traveled (12.5 km h−1; Bradshaw et al. 2002). During the equinox periods (4 March–14 April and 30 August–14 October) estimates of longitude are unaffected, but latitudes could not be estimated due to the invariance of day length. We used linear interpolation of latitude to the next most reliable location to provide an estimate of the daily location. Daily positions were filtered using a state-space Kalman location filter (Sibert et al. 2003). This time-dependent model of the variance in geo-location estimates (Sibert et al. 2003) was used to provide realistic estimates of in situ movement parameters from geo-location positions while the seals were at sea. Light-derived geo-location data have inherent spatial errors up to ±350 km (van den Hoff 2002; Bradshaw et al. 2002) and other parameters derived from them retain these errors.
Use of location data and mean migration parameters
To compare location data among sex/age groups, we calculated the following mean migration parameters per individual: duration of the trip to sea, maximum distance from Macquarie Island, total trip distance and daily rates of travel. We also calculated the bearing to the position of maximum distance to indicate the major directional component of each foraging trip (Bradshaw et al. 2004) which simply represents the path of the migration. We compared these parameters between the different age/sex groups using one-way general linear models (GLM) for only seals (n=42) with complete trips to sea (n=76). Some seals recorded data for consecutive trips to sea within the study period so we compared an individual’s maximum distance reached and duration of different trips using repeated-measures ANOVA, (two trips: n=10; three trips: n=12) to determine if all trips to sea could be included in the analyses. These were found to be the same for sequential trips and so we pooled the data to improve statistical power. We also tested the hypothesis that individuals showed fidelity to foraging areas using the bearing to the location of maximum distance in trip I+1 versus to that of trip I for an individual’s sequential trips to sea using a linear regression model (Bradshaw et al. 2004).
Spatial summary
Once the location data were filtered, they were rasterized onto a 300×300 km grid (IDL 5.0, Research Systems, USA). The size of the grid cells were set to allow for maximum distance that the seals could travel (between an average of 70 and 90 km day−1; Le Boeuf et al. 2000) and the errors associated with geo-location (Bradshaw et al. 2002; van den Hoff et al. 2002). For each grid cell, a central longitude and latitude were produced and the time (h) spent within any grid cell for each individual. The data for all seals (n=48) were split into 14-day blocks for temporal differences in spatial overlap and the mean maximum distance between age/sex groups to be examined using a series of Kruskal-Wallis tests (Bonferroni corrected; p<0.002).
Total area used: age and sex comparisons
We used one-way GLM to test for differences between age and sex groups in the total number of days spent at sea and the total area used. For these analyses, only seals (n=31) with complete data for the period between the end of the annual molt and the following molting season or first breeding season (December–November). There were too few data from each year to examine annual effects statistically, so data from both years were pooled. The areas used by individuals were calculated and analyzed in the same manner as the other migration parameters.
Time spent within oceanographic regions
We calculated the time spent by each individual within five distinct oceanographic regions defined by frontal systems within the study area (Orsi et al. 1995; Rintoul et al. 1997), and compared them using a one-way GLM. The regions were defined as: (1) the sub-tropical zone to the north of the sub-tropical front (STF); (2) the sub-Antarctic zone (SAZ) between the sub-tropical front and the sub-Antarctic front; (3) the polar frontal zone (PFZ) between the sub-Antarctic front (SAF) and the Antarctic polar front (APF); (4) the Antarctic zone (AZ) between the APF and southern boundary of the Antarctic circumpolar current (SBDY); and (5) south of the SBDY as the high Antarctic zone (HAZ).
Results
Annual cycle patterns
For the first mid-year haul-out, the yearlings arrived first (average arrival day = 4 April), followed by the 2-year olds (1 May) and 3-year olds (27 May). Some seals had a second mid-year haul-out, during which the yearlings of both sexes returned, but only a 2-year-old male returned from the older age groups. Here, the mean arrival date for yearlings was 23 July, and 4 August for the 2-year-old male, and mean residence time was almost half that of the first haul-out (Table 1). For the molt, the first to return were the 2-year olds (26 November), followed by the yearlings (1 December) and the 3-year olds (3 December), but the differences were not significant as has been found in other studies with larger sample sizes (Hindell and Burton 1988; Wheatley 2001) . The number of trips to sea varied with age: yearlings made\((\bar X \pm {\text{SD}})\) 2.7±0.46 trips, 2-year olds made 2.2±0.41 trips, and 3-year olds made 1.1±0.32 trips.
At-sea distribution
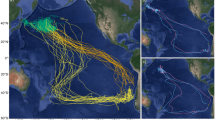
The total area used by all seals over all trips to sea was 16,292,500 km2 (n=83 trips to sea) between 107° and 234°E, and 34° and 71°S (Fig. 1). The seals traveled predominantly to the southeast and southwest (Fig. 2), with a few traveling northwest. There was a significant difference (one-way ANOVA: F2,25=4.03; p<0.03, n=31) between the mean total area used by the different age groups; a post-hoc LSD test indicated that yearlings used a significantly smaller area (2,392,833±533,697 km2) than 2- and 3-year olds, but there was no difference between the two older ages (combined mean area = 3,537,188±1,319,271 km2; Fig. 1). There were no significant sex or interaction effects (one-way ANOVA: F1,25=0.99; p<0.76 and F2,25=0.11; p<0.89, respectively).
Time spent within oceanographic regions
Yearlings spent around 99% of their time in the SAZ, PFZ and AZ, and less than 1% of their time south of the SBDY in the HAZ. Two-year olds spent approximately 89% of their time in the SAZ, PFZ and AZ, and 11% in the HAZ. Finally, the 3-year olds spent 80% in the SAZ, PFZ and AZ, and 20% in the HAZ.
Individual behavior
There were significant differences between migration parameters for the different age/sex groups (Table 2). Older seals traveled farther from Macquarie Island, than younger seals (one-way ANOVA: F2,59=8.770; p<0.001, n=76; Fig. 3). This difference was due largely to the younger seals making shorter trips (duration) than older seals (one-way ANOVA: F2,59=19.581; p<0.001), also males made shorter trips than females (one-way ANOVA: F1,59=9.964; p=0.003) in each age group. Younger seals did not travel as far, (total distance traveled) and used a smaller area than older seals (one-way ANOVA: F2,59=2.126; p<0.001 and F2,59=10.092; p<0.001, respectively), and males had shorter trips and did not travel as far as females (one-way ANOVA: F1,59=5.116; p=0.027) in each age group. However, the daily rate of travel (total distance traveled/trip duration) was similar for all age/sex groups. Seals tracked over sequential migrations had the same direction of travel for each trip regardless of whether the seals made two (linear regression: bearing of trip one to two: F1,9=23.213, p=0.001, R2=0.74, n=10) or three trips to sea (linear regression: bearing of trip one to two: F1,11=26.804, p<0.001, R2=0.73; trip two to three: F1,11=10.281, p=0.009, R2=0.51, n=12).
Temporal variation in regional use
There was a clear pattern of temporal and spatial segregation with age using maximum distance traveled from the island per 14-day time block (Fig. 4). Yearlings and 2-year olds left Macquarie Island in December and moved approximately 1,146±403 km and 1,457±478 km away, respectively. Three-year olds left in January, potentially traveling through the areas used by the younger seals as they left the Island. However, once they reached the middle phase of their trip the overlap was minimal. The 1-year-old seals remained relatively closer to MI at all times and returned earlier for their initial or only mid-year haul-out. The 2-year olds returned for their haul-out later, but traveled farther from MI in their following trip to sea. Due to the low sample size of this age group after their mid-year haul-out, the maximum distance was not significantly different from those of the other age-groups. However, the pattern is consistent with that of the 1- and 3-year-olds. The 3-year-old seals traveled farthest from MI and returned slowly to MI, but only one male was tracked after the mid-year haul-out. Thus, the maximum distance pattern was dominated by females having one trip to sea that returned in September/October to pup. In the mid-year, the mean distance from MI remained large due to the variation in haul-out timings of the individuals of each age group.
Discussion
Niche theory predicts, in an environment with limited or patchy resources, selective pressures promote the evolution of generalist feeding behaviors and the reduction of intra-specific competition (Schoener 1986). Southern elephant seals have large energy requirements (Boyd et al. 1994), so their annual consumption of fish and squid is one of the highest for mammals and birds in the region (Hindell et al. 2003; Bradshaw et al. 2003). Thus, we hypothesize that the ability to find and sequester this considerable biomass from a patchy and unpredictable environment has resulted in the evolution of ontogenetic niche shifts. This process may have reduced local competition that then increased an individual’s probability of foraging success. We found a clear segregation in the use of the Southern Ocean by juvenile southern elephant seals over the course of their annual foraging trips. As the seals aged they made fewer but longer trips to sea, traveled farther and spent more time closer to Antarctica. The lack of strong sex differences in foraging behavior is probably indicative of the lack of dimorphism during the juvenile years. Therefore, it is unlikely that ecological sexual dimorphism is an important factor until closer to breeding age when male and female body sizes diverge dramatically. Exclusion and interference competition are unlikely due to the large range over which this species travels, their presumed solitary feeding behavior, and abundance of suitable haul-out space on the beaches of Macquarie Island (McMahon et al. 2004). However, male and female elephant seals from Heard Island and Iles Kerguelen haul-out at different sites which Burton (1985) suggested reflects foraging-area separation.
The observed haul-out pattern was similar to that described previously for this species (Hindell and Burton 1988; Wheatley 2001), as were the distances traveled (Bradshaw et al. 2004; Field et al. 2001; McConnell et al. 2002; Slip et al. 1994; van den Hoff et al. 2002). However, in contrast to juveniles of the congeneric M. angustirostris, M. leonina juveniles develop foraging patterns similar to their adults later in life. This may be due to the lower age of sexual maturity for M. angustirostris (Le Boeuf et al. 1996). For M. angustirostris, it has been suggested that the direction of migration is set within the first year of life (Stewart 1997). However, for M. leonina the direction of travel is predominantly south-east in the first year followed by a change to south-west and south-east (van den Hoff et al. 2002; McConnell et al. 2002). As adults, female southern elephant seals show remarkable fidelity to foraging regions between years (Bradshaw et al. 2004). Our data suggest that migration directions are fixed as yearlings, as individuals gain experience, but distances traveled are limited by their size, physiology and haul-out pattern. Stewart (1997) also suggested that the divergence in migration between sexes occurs at puberty because of sexual dimorphism and selection pressures through increased energy requirements of males. However, we propose this divergence in migration among age and sex groups is more likely due to intra-specific resource partitioning because the behavior is expressed well before the onset of puberty and sexual dimorphism.
Other studies have demonstrated that both temporal and geographical segregation of activity budgets in marine species do exist. Atlantic humpback whales (Megaptera novaeangliae) segregate their use of the ocean by modifying the timing of their migrations from their geographically distinct foraging areas to common breeding areas (Stevick et al. 2003). Other species such as seabirds (Furness and Birkhead 1984), marine iguanas (Buttemer and Dawson 1993), bowhead whales (Balaena mysticetus) (Cosens and Blouw 2003) and harp seals (Phoca groenlandica) (Sergent 1991) also demonstrate geographical and temporal displacement in their migrations.
We found a clear pattern in both the haul-out and at-sea behavior with increasing age. Although it is unclear what mechanisms drive intra-specific resource partitioning, we hypothesize that the mid-year haul-out functions as a temporal regulator of time at sea, and therefore the foraging ranges of individuals. An important factor in this pattern must be the energetic cost of returning to haul-out. It is unknown whether returning to haul-out during the mid-year incurs any additional costs, but if it does then the costs must be outweighed by the benefits of reduced intra-specific competition. There is support for this view because older, larger individuals can dive deeper and return less often to haul-out than younger seals. Therefore, as seals grow they exploit a greater area of the foraging environment unavailable to younger, smaller age classes. Our data show a delay in the haul-out pattern with age as the seals grow and are able to remain at sea for longer. The ultimate limitation on ontogenetic niche shifts for juvenile seals appears to be rate at which they can grow. Furthermore, older juvenile males (5- and 6-year olds) have a mid-year haul-out (Wheatley 2001) and yet have similar physiology and foraging patterns to adults, so returning to land reduces competition during foraging. Although the mid-year haul-out may serve no particular function directly, it may promote the reduction of intra-specific competition and promote and re-enforce the survival probability of seals participating.
Alternatively, juveniles constrained physiologically and morphologically return to the island to reduce energy consumption or for physiological development. Juvenile seals have a restricted capacity to deal with heat-loss (Thompson et al. 1998), so the mid-year return may provide a better thermal environment for small individuals. Similarly, changes in morphology and physiology with size/age may to lead to reduced intra-specific competition between juveniles (Adams 1996; Wikelski and Wrege 2000; Spina 2000).
Elephant seals are opportunistic generalist feeders with a broad foraging niche (Whitehead et al. 2003). Additionally, the broad spatial scale over which this segregation was observed suggests that reduced foraging niche overlap may be supported by the availability of different prey aggregations relative to oceanographic regions (Field et al. 2001; Bradshaw et al. 2003; Hindell et al. 2003). We suggest that as foraging range increases so does the potential width of the overall foraging niche. Foraging in a patchy and unpredictable environment has resulted in the evolution of behaviors that maximize their probability of foraging successfully and reduces intra-specific competition. If there is an equal probability of locating prey successfully in a patchy environment, then it is likely that the seals would leave their terrestrial haul-outs and disperse toward regions of generally higher productivity. However, this strategy would also be influenced by ontogenetic factors such as morphological and physiological constraints or experience. We suggest that, rather than traveling to specific feeding areas, Macquarie Island elephant seals travel in a general direction and forage opportunistically across the different oceanographic regions until prey patch is found and exploited. Their behavior is then modified further by ontogenetic shifts, through growth and changes in physiology.
We suggest that the different age/size classes of juvenile southern elephant seals have become individual ‘ecological species’ through ontogenetic shifts in foraging niche. This has resulted from both temporal and spatial separation of resource use where individuals avoid intra-specific competition. The most likely mechanism for the development of these behaviors is the modification of the haul-out timing in an environment where there is no additional energetic cost in returning to haul-out, in conjunction with normal developmental restrictions. Thus, this process has allowed these wide-ranging and opportunistically feeding seals to exploit different oceanographic regions and increase their foraging niche width. Future study of southern elephant seal diet, growth, physiology and diving behavior may contribute to a better understanding of the function of the mid-year haul-out and how competition structures the marine community of the Southern Ocean.
References
Adams RA (1996) Size-specific resource use in juvenile little brown bats, Myotis lucifugus (Chiroptera: Vespertilionidae): is there an ontogenetic shift? Can J Zool 74:1204–1210
Baechler J, Beck CA, Bowen WD (2002) Dive shapes reveal temporal changes in the foraging behaviour of different age and sex classes of harbour seals (Phoca vitulina). Can J Zool 80:1569–1577
Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28
Bowen WD, Tully D, Boness DJ, Bulheier BM, Marshall GJ (2002) Prey dependent foraging tactics and prey profitability in a marine mammal. Mar Ecol Prog Ser 244:235–245
Boyd IL, Arnbom TA, Fedak MA (1994) Biomass and energy consumption of the South Georgia population of the southern elephant seals. In: Le Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behaviour, and physiology. University of California Press, Berkeley, pp 98–120
Bradshaw CJA, Hindell MA, Michael KJ, Sumner MD (2002) The optimal spatial scale for the analysis of elephant seal foraging as determined by geo-location in relation to sea surface temperatures. ICES J Mar Sci 59:770–781
Bradshaw CJA, Hindell MA, Best NJ, Phillips KL, Wilson G, Nichols PD (2003) You are what you eat: describing the foraging ecology of southern elephant seals (Mirounga leonina) using blubber fatty acids. Proc R Soc Lond B 270:1283–1292
Bradshaw CJA, Hindell MA, Sumner MD, Michael KJ (2004) Loyalty pays: potential life-history consequences of fidelity to marine foraging regions by southern elephant seals. Anim Behav (in press)
Burns JM (1999) The development of diving behaviour in juvenile Weddell seals: pushing physiological limits in order to survive. Can J Zool 77:737–747
Burton HR (1985) Tagging studies of male southern elephant seals (Mirounga leonina L.) in the Vestfold Hills area, Antarctica, and some aspects of their behaviour. In: Ling JK, Bryden MM (eds) Sea mammals of south latitudes. South Australian Museum, Northfield, pp 19–30
Buttemer WA, Dawson WR (1993) Temporal pattern of foraging and microhabitat use by Galapagos marine Iguanas, Amblyrhynchus cristatus. Oecologia 96:56–64
Constable AJ, Nicol S, Strutton PG (2003) Southern Ocean productivity in relation to spatial and temporal variation in the physical environment. J Geophys Res 108:8079–9000
Cosens SE, Blouw A (2003) Size- and age-class segregation of bowhead whales summering in northern Foxe Basin: a photogrammetric analysis. Ma r Mamm Sci 19:284–296
Field I, Hindell MA, Slip DJ, Michael KJ (2001) Foraging strategies of southern elephant seals (Mirounga leonina) in relation to frontal zones and water masses. Antarct Sci 13:371–379
Field IC, Bradshaw CJA, McMahon CR, Harrington J, Burton HR (2002) Intravenous anaesthesia of elephant seals (Mirounga leonina) using Tiletamine and Zolazepam: effects of age, size, condition and function of haul-out. Vet Rec 151:235–240
Field IC, Hindell MA, Bradshaw CJA, Burton HR (2004) Seasonal use of oceanographic and fisheries management zones by juvenile southern elephant seals (Mirounga leonina) from Macquarie Island. Polar Biol 27:432–440
Furness RW, Birkhead TR (1984) Seabird colony distributions suggest competition for food supply during the breeding season. Nature 311:655–656
Hindell MA, Burton HR (1988) Seasonal haul-out patterns of the southern elephant seal (Mirounga leonina L.) at Macquarie Island. J Mamm 69:81–88
Hindell MA, Slip DJ, Burton HR (1994) Possible causes of the decline of southern elephant seal populations in the southern Pacific and southern Indian Oceans. In: Le Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behaviour, and physiology. University of California Press, Berkeley, pp 68–84
Hindell MA, McConnell BJ, Fedak MA, Slip DJ, Burton HR, Reijnders PJH, McMahon CR (1999) Environmental and physiological determinants of successful foraging by naive southern elephant seal pups during their first trip to sea. Can J Zool 77:1807–1821
Hindell MA, Bradshaw CJA, Sumner MD, Michael KJ, Burton HR (2003) Dispersal of female southern elephant seals and their prey consumption during the austral summer: relevance to management and oceanographic zones. J Appl Ecol 40:703–715
van den Hoff J (2002) Migrations of juvenile southern elephant seals from Macquarie Island. MSc Thesis, University of Tasmania
van den Hoff J, Burton HR, Hindell MA, Sumner MD, McMahon CR (2002) Migrations and foraging of juvenile southern elephant seals from Macquarie Island within CCAMLR managed areas. Antarct Sci 14:134–145
Kato A, Watanuki Y, Nishiumi I, Kuroki M, Shaughnessy P, Naito Y (2000) Variation in foraging and parental behaviour of king cormorants. Auk 117:718–730
Le Boeuf BJ (1994) Variation in the diving pattern of northern elephant seals with age, mass, sex and reproductive condition. In: Le Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behaviour, and physiology. University of California Press, Berkeley, pp 237–252
Le Boeuf BJ, Laws RM (1994) An introduction to the genus. In: Le Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behaviour, and physiology. University of California Press, Berkeley, pp 1–26
Le Boeuf BJ, Morris PA, Blackwell SB, Crocker DE, Costa DP (1996) Diving behaviour of juvenile northern elephant seals. Can J Zool 74:1632–1644
Le Boeuf BJ, Crocker DE, Costa DP, Blackwell SB, Webb PM, Houser DS (2000) Foraging ecology of northern elephant seals. Ecol Monogr 70:353–382
Ling JK, Bryden MM (1981) Southern elephant seal Mirounga leonina Linnaeus, 1758. In: Ridgeway SH, Harrison RJ (eds) Handbook of marine mammals, vol 2. Lavenham Press, Lavenham, pp 297–327
McConnell BJ, Fedak MA, Burton HR, Engelhard GH, Reijnders PJH (2002) Movements and foraging areas of naïve, recently weaned southern elephant seal pups. J Anim Ecol 71:65–78
McMahon CR, Burton HR, Bester MN (2003) A demographic comparison of two southern elephant seals populations. J Anim Ecol 72:61–74
McMahon CR, Bester MN, Burton HR, Hindell MA, Bradshaw CJA (2004) Population status, trends and a re-examination of the hypotheses explaining the recent decreases of the southern elephant seal, Mirounga leonina. Mamm Rev (in press)
Neumann DR (1999) Agonistic behaviour in harbour seals in relation to the availability of haul-out space. Mar Mamm Sci 15:507–525
Orsi AH, Whitworth T, Nowlin WD (1995) On the meridional extent and fronts of the Antarctic circumpolar current. Deep-Sea Res 42:641–673
Pearson D, Shine R, How R (2002) Sex-specific niche partitioning and sexual size dimorphism in Australian pythons (Morelia spilota imbricate). Biol J Linn Soc 77:113–125
Perry G (1996) The evolution of sexual dimorphism in the lizard Anolis polylepis (Iguania): evidence from intraspecific variation in foraging behaviour and diet. Can J Zool 74:1238–1245
Pianka ER (1981) Competition and niche theory. In: May RM (ed) Theoretical ecology: principles and applications. Blackwell, Oxford, pp 114–141
Polis A (1984) Age structure component of niche width and intraspecific resource partitioning: can age groups function as ecological species? Am Nat 123:541–564
Rintoul SR, Donguy JR, Roemmich DH (1997) Seasonal evolution of upper ocean thermal structure between Tasmania and Antarctica. Deep-Sea Res 44:1185–1202
Schoener TW (1986) Resource partitioning. In: Kikkawa J, Anderson DJ (eds) Community ecology pattern and process. Blackwell, Carlton, pp 91–126
Sergent DE (1991) Harp seals, man and ice. Can J Fish Aquat Sci 114:153
Sibert JR, Musyl MK, Brill RW (2003) Horizontal movements of big-eye tuna (Thunnus obesus) near Hawaii determined by Kalman filter analysis of archival tagging data. Fish Oceanogr 12:141–151
Skulason S, Smith TB (1996) The ecology of resource polymorphism in vertebrates—reply. Trends Ecol Evol 11:25–26
Slip DJ (1997) Diving and foraging behaviour of juvenile southern elephant seals from Heard Island. In: Hindell M, Kemper C (eds) Marine mammal research in the Southern Hemisphere, vol 1: status, ecology and medicine. Beatty, Chipping Norton, pp 114–124
Slip DJ, Hindell MA, Burton HR (1994) Diving behaviour of southern elephant seals from Macquarie Island. In: Le Boeuf BJ, Laws RM (eds) Elephant seals: population ecology, behaviour, and physiology. University of California Press, Berkeley, pp 253–270
Spina AP (2000) Habitat partitioning in patchy environment: considering the role of intraspecific competition. Environ Biol Fish 57:393–400
Stevick PT, Allen J, Berube M, Clapham PJ, Katona SK, Larsen F, Lien J, Mattila DK, Palsboll PJ, Robbins J, Sigurjonsson J, Smith TD, Oien N, Hammond PS (2003) Segregation of migration by feeding ground origin in North Atlantic humpback whales (Megaptera novaeangliae). J Zool 259:231–237
Stewart BS (1997) Ontogeny of differential migration and sexual segregation in northern elephant seals. J Mamm 78:1101–1116
Thompson PM, Mackay A, Tollit DJ, Enderby S, Hammond PS (1998) The influence of body size and sex on the characteristics of harbour seal foraging trips. Can J Zool 76:1044–1053
Warren PH (1996) Structural constraints on food web assembly. In: Hochberg ME, Clobert J, Barbault R (eds) Aspects of the genesis and maintenance of biological diversity. Oxford University Press, Oxford, pp 142–161
Wheatley KE (2001) Behavioural and physiological aspects of the mid-year haul-out in southern elephant seals (Mirounga leonina) on Macquarie Island. MPhil Dissertation, University of St. Andrews
Whitehead H, MacLeod CD, Rodhouse P (2003) Differences in niche breadth among some teuthivorous mesopelagic marine mammals. Mar Mamm Sci 19:400–405
Wikelski M, Wrege PH (2000) Niche expansion, body size and survival in Galápagos marine iguanas. Oecologia 124:107–115
Williams RJ, Martinez ND (2000) Simple rules yield complex food web. Nature 404:180–183
Woodward G, Hildrew AG (2002) Body-size determinants of niche overlap and intraguild predation within a complex food web. Ecology 71:1063–1074
Acknowledgements
We thank members of the 51st–53rd Australian National Antarctic Research Expeditions (ANARE) to Macquarie Island for their assistance during fieldwork and the Australian Antarctic Division for logistic support, especially J. van den Hoff. M. Biuw, C. McMahon, J. van den Hoff, K. Wheatley and two anonymous reviewers provided useful comments on the manuscript. Data were collected with Australian Antarctic Animal Ethics Committee approval and Tasmanian Parks and Wildlife Service permits. Funding was provided by the Antarctic Science Advisory Committee and Sea World Research and Rescue Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Field, I.C., Bradshaw, C.J.A., Burton, H.R. et al. Resource partitioning through oceanic segregation of foraging juvenile southern elephant seals (Mirounga leonina). Oecologia 142, 127–135 (2005). https://doi.org/10.1007/s00442-004-1704-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1704-2