Abstract
Calanoid copepods typically exhibit escape reactions to hydrodynamic stimuli such as those generated by the approach of a predator. During the summers of 2000, 2001 and 2004, two small calanoid species, Temora turbinata Dana, 1849 and Paracalanus parvus Claus, 1863 were exposed to a visual predatory fish, the blenny Acanthemblemaria spinosa Metzelaar, 1919, and their predator–prey interactions were recorded using both high-speed and standard videographic techniques. Copepod escape reaction components, including swimming pattern, reactive distance, turning rate, and jump kinetics, were quantified from individual predation events using motion analysis techniques. Among the observed escape reaction components, differences were noted between the species’ swimming patterns prior to attack and their response latencies. Temora turbinata was a continuous cruiser and P. parvus exhibited a hop-and-sink swimming pattern. During periods of sinking, P. parvus stopped beating its appendages, which presumably reduced any self-generated hydrodynamic signals and increased perceptual abilities to detect an approaching predator. Response latency was determined for each copepod species using a hydrodynamic stimulus produced by a 1 ms acoustic signal. Response latencies of T. turbinata were significantly longer than those of P. parvus. Despite some apparent perceptual advantages of P. parvus, the blenny successfully captured both species by modifying its attack behavior for the targeted prey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To evade the immediate threat of an approaching predator, calanoid copepods typically exhibit an escape reaction. This reaction results from the integration of several sensory and behavioral characteristics: (1) degree of sensitivity to stimuli (Fields and Yen 1997; Lenz and Hartline 1999), (2) latency of response (time between onset of the stimulus and initiation of the reaction) (Lenz et al. 2000; Buskey et al. 2002; Buskey and Hartline 2003), and (3) movement kinetics of the response, (i.e., escape speed, and acceleration, direction, and distance traveled) (Buskey et al. 2002; Buskey and Hartline 2003). Variations of these sensory and behavioral traits among copepod species may contribute to differences in escape performance. Furthermore, the importance of individual escape reaction components will vary with the type of predator.
Copepods exhibit various swimming patterns ranging from continuous to intermittent locomotion. Copepods that maintain a nearly constant frontward motion have been termed ‘continuous cruisers.’ Intermittent swimmers can be classified as either ‘hop-and-sink’ swimmers, taking brief forward jumps followed by a short period of sinking when appendage motion ceases, or ‘cruise-and-sink’ swimmers, characterized by longer periods of forward swimming followed by brief sinking phases (Bainbridge 1952). The pausing of motion during intermittent swimming is believed to increase the perceptual abilities of the copepod by reducing any self-generated hydrodynamic noise (O’Brien et al. 1989; Bundy and Paffenhöfer 1996; Yen 2000; Kramer and McLaughlin 2001).
Many vertebrate predators, such as fishes, rely on copepods as a major food source. Fishes possess many sophisticated sensory systems including vision, hearing, and a lateral line. The spinyhead blenny, Acanthemblemaria spinosa, has been referred to as a hemisessile organism, living within crevices of coral skeletons (Kotrschal and Lindquist 1986; Clarke 1992). Because the blenny visually detects its prey, the color, size, and motion of the prey item should significantly affect the prey’s chance of survival. Small, nearly transparent organisms are more difficult to see; however, an irregular or intermittent swimming pattern can counteract this advantage (Zaret 1980; Wright and O’Brien 1982; Peterson and Ausubel 1984; Buskey et al. 1993).
A copepod’s success in escaping from visual vertebrate predators depends on its ability to detect the attacking predator’s approach and to respond quickly and effectively. The present study was designed to evaluate copepod escape performance in response to the blenny Acantheblemaria spinosa and to determine the relative importance of particular escape reaction components in response to a visual, vertebrate predator. As a member of the superfamily Megacalanoidea, Paracalanus parvus is believed to possess myelinated sensory and motor axons which would act to increase the conduction speed of nerve impulses (Ritchie 1984). Temora turbinata belongs to the superfamily Centropagoidea, and although this species has not been examined specifically, Lenz et al. (2000) found the congener T. longicornis has non-myelinated axons. Thus, we predicted that the non-myelinated T. turbinata would have lower escape success from the blenny, since they should have longer response latencies.
Materials and methods
Fish and copepod collection and care
Two calanoid copepod species were chosen from the same habitat, indicating they were acclimated to the same ambient hydrodynamic regime. Individuals of both species, Temora turbinata Dana, 1849 and Paracalanus parvus Claus, 1863, are relatively small with average prosome lengths < 1 mm. The hop-and-sink swimming pattern of P. parvus may provide a perceptual advantage over the continuous cruiser, T. turbinata.
During the summers of 2000, 2001 and 2004, copepods were collected by towing a 0.5 m diameter, 153 μm mesh net from The University of Texas Marine Science Institute pier located on the Aransas Ship Channel (27° 50.3′N; 97° 3.1′W). The net was held within the stream of an incoming or outgoing tide for 5–10 min, depending on current velocity. Contents of the cod end were diluted with unfiltered sea water and returned to the laboratory for live sorting. Under a dissecting microscope, adult Temora turbinata and Paracalanus parvus were isolated using a Pasteur pipette. The species were kept separately in small glass beakers containing 0.2 μm filtered sea water and were used within 1 h of sorting.
The spinyhead blenny, Acanthemblemaria spinosa Metzelaar, 1919 (Osteichthyes: Blenniidae) was collected from Teague Bay Reef on the northeast coast of St Croix, US Virgin Islands (17° 48′N; 64° 53′W) in the summers of 2000 and 2001, and from the northwest corner of Glover’s Reef (16° 99.5′N; 87° 47.5′W) in Belize in the summer of 2004. Collection involved anesthetizing fish with approximately 1 ml of 0.1% quinaldine sulfate and capturing them within test tubes. Immediately following capture, fish were transferred to aerated jars to expedite recovery from the anesthetic. They were kept for up to 1 week in aerated plastic basins before being transported to The Marine Science Institute. The fish were split into groups of three or four and maintained within a flow-through system consisting of 8.3 l aquaria with continuously flowing filtered sea water pumped from the Aransas Ship Channel. Water temperature ranged from 25–28°C. Fish were exposed to a diel 12 h light:12 h dark cycle and were fed newly hatched Artemia salina nauplii twice a day. Their diet was supplemented with natural zooplankton assemblages daily. Fish were provided shelters crafted from Sculpey® polymer clay which were equivalent in size to 1/8th of a sphere with a 4 cm radius. These tube-dwelling blennies were 20–25 mm in total length and they resided within cylindrical holes (21 mm radius, 25 mm long) at the center of each shelter’s curved face. The hemisessile nature of the spinyhead blenny is amenable to predator–prey studies. The blenny remains within its shelter, darting out to attack prey using both suction and ram-feeding (Clarke 1992; Clarke et al. 2005).
Copepod swimming patterns in the absence of a predator
Eight groups of five individuals were sorted per copepod species. One group of Temora turbinata was gently added to a 2.3 × 8.0 × 4.0 cm clear acrylic plastic chamber filled with 0.2 μm filtered sea water. After a 10 min acclimation period, copepod routine swimming behaviors were recorded for 2 min at 30 frames s−1 (FPS) using a Cohu video camera (model 3315) equipped with a Nikon Nikkor 55 mm lens. The camera was positioned to look through a 2.3 cm slice of the water. Illumination of this slice was provided by a fiber optic light above the chamber and a ring of infrared light-emitting diodes behind the chamber. Video observations were repeated on the remaining seven groups of T. turbinata and with all eight groups of Paracalanus parvus.
The 2 min recordings were played through a Motion Analysis VP-110 video-to-digital processor, which digitized the outlines of the observed copepods and sent the information to a computer at a filtered sampling rate of 15 FPS. The video-computer motion analysis system ExpertVision Cell-Trak processed the digitized images and calculated the swimming speed (mm s−1) of each of the observed swimming paths. The mean swimming speed of each 2 min observation period was calculated. A grand mean was calculated for each species by averaging the means of the eight groups of copepods.
Hydrodynamic stimuli
A hydrodynamic stimulus was produced through the use of an acoustic signal. A Philips PM 5712 pulse generator produced a single sine wave pulse of 1 ms duration. The pulse was amplified by an RCA SA-155 integrated stereo amplifier, which allowed adjustment of the signal’s amplitude before being sent to an 8.9 cm dual cone audio speaker. The experimental chamber, rested on a sheet of Plexiglas® placed on top of the speaker cone. The 1 ms acoustic pulse translated into the vibration of the experimental chamber and its contents. High-speed video recording at 1000 FPS was performed using a Redlake MotionMeter® model 1140. The pulse of the signal generator triggered the camera and marked the onset of the stimulus. Response latency (ms), the time between the onset of the stimulus and the initiation of the escape reaction, was determined for each copepod species through slow motion playback of these high-speed recordings.
Predator–prey interactions
Predator–prey interactions were recorded using both standard and high-speed videographic techniques. A Cohu camera (model 3315) with a Nikon Nikkor 55 mm lens was used for standard video recording. High-speed video was recorded at 1000 FPS using the Kodak Motion Corder Analyzer SR-3000. The high-speed recordings were then played back at 30 FPS and recorded using a Panasonic AG1960 videocassette recorder. Blennies were transferred within their shelters to a 10.0 × 10.0 × 10.0 cm clear acrylic plastic container filled with approximately 800 ml of 0.2 μm filtered sea water for standard video recordings or to a 7.1 × 3.3 × 6.1 cm container for high-speed video recordings.
For both standard and high-speed video experiments, blennies were starved 20–24 h prior to experimentation. They were allowed 10 min to acclimate before a group of approximately 100 copepods of a single species was added. Predator–prey interactions were recorded for up to 30 min. Approximately ten interactions were evaluated per replicate to reduce the probability of analyzing the re-attack of the same copepod. Ten replicates were performed per species, using ten different blennies (one trial with each species for each blenny). Experiments involving the same blenny were spaced several days apart.
A fiber optic light centered above the experimental chamber provided illumination for all experiments. Additionally, the shortened exposure time of the high-speed recordings required the use of a ring of infrared light-emitting diodes to provide adequate dark-field illumination to capture the images. A ruler was videotaped at the end of each trial to calibrate distances.
Video analysis
Standard video recordings were reviewed using slow-motion playback and frame-by-frame analysis. Information regarding predation events was gathered and categorized according to a modified version of Holling’s (1959) components of predation model, which can be chronologically summarized as: encounter → approach→ attack → capture → ingestion. During an encounter, the blenny or the copepod perceives the presence of the other as indicated by direct contact or the initiation of an attack or escape motion. Movement of the blenny towards the copepod was termed the approach. An attack occurred when the blenny combined this forward motion with the opening of its mouth and initiation of suction feeding. Copepods exhibited escape reactions to approaches and to attacks.
Several components associated with each species’ escape behavior and detection abilities were examined, including swimming pattern, reactive distance, and jump kinetics. Copepod swimming behavior in the frames immediately prior to a predation event was classified as one of the following: (1) slow forward cruising (produced through the beating of feeding appendages); (2) irregular, fast forward cruising or an escape jump; and (3) sinking (no beating of appendages).
Once an escape reaction is initiated, its kinetics (direction, speed, acceleration, and distance) may influence escape success. To analyze the kinetics, high-speed video recordings of individual escape jumps were played through the Motion Analysis VP-110 video-to-digital processor. Digitized images were processed using the ExpertVision Cell-Trak system. For each of the observed escape reactions, swimming path, speed (mm s−1), acceleration (m s−2), and number of thrusts per jump were calculated.
A second computerized image analysis system, Nikon Metavue®, was used to quantify the speed of the blennies as they approached and attacked copepods. Video segments were converted to digital format, and blenny approach and attack speeds were calculated by tracking a point on the blenny’s upper lip through each video frame. This system was also used to measure copepod reactive distance (mm) from the blenny and escape jump distance (mm) from the standard video recordings. These values may underestimate the distance traveled throughout an escape reaction since copepods often jumped out of the recorded field of view and their escape jumps were rarely perpendicular to the camera. In a few cases, the position of the blenny or the direction of the escape jump made it impossible to quantify copepod reactive distance or jump distance, respectively.
Statistical analysis
Statistical analyses were computed by Systat software (v. 11). Interspecific comparisons of mean swimming speeds of the copepods, reactive distance to the blenny, response latency to the near-field hydrodynamic stimulus, and blenny attack speed were made using a Student’s t test. Statistical analyses were stratified by blenny to control for variation among individual predators. Swimming pattern within a species was analyzed using Pearson chi-square tests.
Interspecific comparisons of escape reaction kinetics in response to the blenny were performed using multivariate analysis of variance (MANOVA), and the same was done for the escape reaction kinetics in response to the near-field hydrodynamic stimulus. A two-way MANOVA was run to compare the kinetics of each species’ escape reactions between the blenny and the near-field hydrodynamic stimulus.
Results
Copepod swimming patterns
Temora turbinata displayed the swimming pattern of a continuous cruiser while, Paracalanus parvus demonstrated a typical hop-and-sink swimming pattern (Fig. 1). Mean (±SE) swimming speeds of T. turbinata, 1.81 ± 0.19 mm s−1, and P. parvus, 1.83 ± 0.17 mm s−1, were not significantly different (Student’s t test, P = 0.92). However, the mean (±SE) swimming speed of T. turbinata was significantly different than the mean speed of P. parvus during ‘hops’, 3.26 ± 0.37 mm s−1 (Student’s t test, P = 0.005), and mean sinking speed 0.99 ± 0.11 mm s−1 (Student’s t test, P = 0.004).
Predator–prey interactions
Over 100 interactions were observed between blennies and each copepod species (Temora turbinata, n = 158; Paracalanus parvus, n = 138). No difference between species was found in escape success and 79.8 and 78.3% of encounters resulted in capture for T. turbinata and P. parvus, respectively (Mantel-Haenzel Chi-Square Test, P = 0.86).
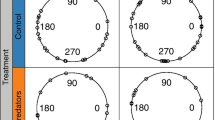
Temora turbinata and Paracalanus parvus display characteristic swimming patterns of a continuous cruiser and a hop-and-sink swimmer, respectively. Temora turbinata displayed similar swimming patterns prior to both captures and escapes (Fig. 2a; Pearson Chi-Square Test, P = 0.23). The majority of all interactions were preceded by slow swimming (Fig. 2a). In contrast, P. parvus escaped significantly more often when passively sinking prior to attack (Fig. 2b; Pearson Chi-Square Test, P ≤ 0.0005).
There was no significant difference in reactive distance between the species (Fig. 3; Student’s t test, P = 0.70) and no relationship was found between reactive distance and the escape speed of the associated jump (Pearson correlation, r = −0.25). The successful escape of Paracalanus parvus was usually preceded by passive sinking. The average reactive distance of P. parvus during interactions preceded by periods of passive sinking (1.85 mm, n = 17) was slightly longer than when preceded by active swimming behavior (1.29 mm, n = 4; Student’s t test, P = 0.056). Temora turbinata displayed significantly longer jump distances than P. parvus (Fig. 4; Mann–Whitney rank sum test, P = 0.045). Swimming behavior of P. parvus did not affect jump distance (Student’s t test, P = 0.37).
Blenny approach speed was examined over 20 ms prior to either capture or escape events. Approach speeds prior to capture and escape events (n = 21 for both) showed little variation until the blenny was within 8 ms of the attack (Fig. 5). Blennies that successfully captured the copepod rapidly accelerated from a mean speed (±SE) of 28.9 ± 3.1 mm s−1 to 173.3 ± 13.6 mm s−1. Unsuccessful attacks resulted when the blenny maintained a constant approach speed of approximately 28 mm s−1. Blenny approach speed was not correlated with reactive distance (r = 0.37 for Temora turbinata, r = 0.40 for Paracalanus parvus) or the jump distance (r = 0.09 for T. turbinata, r = 0.30 for P. parvus). The blenny exhibited significantly slower mean (±SE) speeds when successfully capturing T. turbinata (130.83 ± 9.56 mm s−1) versus P. parvus (225.32 ± 13.66 mm s−1) (Fig. 6; Student’s t test, P < 0.0005).
The kinetics of successful escape reactions did not differ between species (Table 1). During escape reactions, Temora turbinata and Paracalanus parvus attained maximum speeds exceeding 850 and 700 mm s−1, respectively and accelerations exceeding 300 m s−2 for both species (Table 1).
Hydrodynamic stimuli
The hydrodynamic stimulus elicited escape reactions by both copepod species. Response latencies for Temora turbinata were highly variable and ranged between 3 and 50 ms (Fig. 7a). The majority of escape reactions (80%) occurred within 30 ms of stimulus onset. Mean response latency for Paracalanus parvus was 3.32 ± 0.19 ms (Fig. 7b).
Differences between species were also noted for the escape reactions elicited by the hydrodynamic stimulus. Temora turbinata displayed significantly lower maximum escape speeds, average escape speeds, and maximum accelerations than P. parvus (Table 2). In fact, values recorded for all measures of jump kinetics in response to the hydrodynamic stimulus were significantly lower than those recorded in response to the blenny (Table 3).
Discussion
Paracalanus parvus exhibits a hop-and-sink pattern of swimming while Temora turbinata displays a continuously cruising pattern. Paracalanus parvus is captured significantly more often during bouts of swimming, and escapes significantly more often when attacked during periods of sinking. The intermittent locomotion of P. parvus appears to promote detection of hydrodynamic stimuli, providing them with a slight perceptual advantage over the continuously swimming T. turbinata (Fig. 2b). During successful escape reactions from the blenny, Temora turbinata and Paracalanus parvus achieved similar escape speeds, accelerations, and jump distances.
Prey swimming behavior affects their conspicuousness to visual predators (Zaret 1980; Wright and O’Brien 1982). Copepod swimming behavior ranges between continuous and intermittent locomotion. While irregular swimming patterns may increase an organism’s probability of being detected by planktivorous fish (Buskey et al. 1993), short pauses within a smooth pattern of motion may provide prey with brief moments of invisibility to predators (Kramer and McLaughlin 2001). Pauses in copepod propulsion also result in sinking, which decrease the amount of self-generated hydromechanical noise and enhance their perceptual abilities (Fig. 2b; Yen 2000; Kramer and McLaughlin 2001).
Although characteristically longer response latencies were measured for Temora turbinata, the short latencies of Paracalanus parvus did not enhance their escape success during predator–prey interactions. These latencies were measured in response to a hydrodynamic stimulus that produced flow disturbances varying both spatially and temporally from those created by an approaching blenny. Within the 8 ms prior to a successful attack, the blenny rapidly accelerated from speeds of only 21 to 176 mm s−1. If P. parvus has a similar response latency (ca. 3 ms) to both the hydrodynamic stimulus and the approaching blenny, then P. parvus would have ample time to detect and respond to the velocity gradients created by the approaching blenny. Temora turbinata may have the capacity to detect an approaching blenny, but had response latencies > 10 ms to the hydrodynamic stimulus, thereby reducing their ability to elicit an escape reaction before being caught. This may explain why blennies adopted slower attack speeds during interactions with T. turbinata. Faster attack speeds were required to capture P. parvus.
Escape performance of both species was significantly reduced when exposed to the hydrodynamic stimulus further demonstrating the differences in the flow disturbances created by the hydrodynamic stimulus and the blenny. Significantly different jump kinetics were measured for Temora turbinata and Paracalanus parvus. Paracalanus parvus exhibited greater escape speeds and accelerations than T. turbinata. However, in response to the blenny attack, no differences were noted between the two species’ jump kinetics. This suggests that blennies present a different stimulus to each species, perhaps a reduced hydrodynamic stimulus when attacking prey with greater perceptual abilities or shorter response latencies.
Additionally, copepod species appear to modify their escape behavior in relation to the stimulus strength they encounter. A discrepancy between copepod escape velocities was found for both Temora turbinata and Paracalanus parvus in response to the hydrodynamic stimulus and the blenny. Similarly, Suchman (2000) observed faster escape velocities by Acartia hudsonica in response to Cyanea sp. than responding to Aurelia aurita, thus indicating that Cyanea sp. provided a stronger stimulus than A. aurita. Viitasalo et al. (1998) compared the escape behavior of two calanoids, Eurytemora affinis and Temora longicornis, to three hydrodynamic stimuli: (1) mysid shrimps, (2) juvenile sticklebacks (fish), and (3) an artificial flow field (siphon-generated flow field). Eurytemora affinis reacted at longer distances from mysid attacks than from sticklebacks and had higher escape speeds in response to mysid encounters. Both species displayed lower escape velocities to the artificial flow field and their reaction distances were greater than those prior to sticklebacks (Viitasalo et al. 1998).
To fully understand the process of predation, it is necessary to consider predator–prey interactions from the perspective of both the predator and the prey. Just as we analyze predation events along certain ‘components of predation,’ we must examine the individual components comprising an organism’s escape behavior. Focusing on a single component, e.g., escape speed or reaction distance, may provide only a partial picture of the predator–prey relationship. The perceptual and behavioral components measured here suggested that both the increased sensitivity to hydrodynamic stimuli resulting from a hop-and-sink swimming pattern and the short (3.32 ms) response latency of Paracalanus parvus enhanced its escape success from a visual, vertebrate predator. However, the blenny compensated for these adaptations of the copepods resulting in equal success in capturing both copepod species equally.
References
Bainbridge R (1952) Underwater observations on the swimming of marine zooplankton. J Mar Biol Ass U K 31:107–112
Bundy MH, Paffenhöfer GA (1996) Analysis of flow fields associated with freely swimming calanoid copepods. Mar Ecol Prog Ser 133:99–113
Buskey EJ, Hartline DK (2003) High speed video analysis of the escape responses of the copepod Acartia tonsa to shadows. Bio Bull 204:28–37
Buskey EJ, Coulter C, Strom S (1993) Locomotory patterns of microzooplankton: potential effects on food selectivity of larval fish. Bull Mar Sci 53:29–43
Buskey EJ, Lenz PH, Hartline DK (2002) Escape behavior of planktonic copepods in response to hydrodynamic disturbances: high speed video analysis. Mar Ecol Prog Ser 235:135–146
Clarke RD (1992) Effects of microhabitat and metabolic rate on food intake, growth and fecundity of two competing coral reef fishes. Coral Reefs 1:199–205
Clarke RD, Buskey EJ, Marsden KC (2005) Effects of water motion and prey behavior on zooplankton capture by two coral reef fishes. Mar Biol 146:1145–1155
Fields DM, Yen J (1997) The escape behavior of marine copepods in response to a quantifiable fluid disturbance. J Plankton Res 19:1289–1304
Holling CS (1959) The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91:293–320
Kotrschal K, Lindquist DG (1986) The feeding apparatus of four Pacific tube blennies (Teleostei: Chaenopsidae): lack of ecomorphological divergence in syntopic species. Mar Ecol 7:241–253
Kramer DL, McLaughlin RL (2001) The behavioral ecology of intermittent locomotion. Am Zool 41:137–153
Lenz PH, Hartline DK (1999) Reaction times and force production during escape behavior of a calanoid copepod Undinula vulgaris. Mar Bio 133:249–258
Lenz PH, Hartline DK, Davis A (2000) The need for speed. I. Fast reactions and myelinated axons in copepods. J Comp Physiol A Sens Neural Behav Physiol 186:337–345
O’Brien WJ, Evans BI, Browman HI (1989) Flexible search tactics and efficient foraging in saltatory searching animals. Oecologia 80:100–110
Peterson WT, Ausubel SJ (1984) Diets and selective feeding by larvae of Atlantic mackerel Scomber scombrus on zooplankton. Mar Ecol Prog Ser 17:65–75
Ritchie JM (1984) Physiological basis of conduction in myelinated nerve fibers. In: Morrell P (ed) Myelin. Plenum Press, New York, pp 117–145
Suchman C (2000) Escape behavior of Acartia hudsonica copepods during interactions with scyphomedusae. J Plankton Res 22:2307–2323
Viitasalo M, Kiørboe T, Flinkman J, Pedersen L, Visser A (1998) Predation vulnerability of planktonic copepods: consequences of predator foraging strategies and prey sensory abilities. Mar Ecol Prog Ser 175:129–142
Wright DI, O’Brien WJ (1982) Differential location of Chaoborus larvae and Daphnia by fish: the importance of motion and visible size. Am Midl Nat 108:68–73
Yen J (2000) Life in transition: balancing inertial and viscous forces by planktonic copepods. Bio Bull 198:213–224
Zaret TM (1980) The effect of prey motion on planktivore choice. In: Kerfoot WC (ed) Evolution and ecology of zooplankton communities. University Press of New England, Hanover, pp 594–603
Acknowledgments
Funding for this research was provided by the National Science Foundation through Grant OCE-9910608 and The University of Texas Marine Science Institute Lund Fellowship. We thank R.D. Clarke for collecting the blennies and his assistance in their care and maintenance. We are grateful to P.H. Lenz and D.K. Hartline who shared both their advice and knowledge regarding sensory abilities of calanoid copepods. Technical assistance was provided by C. Hyatt. Guidelines of The University of Texas Institutional Animal Care and Use Committee were followed in the use of the blennies. This is The University of Texas Marine Science Institute contribution number 1418.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Waggett, R.J., Buskey, E.J. Calanoid copepod escape behavior in response to a visual predator. Mar Biol 150, 599–607 (2007). https://doi.org/10.1007/s00227-006-0384-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0384-3