Abstract
Water motion is an important factor affecting planktivory on coral reefs. The feeding behavior of two species of tube-dwelling coral reef fish (Chaenopsidae) was studied in still and turbulent water. One species of blenny, Acanthemblemaria spinosa , lives in holes higher above the reef surface and feeds mainly on calanoid copepods, while a second, A. aspera , lives closer to the reef surface, feeds mainly on harpacticoid copepods, and is exposed to less water motion than the first. In the laboratory, these two blenny species were video recorded attacking a calanoid copepod ( Acartia tonsa, evasive prey) and an anostracan branchiopod (nauplii of Artemia sp., passive prey). Whereas A. spinosa attacked with the same vigor in still and turbulent water, A. aspera modulated its attack with a more deliberate strike under still conditions than turbulent conditions. For both fish species combined, mean capture success when feeding on Artemia sp. was 100% in still water and dropped to 78% in turbulent water. In contrast, when feeding on Acartia tonsa, mean capture success was 21% in still water and rose to 56% in turbulent water. We hypothesize that, although turbulence reduces capture success by adding erratic movement to Artemia sp. (passive prey), it increases capture success of Acartia tonsa (evasive prey) by interfering with the hydrodynamic sensing of the approaching predator. These opposite effects of water motion increase the complexity of the predator-prey relationship as water motion varies spatially and temporally on structurally complex coral reefs. Some observations were consistent with A. aspera living in a lower energy benthic boundary layer as compared with A. spinosa: slower initial approach to prey, attack speeds modulated according to water velocity, and lower proportion of approaches that result in strikes in turbulent water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs harbor a diversity of planktivores ranging from sessile benthic corals to active nektonic fishes. Whereas the sessile organisms must passively capture plankton as it is delivered to them by water movement (Sebens et al. 1998; Gardella and Edmunds 2001), most planktivorous coral reef fishes are able to seek out plankton where it is most concentrated (Jones 1987; Hamner et al. 1988) and actively capture one to several individuals at a time. Sessile organisms are subject to local variations in water motion, which may be reduced in the benthic boundary layer (Shashar et al. 1996) or increased by the channeling effect of reef structures. Adult fishes, while generally less affected by water motion, have to contend with active anti-predator behaviors of their zooplankton prey (Drenner et al. 1978; Kiørboe and Visser 1999). Although less obvious, antipredator behaviors are also operational for prey of sessile benthic feeders (Trager et al. 1994) and water motion will have an effect on prey capture by fishes (Hobson 1991).
The chaenopsid blennies of the genus Acanthemblemaria comprise 20 described species occurring in the western Atlantic and eastern Pacific (Almany and Baldwin 1996; Williams 2003). A. spinosa and A. aspera are the two most widespread species in the western Atlantic (Smith-Vaniz and Palacio 1974). They are small fish, generally 20–25 mm in standard length, which reside permanently in close-fitting cavities in coral. They spend most of their time scanning their surroundings for small crustaceans which they capture by darting rapidly from their holes. A. spinosa generally occupies shelters in corals 0.5–1.5 m above the reef surface and feed primarily on calanoid copepods (planktonic, elusive prey) whereas A. aspera occupies shelters 0–0.3 m above the reef surface and feed primarily on harpacticoid copepods (benthic, non-evasive prey; Clarke 1989, 1996, 1999). A. spinosa has a standard metabolic rate 1.55 times higher than that of A. aspera (Clarke 1999); this higher metabolism may make them better adapted for active pursuit of evasive, planktonic prey.
Several behavioral adaptations of zooplankton affect their interactions with potential predators, including avoidance and escape responses (reviewed in Ohman 1988). Once encountered by a predator, zooplankton species and their various developmental stages can differ considerably in their escape capabilities (Singarajah 1969, 1975; Landry 1978). Calanoid copepods have some of the most vigorous escape responses found in the aquatic environment, with initial accelerations of over 200 m s−2 and reaching escape speeds of over 500 body lengths s−1 (Buskey et al. 2002). They respond to small hydrodynamic disturbances in the water (Fields and Yen 1997) with rapid responses within a few milliseconds of stimulation (Lenz and Hartline 1999). Not all zooplankton species possess active escape behaviors comparable to those of calanoid copepods; for example many cladocerans use a passive sinking response for evading capture (Kerfoot et al., 1980). The nauplii of the brine shrimp Artemia sp. swim continuously but have no active escape responses to predators (Buskey et al. 1993, unpublished observations).
In addition to delivering plankton, water motion, particularly turbulence, may influence capture success. The exact impact of turbulence on capture success is difficult to predict because it has two opposite effects: (1) by creating erratic movement of prey particles, a fish is more likely to abort a pursuit or miss during a strike (MacKenzie and Kiørboe 2000) and (2) by generating variable water movements, turbulence may mask the signals that prey use to avoid capture (e.g., reaction distances of Acartia tonsa to a standard stimulus is greater in still than in turbulent water, O. Gilbert and E. Buskey, unpublished data). The significance of these effects is likely to be different for passive and evasive prey. In this paper, we investigate the interaction of water motion and prey behavior on capture success of a pair of fishes that live in different microhabitats and dwell in cavities, but actively capture their prey.
Materials and methods
The organisms
In the summers of 2000 and 2001, 32 Acanthemblemaria spinosa and 32 A. aspera were collected on Tague Bay Reef on the northeast coast of St. Croix, U.S. Virgin Islands (17°48′N, 64°53′W). All fish were mature, with equal numbers of each sex and both species in the same size range (21–25 mm SL). Each fish was captured by delivering ca. 1 ml of 0.1% quinaldine sulfate to the mouth of its shelter that was immediately covered with a 15 mm diameter test tube. After leaving the shelter and becoming tranquilized in the test tube, the fish was immediately placed in a ventilated jar, minimizing its exposure to the anesthetic. The fish were maintained for a maximum of 1 week in plastic basins with aeration and water changes every second day. The fish were placed in groups of four in 13×26 cm polyethylene bags containing ca. 100 ml water and 600 ml of air. They were transported to the laboratory in Texas by air in an insulated container as hand-carried luggage. They were subsequently maintained for 3 weeks in groups of four in 19 l aquaria with flowing seawater. They were fed Artemia sp. nauplii several times a day. The fish were provided with shelters constructed from Sculpey polymer clay, a plastic material that hardens with heating. Each shelter was an eighth sphere with a 4 cm radius. A cylindrical cavity 4.2 mm in diameter and 25 mm deep was located at the center of the curved face.
Copepods were captured with a 0.5 m diameter plankton net (153 µm mesh) deployed during mid-ebb tide from the University of Texas Marine Science Institute pier in the Aransas Ship Channel, on the coast of Texas (27°50′N, 97°03′W). They were diluted in whole seawater and maintained in a plastic bucket of mixed plankton with aeration and used within 18 h of capture. Adult females of the calanoid copepod Acartia tonsa were individually picked out from the mixed plankton after being lightly anesthetized with MS−222 for the first year experiments (feeding volume) and without anesthetic for the second year experiments (capture success, effects of turbulence). Brine shrimp, Artemia sp., were hatched in 1:1 diluted seawater from commercially packaged cysts and used within 12 h of hatching. The Acartia tonsa were translucent, ca. 840 µm long, and exhibited strong predator avoidance behavior (Buskey et al. 2002) whereas the Artemia sp. were opaque, ca. 600 µm long, and showed no response to predators.
Blennies were not fed on the days they were used for experiments. For all experiments, the fish were moved into the test chambers with their shelters thus minimizing the disruption. They exhibited normal behavior, including feeding, immediately on placement in the test chambers. They were given an adjustment period of 10 min. before being fed and their behavior videotaped. We carried out three different tests: feeding volumes, to determine if the two fish species were responding to prey at the same distances and directions; capture success, to determine how water motion and prey escape behavior affected prey capture for the two fish species; and attack speeds, to determine if different attack strategies explain differences in capture success of the two fish species.
Feeding volumes
We placed individual fish with their shelters in a 15×15×10 cm glass chamber with a mirror placed at 45° resting on the rear upper edge; light was provided by two incandescent bulbs at an intensity of 47.6 µmol photons m−2 sec−1. After a 10 min adjustment period, the fish were videotaped for 20 min as they fed on Artemia sp. nauplii. The recorded field included the mirror above the chamber. Plastic transparencies were taped to a video monitor and the maximum distance of each attack was marked both in the direct image and the one in the mirror. We used a coordinate system in which x and y are the horizontal axes and z is the vertical axis. The distances from the shelter holes were then measured in the x and z axes from the direct images and the y -axis from the mirror image. A grid placed on the back and bottom of the chamber was used to determine scale factors for the direct and mirror images.
The available volumes for feeding differed with geometric quadrant because the shelters occupied varying amounts of the quadrant volume. The available volumes were calculated by assuming that feeding occurred in a sphere including 95% of the strikes (2.8 cm radius, see Results), subtracting from that the intersecting volume of the shelter (an eighth sphere of 4 cm radius with different center, Fig. 2) and determining the remaining free space in each of eight octants. For each x – z segment, the two mirror-image octants along the y -axis were combined, providing quadrants for further analysis.
Water motion
To determine the effects of water motion on attack speeds and capture success of the blennies, we used a 6×14×6 cm clear acrylic chamber and placed a 6×3.5×6 cm submersible water pump at one end (Aquarium Systems MN404). The 1.3 l min−1 output was directed through plastic tubing and split in two. The two laminar streams were directed along the sides of the chamber towards the opposite end where they were deflected back towards the center, interacting to create turbulent water movements (Fig. 1) that were in the range that occurs on reefs (see Discussion). Plankton netting (153 µm mesh) was placed in the path of the return flow to prevent the prey from passing through the pump. Each blenny to be tested was placed in its shelter facing the end wall from which the water was deflected thus experiencing turbulence in its feeding volume directly in front of the shelter opening. We also determined feeding success in still conditions in the same chamber with the pump turned off.
Top view of turbulence chamber with representative water flows in “freeze frame”. Flow characteristics are based on video recording of hydrated Artemia sp. cysts. Outflow of submersible pump is directed through tubing down to a T divider and to the sides of the chamber at the level of the hole in which the blenny resides. Return flow to the pump intake passes through 153 µm plankton netting
We measured the magnitude of turbulence by video-recording hydrated Artemia sp. cysts at 125 frames s−1 as they were carried by the moving water. The cysts were illuminated with a 2 mm wide red laser sheet (Lasiris Model 670–5) to limit recording to the x – z plane (Stamhuis and Videler 1995). We analyzed the images using an Expertvision Cell-Trak motion analysis system, which provided a series of x - and z -axis velocities. Using the method of Saiz (1994), we calculated the fluctuating root mean square velocity, the “temporal velocity autocorrelation function”, and from the latter, the integral scale length. From this, we calculated the energy dissipation rate.
To test the effects of exposure to turbulence on the behavior of the copepods, the swimming behavior of eight groups of 30 adult female copepods was quantified before and after exposure to the turbulence chamber without a blenny present. After being sorted from the plankton, each group of copepods was allowed to adapt for a minimum of 1 h before their spontaneous swimming behavior was videotaped for 5 min in still water under the same conditions as used in the feeding studies. The pump was then turned on for 15 min to allow the copepods to experience the turbulence created under experimental conditions, and the copepods were then given a 15-min recovery period in still water before being videotaped again for 5 min. The videotaped swimming behavior was quantified using the Expertvision Cell-Trak motion analysis system, and their swimming speeds compared before and after exposure to turbulence.
Attack speeds
The blennies were video recorded at 250 frames s−1 (Kodak Motion Corder Analyzer Model SR-3000 equipped with a 50 mm f1.4 Nikkor lens). The tanks were backlit with infrared light-emitting diodes, creating a darkfield lighting that provided high contrast of the copepod images on the video screen. The recording was then played back at 30 frames s−1 and recorded on a Panasonic AG-6300 video-cassette recorder. The attacks on prey were analyzed frame by frame by tracing the positions of the fish (and sometimes the prey) onto transparencies taped onto a video monitor. Distance between adjacent points were measured with calipers and converted to swimming speeds. To avoid spatial distortions, only attacks normal to the camera axis (in the x – z plane) were analyzed.
Capture success
Using the setup described above, the blennies were video recorded at 125 frames s−1 and the images were transferred to videotape at a rate of 30 frames s−1. For analysis, the videotapes were played back on a JVC HR-S9800U videocassette recorder with single frame advance. For each fish, the results of each of the first 30 approaches to prey were determined.
Results
Feeding volumes
A total of 578 attacks on Artemia sp. in still water by 9 A. spinosa and 9 A. aspera were recorded. The distribution of attacks was determined by dividing the feeding areas into quadrants as projected onto vertical (Fig. 2) and horizontal (Fig. 3) planes. The areas available for feeding were not the same in each quadrant because the shelters filled space within the quadrants. The feeding intensity in each quadrant was therefore expressed on a unit volume basis by dividing the number of strikes by the available volume (Table 1, see Materials and methods). To determine if the fishes attacked more frequently upwards than downwards, the ratio of the attacks in quadrants 2+3 to 1+4 were subtracted from the expected ratio (the ratio of the available volumes) and the signs of these differences were added. Neither species demonstrated a preference (+:- ratio = 5:4 for A. spinosa and 4:5 for A. aspera, P =0.50 for each, sign test). A similar analysis of forward: backward (quadrants 3+4 to 1+2) demonstrated no preference for A. aspera (5:4, P =0.50, sign test) but a clear preference for forward in A. spinosa (9:0, P =0.039, sign test). This result is consistent with a lack of difference between the species for vertical distribution of attacks (2+3:1+4 ratios, U =30, n = m =9, P =0.39, Mann-Whitney U test) and a clear difference for forward/rearward attacks (3+4:1+2 ratios, U =10, n = m =9, P =0.0056, Mann-Whitney U test). This difference is apparent in Fig. 3, where A. spinosa shows a concentration of attacks 1 cm ahead whereas A. aspera does not show any concentration.
Acanthemblemaria spinosa, A. aspera. Location of maximum extent of attacks by fish on Artemia sp. nauplii in a saggital plane. The entrance of the shelter cavity is in the center and the circle represents a radius of 2 cm around the entrance. The quarter circle centered in the lower left corner represents the average profile of the clay shelter. Quadrant numbers are indicated in A. spinosa panel
Acanthemblemaria spinosa, A. aspera. Same data as in Fig. 2 plotted in a frontal plane. The shape on the horizontal axis represents the location and size to scale of the fish; the circle represents a radius of 2 cm around the fish. Attacks for each species occurred equally on the left and right, so the distributions were “folded over”, with all A. aspera attacks shown on the right side ( lower half) and all A. spinosa attacks on the left ( upper half). Based on 238 attacks by 10 A. spinosa and 340 attacks by 10 A. aspera
The number of attacks varied from 2 to 70 per 20 min with those fish exhibiting the fewest attacks having the shortest attack distances ( d =13.3+0.13 n and d =15.4+0.14 n, where d is mean distance and n is number of attacks; r =0.72 and 0.58, P =0.018 and 0.077, for A. spinosa and A. aspera respectively). The mean distance of feeding darts varied slightly with direction of attack but A. aspera averaged greater distances than A. spinosa in all cases (Fig. 4). Overall, mean attack distance was 19.1 mm for A. spinosa and 20.8 mm for A. aspera. The 95th percentiles for attack distances were 26.1 mm for A. spinosa and 30.1 mm for A. aspera.
Water motion
Measurement of water motion in the turbulence chamber was based on 109 tracks of hydrated Artemia sp. cysts that lasted for 30 or more frames for a total of 4,378 velocity measurements. Turbulence was close to isotropic with horizontal turbulence being slightly greater than vertical turbulence (Table 2). The values for water velocity given in Table 2 are for the horizontal and vertical vectors; the true mean speed of water was 20.2 cm s−1.
There was no difference in the swimming behavior of the copepod Acartia tonsa before and after being exposed to turbulence (mean swimming speeds 0.827 and 0.868 mm sec−1 respectively, P =0.447, df =7, paired comparison t -test).
Attack speeds
Blenny prey capture behavior ranged from (1) remaining in place and engulfing food items as they moved immediately in front of their mouths to (2) rapid excursions of greater than one body length (Fig. 5a, b) with several adjustments as the prey was carried by turbulence or engaged in escape movements (Fig. 5c, d). Blennies rarely captured Acartia tonsa when the copepods initiated escape movements. Success on the part of the blennies involved approaching copepods and striking without stimulating the prey to perform escape behaviors.
Acanthemblemaria spinosa, A. aspera. Traces from video monitor of blennies attacking Acartia tonsa in turbulent water. a A. aspera at maximum extension about to engulf a copepod. b Tracks of blenny and copepod in a. Large dots represent the position of the fish at 0.02 s intervals (5 frames) during its outbound movement and small dots represent the position of the copepod at the same times. Arrow represents the direction of copepod movement. c, d are similar traces for two A. spinosa in which the copepod was captured ( c) and escaped ( d). All panels are to same scale
Under the least challenging conditions, passive prey in still water, A. spinosa attacked Artemia sp. nauplii with an immediate burst of speed followed by a slight decrease in speed and finished with a rapid strike (Fig. 6b). In contrast, A. aspera advanced very slowly at first and accelerated continuously until the strike (Fig. 6d). Because the curves in Fig. 6 represent the means of many attacks, and peak speeds are achieved at varying points in the attack, the peaks in Fig. 6 are lower than the mean maximum speeds attained. A. spinosa achieved mean maximum speeds almost twice as great as A. aspera (Table 3).
Acanthemblemaria spinosa, A. aspera. Mean swimming speeds during attacks. Time is measured from the point of maximum extension, so negative times indicate movement away from the shelter and positive times indicate return to shelter. Vertical dashed lines are reference marks at −0.2 s; horizontal dashed lines are reference marks at 14 cm s−1. Sample sizes as follows (panel, n): a 6, b 19, c 7, d 14
Under more challenging conditions, attacking evasive prey ( Acartia tonsa) in turbulent water, A. spinosa reached similar speeds as when attacking passive prey ( Artemia sp.) in still water. In contrast, A. aspera reached much greater mean maximum speeds when attacking evasive prey in turbulent water as compared to passive prey in still water (Fig. 6c, d, Table 3). A. aspera had a much slower initial approach than A. spinosa, just as they did when capturing Artemia sp. in still water, but in this case they did not start sooner than A. spinosa. Both species struck at prey with much greater acceleration under turbulent conditions, especially A. aspera.
Complete attack cycles averaged 0.25 s for A. spinosa and 0.40 s for A. aspera when feeding on Artemia sp. in still water and 0.32 s and 0.24 s respectively when feeding on Acartia tonsa in turbulent water. The difference in attack duration in still water was due to the slow initial approach of A. aspera; the mean return times were the same (Table 4). The difference in attack duration in turbulent water was due to the shorter return times of A. aspera; the mean start times were the same (Table 4).
Capture success
Prey capture success rates were determined from videotapes of blennies feeding on Acartia tonsa. Predatory behavior was broken into three categories: (1) approach: fish orients and begins moving towards prey, (2) strike: fish lunges towards prey simultaneously opening the mouth, expanding the opercula, and closing the mouth (Fig. 5a), and (3) capture: prey is engulfed and disappears from the video screen. Approaches and strikes sometimes resulted in copepods initiating rapid escape responses in which case they suddenly appeared elsewhere on the screen, usually with a blurred path evident on stopped frames (at 30 frames s−1). Approaches ceased when the prey moved away but strikes seemed to be fixed action patterns that were completed even when the prey escaped.
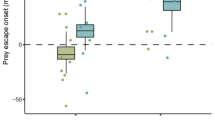
We followed the progress of attacks by each blenny species on Acartia tonsa and Artemia sp. under still and turbulent water conditions (Fig. 7). The total capture success rates (percent of approaches that result in capture) indicate that turbulence reduces success when blennies are feeding on passive prey ( t =3.86, df =10, P =0.003 and t =8.64, df =10, P <0.0001 for A. spinosa and A. aspera respectively; calculations based on arcsin square root transformations of proportions to normalize data) but increases success when they are feeding on evasive prey ( t =3.48, df =10, P =0.006 and t =2.76, df =10, P =0.02 respectively; same transformations as above). Consequently, the highest and lowest capture success rates occurred in still water (Fig. 7). Note also that A. spinosa has the higher capture success in turbulent water and A. aspera has the higher capture success in still water, although the only significant difference is for Artemia sp. in turbulent conditions ( t =3.02, df =10, P =0.01; same transformations as above). The higher capture success of evasive prey in still water by A. aspera is related to attack speed; high speed video records of A. spinosa showed that 100% of attacks at speeds <1 cm s−1 were successful as compared to 9% of attacks >3 cm s−1 (Table 5). These last rates are based on 134 attacks by 11 fish but cannot be tested statistically because the number of attacks by individual fish ranged from 2 to 36.
Acanthemblemaria spinosa, A. aspera. Mean capture success (percent of approaches that result in captures) for A. spinosa ( light shading) and A. aspera ( dark shading) feeding on passive and evasive prey in still and turbulent water. n =6 for each bar and the error bars indicate standard error. All calculations based on arcsin square root transformations of proportions to normalize data
If we examine the components of total capture success, the percent of approaches that result in strikes and the percent of strikes that result in captures, we see that for passive prey, A. spinosa have greater success than A. aspera in turbulent water for both components (only strikes as a percent of approaches is significant), and that in still water, they are essentially identical for both components (Table 6). For evasive prey, A. spinosa also has higher success than A. aspera in turbulent water for both components, but not significantly so. In still water, A. spinosa has greater success for strikes as a percent of approaches but A. aspera has the greater success for captures as a percent of strikes. Although differences between species were generally not statistically significant, differences between water motion treatments were always significant (Table 6). For evasive prey, both species experienced greater success in both components in turbulent water whereas for passive prey, both species experienced greater success in both components in still water
Discussion
Coral reefs are known for their high species diversity of many taxa (Reaka-Kudla 1997), including fishes (Bellwood and Wainwright 2002). The conditions allowing coexistence of so many species have been debated for over 30 years. We have learned a great deal about the ecology of reef fishes in that time and we now see that the answer involves a multiplicity of mechanisms. Among these is fine partitioning of space within habitats, a mechanism that probably has its strongest influence on small fishes such as blennies (Greenfield and Johnson 1990). The adaptations that foster such precise division of space are only now being explored. A. spinosa and A. aspera live in different microhabitats and concentrate their diets on different types of copepods. We found a number of differences in behavior under controlled conditions that correlate with the apparently more energetic microhabitat of A. spinosa as compared with that of A. aspera: A. aspera attacks at greater distances and is more inclined to strike at prey that are behind it; A. aspera approaches prey more slowly and modulates its strike speeds with changes in water movement; A. spinosa approaches to passive prey in turbulent water lead to more strikes than do A. aspera approaches.
A. spinosa and A. aspera display similar reactive distances at all angles of attack (Fig. 4) whereas free-swimming fish display shorter reactive distances to the side and behind (Luecke and O’Brien 1981; Kiflawi and Genin 1997). Blenny eyes are very mobile and move independently (personal observation) allowing them to scan through a greater range of angles than the relatively fixed eyes of free-swimming fish. This greater sensory scanning area conforms to the greater flexibility of the body giving tube blennies the capacity to capture prey at a greater range of angles than free-swimming fish.
Although the shapes of the feeding volumes were very similar for A. spinosa and A. aspera, the density of strikes differed within those volumes (Figs. 2,3). The significantly greater proportion of backward strikes by A. aspera could be related its normally feeding on harpacticoid copepods (Clarke 1999). These benthic organisms, while “...relatively easy to catch (if you’re a fish!)” (Hicks and Coull 1983), would require great flexibility on the part of a “hemisessile” (Kotrschal and Lindquist 1986) fish. Whereas free-swimming fish can orient their whole bodies towards harpacticoids as they pick them off surfaces, and plankton-eating A. spinosa can choose the location to strike as prey are carried past them, A. aspera must be able to bend sideways and backwards to pick harpacticoids off surfaces in the vicinity of the shelter hole. This speculation assumes that harpacticoids are generally available on a largely two-dimensional surface and that they are presented at a lower rate than plankton because they are not continuously carried into the feeding volume by water movement. This latter assumption is supported by the observations that in six species of the genus Coralliozetus, females, which are mobile, have higher feeding rates than males, which reside in shelters (Hastings 2002).
The mean water speed of 20.2 cm s−1 attained in the turbulence chamber was similar to the maximum laminar speed of 18 cm s−1 in a flume used by Kiflawi and Genin (1997) to study feeding by two planktivorous fish. It was also in the range reported on natural reefs. Maximum flow rates vary from ~70 cm s−1 on the “seaward portion of the reef flat” (Williams and Carpenter 1998), to <4 to 32 cm s−1 1 cm above coral surfaces at 10 m on the forereef (Sebens et al. 1998) to ~12 cm s−1 at 15 cm above the substrate at 20 m on the forereef (Helmuth and Sebens 1993). The mean turbulent intensity of 0.17 similarly falls in the range reported for natural reefs: 0.81 (Williams and Carpenter 1998) and 0.19 (Helmuth and Sebens 1993). Hearne et al. (2001) noted that turbulent energy on coral reef flats “is some three orders of magnitude higher than anywhere else in the ocean” and they calculated ε~10−2 W kg−1 (=100 cm2 s−3) for Kaneohe Reef, Hawaii, similar to the 160 cm2 s−3 attained in the turbulence chamber. These comparisons suggest that the turbulence experienced by the blennies in these experiments was in the same range as the conditions they would experience in the field. Preliminary measurements in the feeding volumes of A. spinosa and A. aspera yield maximum water speeds of 40 cm s−1 (C. Finelli and R. Clarke, unpublished data). Additionally, we only tested one level of turbulence. We need to repeat this study with several levels of turbulence to determine if there is a dome-shaped response similar to that seen in larval fishes (MacKenzie et al. 1994).
Subjective field observations indicate that blennies pursuing prey generally dart one to two body lengths, or 2–5 cm, from their shelters (Clarke 1996). The chamber in which feeding volumes were determined provided a distance to the glass walls of 7.5 cm on the sides and 12 cm ahead. Within this space, the fishes attacked at a mean distance of 2.0 cm and 95% of attacks occurred within 2.8 cm. Because the distance of forward attacks was no greater than sideways attacks (Fig. 4), and the measured distances are consistent with field observations, we are confident that the results were not greatly impacted by the size of the test chamber. The test chambers in which attack speeds and capture success were measured were smaller, providing 3 cm on the sides and 6 cm ahead. Only attacks that were directed ahead were used for speed measurements, so the distance to the forward wall was >2 times the 95th percentile for attack distances as measured in the large chamber. The fish appeared to behave in the same manner in these chambers, frequently emerging fully from the shelters to attack prey. We did not use these chambers to measure distances and believe that attack speeds and capture success were not affected by chamber size.
The similarity in swimming speeds of Acartia tonsa that had and had not been subjected to a turbulence treatment suggests that they were neither damaged nor was their behavior modified in a permanent manner by exposure to turbulence in a small chamber. This observation combined with the above discussion of chamber size gives us confidence that the results are not unduly affected by the experimental conditions and are applicable to the natural environment.
The attack patterns of A. aspera can be characterized as deliberate, with a slow initial approach followed by a rapid strike averaging 20.3 cm s−1 when attacking Acartia tonsa under turbulent conditions. When attacking Artemia sp. in still water, they struck at almost half that speed, 12.4 cm s−1. These values are remarkably similar to attack speeds of juvenile Sacramento perch ( Archoplites interruptus) of similar length to the blennies. They attacked evasive Diaphanosoma brachyurum at 23.0 cm s−1 and nonevasive Daphnia magna at 11.3 cm s−1 (Vinyard 1982). A similar modulation of attack behavior has been observed in other fishes (Kiflawi and Genin 1997; Nemeth 1997; Wainwright et al. 2001). In contrast, A. spinosa attacked at the same high speed under both conditions (20.1 and 22.7 cm s−1 respectively). This ability of A. aspera to modulate its attack speeds, may be related to its normal diet of harpacticoids. Picking prey off surfaces would entail more suction than ram feeding (Nemeth 1997), otherwise the fish may damage their mouthparts. A. spinosa may not have this ability because they feed almost exclusively on planktonic prey, which may be more susceptible to ram feeding (Nemeth 1997), as exemplified in the rapid jaw protrusion of the sling-jaw wrasse, Epibulus insidiator (Wainwright and Bellwood 2002). Indeed, ram feeding is associated with elusive prey as exemplified by the long-snouted butterflyfish, Forcipiger longirostrus (Ferry-Graham et al. 2001) and Chromis viridis, which use primarily suction feeding on Artemia sp. (passive prey) and ram feeding on Eucalanus (an evasive calanoid copepod, Coughlin and Strickler 1990).
Although they struck at the same speed under both conditions, A. spinosa approached more slowly when attacking Acartia tonsa in turbulent water as compared to Artemia sp. in still water (Fig. 6a, b). This is probably a result of their having to reorient several times as they approached the erratically moving prey (Fig. 5d). Even with this slowdown, A. spinosa approaches were still about three times faster than those of A. aspera under the same conditions (ca. 6 vs 2 cm s−1 respectively). This may be why 13% of A. spinosa approaches did not result in strikes whereas the comparable figure for A. aspera was 2%. Aborted approaches occurred when Acartia tonsa performed escape movements, which occurred more frequently at faster approaches (Table 5).
While approach speeds may reflect reorientations as blennies advance towards prey in turbulent water, they also affect capture (escape) success in still water. A. spinosa capture success is inversely related to approach speed (Table 5). Acartia tonsa responds to hydrodynamic disturbance with swimming speeds of 50 cm s−1 and a response time of 4 ms (Buskey et al. 2002) whereas A. spinosa strikes are generally <20 cm s−1. At a swimming speed of 20 cm s−1 an A. spinosa would travel 0.8 mm in 4 ms, which means that a hydrodynamic disturbance (“bow wave”) >0.8 mm ahead of a swimming blenny would give a copepod time to respond and avoid capture. We have no information on the nature and magnitude of disturbances caused by swimming blennies. It would be especially useful to know how the length of the “bow wave” varies with swimming speed.
Given the ability of Acartia tonsa to detect attacks by sensing hydrodynamic disturbances, water motion potentially can interfere with this detection system. Figure 7 shows that both blennies have greater success capturing Acartia tonsa when under turbulent than still conditions. The fact that they have lower success capturing passive Artemia sp. under turbulent than still conditions demonstrates that the movement of prey hinders their capture. For blennies feeding on Acartia tonsa, this predator handicap is overbalanced by the reduced detection of blennies by copepods (prey handicap). The explanation for this handicap could be habituation of the escape response due to exposure to frequent hydrodynamic stimuli (Hwang and Strickler 1994; Hwang et al. 1994). Another possible mechanism is the masking effect of turbulent water; the varying pressure waves created by fluctuating water velocities may make enough noise to obscure the signal generated by an approaching fish. A similar effect occurs in non-web-building Portia spiders, which feed on web-building spiders. They walk rapidly towards the prey spider under windy conditions taking advantage of a “vibratory smokescreen” which masks the signals created by their walking on the prey spider’s web (Jackson and Wilcox 1998). As with blennies, Portia attacks are more successful in windy than still conditions; there is evidence that the mechanism is the interference with the prey spider’s ability to detect the approaching predator (Wilcox et al. 1996). Portia spiders engage in deceptive behaviors only in still air. This flexibility is analogous to the slower approaches of A. aspera in still than in turbulent water.
The ability of fish predators to capture prey and the ability of copepod prey to elude their predators is affected not only by the strategies of each player (O’Brien 1979), but is also contingent on the hydrodymamic environment (Landry et al. 1995; Lough and Mountain 1996). This adds a great deal of complexity to the predator-prey relationship because the temporally and spatially varying degree of water motion on coral reefs will result in temporally and spatially varying probabilities of “success” for predators and prey. Consequently, we need to determine the magnitude of water motion in various reef habitats, including the degree of variation in space and time within habitats.
References
Almany GR, Baldwin CC (1996) A new Atlantic species of Acanthemblemaria (Teleostei: Blennioidei: Chaenopsidae): morphology and relationships. Proc Biol Soc Wash 109:419–429
Bellwood, DR, Wainwright PC (2002) The history and biogeography of fishes on coral reefs. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic, San Diego, pp 5–32
Buskey EJ, Coulter CJ, Strom S (1993) Locomotory patterns of microzooplankton: potential effects on food selectivity of larval fish. Bull Mar Sci 53:29–43
Buskey EJ, Lenz PH, Hartline DK (2002) Escape behavior of planktonic copepods in response to hydrodynamic disturbances: high speed video analysis. Mar Ecol Prog Ser 235:135–146
Clarke RD (1989) Population fluctuation, competition and microhabitat distribution of two species of tube blennies, Acanthemblemaria (Teleostei: Chaenopsidae). Bull Mar Sci 44:1174–1185
Clarke RD (1996) Population shifts in two competing fish species on a degrading coral reef. Mar Ecol Prog Ser 137:51–58
Clarke RD (1999) Diets and metabolic rates of four Caribbean tube blennies, genus Acanthemblemaria (Teleostei: Chaenopsidae). Bull Mar Sci 65:185–199
Coughlin DJ, Strickler JR (1990) Zooplankton capture by a coral reef fish: an adaptive response to elusive prey. Environ Biol Fish 29:35–42
Drenner RW, Strickler JR, O’Brien WJ (1978) Capture probability: the role of zooplankton escape in the selective feeding of planktivorous fish. J Fish Res Board Can 35:1370–1373
Ferry-Graham LA, Wainwright PC, Bellwood DR (2001) Prey capture in long-jawed butterflyfishes (Chaetodontidae): the functional basis of novel feeding habits. J Exp Mar Biol Ecol 256:167–184
Fields DM, Yen J (1997) The escape behavior of marine copepods in response to a quantifiable fluid mechanical disturbance. J Plankton Res 19:1289–1304
Gardella DJ, Edmunds PJ (2001) The effects of flow and morphology on boundary layers in the scleractinians Dichocoenia stokesii (Milne-Edwards and Haime) and Stephanocoenia michilini (Milne-Edwards and Haime). J Exp Mar Biol Ecol 256:279–289
Greenfield DW, Johnson RK (1990) Heterogeneity in habitat choice in cardinalfish community structure. Copeia 1990:1107–1114
Hamner WM, Jones MS, Carleton JH, Hauri IR, Williams DMcB (1988) Zooplankton, planktivorous fish, and water currents on a windward reef face: Great Barrier Reef. Bull Mar Sci 42:459–479
Hastings PA (2002) Evolution of morphological and behavioral ontogenies in females of a highly dimorphic clade of blennioid fishes. Evolution 56:1644–1654
Hearn CJ, Atkinson MJ, Falter JL (2001) A physical derivation of nutrient-uptake rates in coral reefs: effects of roughhness and waves. Coral Reefs 20:347–356
Helmuth B, Sebens K (1993) The influence of colony morphology and orientation to flow on particle capture by the scleractinian coral Agaricia agaricites (Linnaeus). J Exp Mar Biol Ecol 165:251–278
Hicks GRF, Coull BC (1983) The ecology of marine meiobenthic harpacticoid copepods. Oceanogr Mar Biol Annu Rev 21:67–175
Hobson ES (1991) Trophic relationships of fishes specialized to feed on zooplankters above coral reefs. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic, San Diego, p 69–95
Hwang J-S, Strickler JR (1994) Effects of periodic turbulence events upon escape responses of a calanoid copepod, Centropages hamatus. Bull Plank Soc Japan 41:117–130
Hwang J-S, Costello JH, Strickler JR (1994) Copepod grazing in turbulent flow: elevated foraging behavior and habituation of escape responses. J Plank Res 16:421–431
Jackson RR, Wilcox RS (1998) Spider-eating spiders. Am Sci 86:350–357
Jones GP (1987) Competitive interactions among adults and juveniles in a coral reef fish. Ecology 68:1534–1547
Kerfoot WC, Kellogg DL Jr, Strickler JR (1980) Visual observations of live zooplankters: evasion, escape and chemical defenses. Am Soc Limnol Oceanogr Spec Symp 3:10–27
Kiflawi M, Genin A (1997) Prey flux manipulation and the feeding rates of reef-dwelling fish. Ecology 78:1062–1077
Kiørboe T, Visser A (1999) Predator and prey perception in copepods due to hydromechanical signals. Mar Ecol Prog Ser 179:97–111
Kotrschal K, Lindquist DG (1986) The feeding apparatus in four Pacific tube blennies (Teleostei: Chaenopsidae): lack of ecomorphological divergence in syntopic species. PSZNI Mar Ecol 7:241–253
Landry F, Miller TJ, Leggett WC (1995) The effects of small-scale turbulence on the ingestion rate of fathead minnow ( Pimephales promelas) larvae. Can J Fish Aquat Sci 52:1714–1719
Landry MR (1978) Predatory feeding behavior of a marine copepod, Labidocera trispinosa. Limnol Oceanogr 23:1103–1113
Lenz PH, Hartline DK (1999) Reaction times and force production during the escape behavior of a calanoid copepod Undinula vulgaris. Mar Biol 133:249–258
Lough RG, Mountain DG (1996) Effect of small scale turbulence on feeding rates of larval cod and haddock on stratified water on Georges Bank. Deep Sea Res 43:1745–1772
Luecke C, O’Brien JO (1981) Prey location volume of a planktivorous fish: a new measure of prey vulnerability. Can J Fish Aquat Sci 38:1264–1270
MacKenzie BR, Kiørboe T (2000) Larval fish feeding and turbulence: a case for the downside. Limnol Oceanogr 45:1–10
MacKenzie BR, Miller TJ, Cyr S, Leggett WC (1994) Evidence for a dome-shaped relationship between turbulence and larval fish ingestion rates. Limnol Oceanogr 39:1790–1799
Nemeth DH (1997) Modulation of attack behavior and its effect on feeding performance in a trophic generalist fish, Hexagrammos decagrammus. J Exp Biol 200:2155–2164
O’Brien WJ (1979) The predator-prey interaction of planktivorous fish and zooplankton. Am Sci 67:572–581
Ohman MD (1988) Behavioral responses of zooplankton to predation. Bull Mar Sci 43: 530–550
Reaka-Kudla, ML (1997) The global biodiversity of coral reefs: a comparison with rain forests. In: Biodiversity. II: Understanding and protecting our biological resources. Joseph Henry, Washington pp 83–108
Saiz E (1994) Observations of the free-swimming behavior of Acartia tonsa: effects of food concentration and turbulent water motion. Limnol Oceanogr 39:1566–1578
Sebens KP, Grace SP, Helmuth B, Maney EJ, and Miles JS (1998) Water flow and prey capture by three scleractinian corals, Madracis mirabilis , Montrastrea cavernosa and Porites porites, in a field enclosure. Mar Biol 131:347–360
Shashar N, Kinane S, Jokiel PL, Patterson, MR (1996) Hydromechanical boundary layers over a coral reef. J Exp Mar Biol Ecol 199:17–28
Singarajah KV (1969) Escape reactions of zooplankton: avoidance of a pursuing siphon tube. J Exp Mar Biol Ecol 3:171–178
Singarajah KV (1975) Escape reactions of zooplankton: effects of light and turbulence. J Mar Biol Assoc UK 55:627–639
Smith-Vaniz WF, Palacio FJ (1974) Atlantic fishes of the genus Acanthemblemaria, with description of three new species and comments on Pacific species (Clinidae: Chaenopsinae). Proc Acad Sci Philadelphia 125:197–224
Stamhuis EJ, Videler JJ (1995) Quantitative flow analysis around aquatic animals using laser sheet particle image velocimetry. J Exp Biol 198:283–294
Trager G, Achituv Y, Genin A (1994) Effects of prey escape ability, flow speed, and predator feeding mode on zooplankton capture by barnacles. Mar Biol 120:251–259
Vinyard GL (1982) Variable kinematics of Sacramento Perch ( Archoplites interruptus) capturing evasive and nonevasive prey. Can J Fish Aquat Sci 39:208–211
Wainwright PC, Bellwood DR (2002) Ecomorphology of feeding in coral reef fishes. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, Calif., pp 33–55
Wainwright PC, Ferry-Graham LA, Waltzek TB, Carroll AM, Hulsey CD, Grubich JR (2001) Evaluating the use of ram and suction during prey capture by cichlid fishes. J Exp Biol 204:3039–3051
Wilcox RS, Jackson RR, Gentile K (1996) Spiderweb smokescreens: spider trickster uses background noise to mask stalking movements. Anim Behav 51:313–326
Williams JT (2003) Three new species of blennioid shore fishes discovered at Navassa Island, Caribbean Sea. Aqua 6:11–16
Williams SL, Carpenter RC (1998) Effects of unidirectional and oscillatory water flow on nitrogen fixation (acetylene reduction) in coral reef algal turfs, Kaneohe Bay, Hawaii. J Exp Mar Biol Ecol 226:293–316
Acknowledgements
This research was supported by a Research Opportunities Award supplement to National Science Foundation Grant OCE-9910608 to E.J.B., National Science Foundation Grant OCE-0324724 to R.D.C., and National Science Foundation Grant OCE-0324413 to E.J.B. We wish to thank S. Litner and R. Warner who provided facilities in St. Croix, S. Clarke and J. Clarke who helped with blenny collection, C. Hyatt who assisted with sorting copepods, E. Saiz who gave advice on turbulence calculations, and D. King who calculated available feeding volumes by quadrant. Use of fishes for this study follows the guidelines of the University of Texas, Austin, Institutional Animal Care and Use Committee. This is University of Texas Marine Science Institute Contribution Number 1331.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P.W. Sammarco, Chauvin
Rights and permissions
About this article
Cite this article
Clarke, R.D., Buskey, E.J. & Marsden, K.C. Effects of water motion and prey behavior on zooplankton capture by two coral reef fishes. Marine Biology 146, 1145–1155 (2005). https://doi.org/10.1007/s00227-004-1528-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1528-y