Abstract
Marine organisms are currently challenged by multiple and interactive environmental stressors. In the subtropics, warming and intensified precipitation, and hence, reduced salinity, are particularly relevant. Using the sea urchin, Heliocidaris crassispina, we investigated the effects of warming and low salinity on fertilization success and early development. These planktonic developmental stages play significant roles in shaping population dynamics. Gametes were exposed to a temperature gradient (28–43 °C) while being held at two salinities (24 and 32). Fertilization had a higher critical temperature (LT50), the temperature at which 50% individuals reached the designated stage, of 39 °C than that of blastula formation at 31 °C for both salinities, suggesting between-stage variations in sensitivity. The LT50 for blastula formation was very close to present-day recorded maximum sea surface temperature of 31 °C suggesting a small thermal safety factor. Larvae were also reared to the eight-arm stage in one of the four combinations of temperatures (24 and 28 °C) and salinities (24 and 32), which correspond to sea surface temperatures and salinities observed during the urchin’s spawning season. Low salinity and high temperature had interactive effects in reducing larval survivorship. However, amongst larvae that survived the combined stress, warming reduced the negative impact of reduced salinity on arm growth. Unexpected release of blastula-like particles was documented in all treatments except the control (24 °C and salinity 32). Incomplete separations that resulted in conjoined twins, however, were only found at 28 °C. There were significantly different responses in fertilization success and larval growth between maternal lineages. Such intra-specific variations highlight the presence of phenotypic plasticity and could imply the presence of genetic variations in response to thermal and salinity stress. Such plasticity suggests that although purple urchins are experiencing extreme conditions that are stressful at present, they may be able to cope with the future ocean conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global climate change is one of the greatest threats to marine organisms (Halpern et al. 2008; Deutsch et al. 2015; McCauley et al. 2015). Average sea surface temperature is rising at an unprecedented rate and is predicted to increase by 0.6–2 °C by the turn of this century (IPCC 2014). Organisms are not only challenged by the increasing ocean temperatures and acidification caused by excess atmospheric carbon dioxide, they are also experiencing an increase in frequency and intensity of extreme climatic events, including pulses of intense rainfall and terrestrial runoff (Harley et al. 2006; Todgham and Stillman 2013; IPCC 2014). Often, the combined effects of these interactive stressors are synergistic, i.e., greater than the sum of individual effects (Folt et al. 1999; Przeslawski et al. 2015). Therefore, multifactorial studies are needed to help better understand how organisms may cope with the future multi-stressor environment.
Responses to multiple stressors often vary. Some of these variations could be attributed to different combinations of stresses, but also to differences in sensitivity between taxonomic groups (Crain et al. 2008; Karelitz et al. 2017), between populations or families of the same species (Chan et al. 2013; Kelly et al. 2013), between developmental stages (Delorme and Sewell 2014; Karelitz et al. 2017), and between performance metric studies [e.g., growth, survival, and behavior (Chan et al. 2015)]. An example of interspecific variation in response to fluctuations in temperature and salinity is polyembryony, the production of multiple offspring from a single zygote (Craig et al. 1997). This phenomenon was a documented response of larval sand dollars (Echinarachnius parma) to fluctuations in temperature and salinity, but not for larval urchins [Lytechinus variegatus (Allen et al. 2015)] nor crown-of-thorns seastar [Acanthaster planci (Allen et al. 2017)]. Within a single urchin species, pre-exposure of adults (mothers) affects larval growth and survival to salinity and temperature stress (Roller and Stickle 1993). Stress responses are often maternal lineage dependent, e.g., the offspring of certain sand dollar females were more likely to clone, a proposed size reduction mechanism to lower visual predation risk, than others when a predator cue was present (Vaughn 2009, 2010). In addition to conferring phenotypic plasticity through differences in maternal conditions (Kelly et al. 2013; Morley et al. 2016), other genotype studies using full-factorial crosses also suggested that there are heritable components to thermal tolerance such that some families are more resilient than others (Applebaum et al. 2014; Delorme and Sewell 2016; Foo et al. 2016; Sparks et al. 2017). Understanding these variations in responses to multiple stressors is, therefore, essential for predicting population- and community-level responses under conditions of global climate change.
For shallow water and intertidal organisms, including sea urchins, temperature and salinity are essential factors that shape their physiological performance and distributions (see reviews by Tomanek and Helmuth 2002; Helmuth et al. 2005). Combined salinity and temperature stress have been shown to compromise larval urchin survival, development, and physiological performance (e.g., respiration and proton pumping, Greenwood and Bennett 1981; Roller and Stickle 1985, 1993; Metaxas 1998; Carballeira et al. 2011; Delorme and Sewell 2014; see review of other stressors by Przeslawski et al. 2015). Here, we focus on a subtropical urchin because species currently living close to the tropics, in which ambient temperatures reach close to their upper thermal limits, may be particularly at risk of global warming (Deutsch et al. 2008, 2015; Burrows et al. 2014; Collin and Chan 2016).
In subtropical Hong Kong (22.3°N, 114.2°E), sea surface temperatures vary spatial-temporally. Eastern waters of Hong Kong are strongly influenced by the Pearl River Estuary discharge and has a lower salinity of 25 compared to 30 in the more oceanic western waters during the summer wet season (Chan et al. 2001). The Environmental Protection Department (Hong Kong Government) monitors marine water quality monthly at 76 stations distributed across the territory (data available at http://epic.epd.gov.hk/EPICRIVER/marine/). In Port Shelter in eastern waters, a known habitat and sampling site for our focal organism, the mean sea surface temperature was 23.7 °C (range from 13 to 31 °C) and the mean salinity was 31.8 (range from 21 to 36) from 1986 to 2015. The average air temperature in Hong Kong is projected to increase by 3.1–5.5 °C, while the mean annual rainfall is projected to increase by 10% under the business as usual emission scenario at the end of this century (Hong Kong Observatory, data available at http://www.hko.gov.hk/climate_change/future_climate_e.htm). Given a predicted increase in the number of days with intense rainfall during the hot summer months, exposure of coastal organisms to warming water temperatures and reduced salinities is plausible. Since shallow water species in Hong Kong are currently already experiencing a broad range of environmental conditions, they are good candidates to test the hypothesis that a large variability in present-day extreme abiotic conditions preselects organisms to better cope with warmer and more variable salinities in the future.
Earlier studies of salinity and temperature effects on subtropical species in Hong Kong have focused on fouling [e.g., Balanus amphitrite, Hydroides elegans (Qiu and Qian 1999; Pechenik et al. 2007)] or invasive species [e.g., Crepidula onyx (Zhao 2002)]. Often, larvae were only studied with the use of a common garden approach (Qiu and Qian 1999; Zhao 2002). Informed by these previous works, we designed our study to investigate the interactive effect of salinity and temperature on an ecologically and economically important native species, the purple sea urchin, Heliocidaris crassispina. The spawning period of this species is long in Hong Kong, lasting 8 months from March to October, including the hot, summer wet season (Urriago et al. 2016). In a short-term exposure experiment, we tested the hypothesis that reduced salinity decreases the thermal tolerances of different early development stages (fertilization and blastula formation). In a rearing experiment, we tested for the effect of interactive warming and reduced salinity stress on the survival and growth of pluteus larvae and if these responses differed between maternal lineages.
Materials and methods
Study species
Heliocidaris crassispina (A. Agassiz, 1864, formerly Anthocidaris crassispina) ranges from the rocky coasts of Japan and Korea to China and is commonly observed in the intertidal zone of Hong Kong (Chiu 1985). This species plays an important role in controlling the community dynamics of benthic algae in low shore rock pools in Hong Kong (Wai and Williams 2005). Heliocidaris crassispina is also one of the most commonly harvested and farmed sea urchin species in China (Ding et al. 2007). Protocols to obtain and fertilize gametes and for larval rearing of this species are well established, and its larvae have been the subject of various ecotoxicology studies (Vaschenko et al. 1999; Lu and Wu 2005).
Adult collection and spawning
Adults of H. crassispina were procured from a sea urchin farm in High Island, Port Shelter, Hong Kong, and transferred immediately to the Coastal Marine Laboratory at Hong Kong University of Science and Technology (< 1 h). They were kept in a flow-through system at ~ 24 °C and salinity 32 and fed ad libitum with pre-dried kelp (Laminariaceae) prior to use in experiments (for up to 2 months). Injection of 0.5–1 ml of 0.35 M KCl into the coelomic cavity induced urchin spawning (Strathmann 1987). Sperm were collected dry and kept on ice. Eggs were collected in filtered seawater (FSW, 0.22 μm filtered) at control (32) and experimental salinities (24) by moving the adult urchins quickly into different beakers (< 5 min). The order of salinity presented was randomized between females. Collected eggs were subsequently washed through a 154-μm sieve.
Short-term exposure: critical temperature of fertilization and blastula development
A metal “heat block” modeled after Kuo and Sanford (2009), and Collin and Chan (2016), was used to create a thermal gradient. This heat block is a sheet of aluminum (91 cm × 25 cm × 15 cm) with equally spaced holes (2.7 cm in diameter). There are four rows of ten holes, arranged length-wise thus enabling us to measure four replicate sets during each trial. A temperature gradient ranging from 28 to 43 °C was created by running a water chiller (HS-90A, Hailea, China) and a water bath (195001465007, Pharmacia Biotech, Piscataway, USA) at each end. Temperature of the heat block was measured with a thermocouple (HH-20A, Omega, Norwalk, CT, USA) at the beginning and end of the experiment.
Washed eggs from individual females were diluted with the corresponding salinity water to a density of 40 eggs ml−1. 200 eggs were placed in glass vials containing 10 ml FSW at the two salinities [24 and 32; density chosen after Collin and Chan (2016)]. Sperm mixtures were prepared by adding concentrated sperm from at least two males (2–4 individuals) to Falcon tubes containing the appropriate salinity seawater. Sperm were enumerated with duplicate hemocytometer counts and added to the eggs at a final concentration of 1000 sperm ml−1 within the first 20 min of spawning. Each female served as a biological replicate with one set of control salinity (32) and one set of treatment salinity (24) vials. Two replicates, i.e., eggs from two females, were run on a single heat block trial. The vials were placed into the heat block starting from the coolest end, two biological replicates at a time (4 vials, 2 vials from each female with 1 vial at salinity 32 and the other at 24). Inserting vials column-wise ensured equal incubation times at a given temperature. The whole block was filled within 3 min. Two hours after placing in the first set, 2–3 drops of 37% paraformaldehyde (PFA) were added to each vial in sequence, starting from the coolest end, such staggered fixation ensured that the time exposed at each temperature was identical. Two separate trials were performed with a total of four females. Developmental stage (fertilization defined by the formation of fertilization envelope, first cleavage, morula and blastula) of the 30 haphazardly chosen individuals was noted for each vial (staging after Collin and Chan 2016).
Critical temperatures (LT50) for fertilization and blastula formation, defined as the temperature at which 50% fertilization/blastula formation was accomplished, were computed with a logistic regression. The best-fit curves for LT50 were compared between salinity and developmental stage with extra sum of squares F test with the GraphPad Prism software version 6 (California, USA).
Larval rearing experiment: experimental setup, survival and growth estimates
We performed three replicated larval rearing experiments with a total of 10 females, at least three females and two males were used during each experiment (trial 1: 3♀ × 3♂; trial 2: 3♀ × 2♂; trial 3: 4♀ × 3♂). Eggs from individual females were kept separated and used as biological replication, i.e., 10 replicates for each treatment. These eggs were fertilized by the addition of mixed sperm solution at a concentration of ~ 1000 sperm ml−1, creating maternal half siblings in each experiment. Over 95% fertilization success was confirmed by the presence of a fertilization envelope 15-min post-fertilization. Fertilized eggs from each female were divided into four treatments: salinity 32 at 24 and 28 °C, salinity 24 at 24 and 28 °C, at a density of 5 individuals ml−1 in 1.5-l glass jars containing FSW with gentle aeration. All jars were maintained in temperature-controlled sea tables with heaters and chillers. Larvae were fed Rhodomonas sp. at a concentration of ~ 5000 cells ml−1 daily, starting from 1 day post-fertilization, and complete water changes were performed every other day.
Duplicate 10 ml subsamples from each jar were taken daily to monitor the larval density (number of individual ml−1) for 7 days. Subsamples were fixed with 1–2 drops of 37% PFA solution and counted on the same day, and subsequently stored in 2% buffered PFA solution (pH 8.2) for further measurements. Presence of detached blastula-like particles was recorded. These blastula-like particles (Suppl. Figure 1) resemble the exogastrulation observed when urchin larvae were exposed to other stressors, e.g., lithium, chilling, low calcium and estrogen (Okazaki 1956; Takahashi et al. 1977; Ishihara et al. 1982), but were considered evidence for “budding” in an ocean acidification study (Chan et al. 2013).
For larval growth measurement, photographs of fifteen larvae per jar were taken daily under a compound microscope at 10× using a Nikon D5300 digital camera. Using photographs of a stage micrometer for calibration, total body lengths (TBL) and posterodorsal arm lengths (Fig. 1a) were measured with Fiji ImageJ (Schindelin et al. 2012).
Larval growth was estimated by the increase in total body length (TBL) and average posterodorsal arm length on larval H. crassispina (a). Abnormal early development was observed in both low-salinity and warming treatments throughout the experimental period (b) and in the 28 °C treatments conjoined individuals were found and developed into late-stage larvae (c, d). All scale bars are 100 µm
There was no significant difference between experimental trials and the measurements made during different trials were pooled with female as the unit of replication. After confirming the data met the assumptions of normality and homogeneity with Shapiro–Wilk test and Levene’s test, the effects of age, maternal lineage, salinity, and temperature on larval survival, body length, and arm length were tested with ANOVA. These statistical analyses were conducted with SPSS 13 (IBM).
Results
Short-term exposure: critical temperature of fertilization and blastula formation
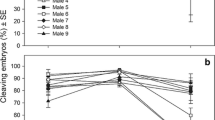
The resulting temperature gradient of the heat block ranged from 28 ± 0.5 to 43 ± 0.5 °C and all logistic regressions of percent fertilized/developed across the temperature gradient were statistically significant with r2 values ranging from 0.59 to 0.96 (Fig. 2, Table 1). Regression curves for fertilization and blastulae at salinity 24 and 32 were significantly different from each other (F test, F6,152 = 309.8, p < 0.0001). The critical temperature (LT50) for fertilization was 39.2 ± 2.4 °C at salinity 24, which is lower than that of salinity 32 at 41.3 ± 2.5 °C (F test, F2,76 = 4.138, p = 0.0197). Similarly, the LT50 for blastulae formation was lower at salinity 24 (31.5 ± 6.9 °C) than that of salinity 32 (32.0 ± 5.6 °C, F2,76 = 10.31, p = 0.0001). At a given salinity, the LT50 was significantly higher for fertilization than that of blastula development (F2,76 = 430.7 and 500.5 for salinity 24 and 32, respectively, p < 0.0001 for both salinities).
Fertilization success (a) and blastula development (b) decreased with increasing temperature after 2 h of incubation in an aluminum heat block (mean ± standard deviation for 4 females). The LT50, temperature at which 50% development was observed was lower for blastula formation than that for fertilization. Reduction in salinity reduced the LT50 for a given developmental stage (squares for salinity 32 and circles for salinity 24)
Larval rearing experiment: survival and release of blastula-like particles
On average, larval density decreased over time, and hence, age (days post-fertilization) had a significant effect on survival (ANOVA, F1,173 = 254.41, p < 0.001, Fig. 3a, b, Table 2). Salinity (32 and 24) alone had a significant effect on larval survival (ANOVA, F1,170 = 5.92, p = 0.016) while temperature (24 and 28 °C) alone did not (ANOVA, F1,173 = 1.87, p = 0.175). There was significant interaction between these two stressors (ANOVA, F1,173 = 6.33, p = 0.013), such that larvae reared under the control treatment of temperature 24 °C and salinity 32 had the highest survivorship (~ 60%) compared to the other treatments, 24 °C with salinity 24 (~ 20%), 28 °C with salinity 32 (~ 20%), and 28 °C with salinity 24 (~ 10%) after 7 days.
Warming and salinity reduction had a synergistic effect on larval survival which was estimated by the change in larval density over time (mean ± standard error of all ten females). Salinity alone had a significant effect on larval survival but temperature did not (a, b). Warming and salinity reduction had no interactive effect on change in total body length (population mean ± standard error, all ten females and 15 individuals from each female c, d). The temperature and salinity interactions had a significant effect on averaged arm length (e, f). Low salinity alone reduced growth but warming promoted growth
A significant difference in larval density was observed between the ten maternal lineages studied (ANOVA, F9,170 = 6.05, p < 0.001), the responses of larvae from different females differed from each other even if they were exposed to the same treatment (Fig. 4a–d). There were significant interactions for female × salinity (ANOVA, F9,173 = 4.33, p < 0.001), female × temperature (ANOVA, F9,173 = 3.83, p < 0.001), and female × temperature × salinity (ANOVA, F9,173 = 2.58, p = 0.008) on larval density.
In several jars, the number of individuals increased overnight (e.g., up to 150 and 300% of the stocking density, Fig. 4b, c) and small-sized individuals were repeatedly observed in the mix of normal-sized larvae throughout the experiment in different jars (Suppl. Figure 1). These particles could be a result of cloning through budding, or extrogastrulation due to stress. These particles were only found in high-temperature (28 °C) or low-salinity (salinity 24) treatments but not in the control. However, incomplete separations, resulting in conjoined individuals were observed only in the 28 °C treatment (Fig. 1b–d).
Larval rearing experiment: larval growth
As larval urchins grew, total body length and average arm length increased over time, such that age had a significant effect on both metrics (ANOVA, F5,1855 = 556.56 and 938.61, respectively, p < 0.0001, Table 2, Fig. 3c–f). Salinity alone had a significant effect on both metrics and larvae reared at salinity 32 had greater total body length and arm length (ANOVA, F1,1855 = 151.64 and 462.70, respectively, p < 0.0001). Seven days post-fertilization, the average total body length across maternal lineage and temperature was 250.3 ± 50.9 µm at salinity 24 but 287.2 ± 50.9 µm at salinity 32. Temperature alone also had a significant effect on both metrics and larvae reared at warmer temperatures had longer total body length and arm length (ANOVA, F1,1855 = 71.67 and 235.67, respectively, p < 0.0001). Seven days post-fertilization, average total body length across lineages and salinity was 269.5 ± SD 50.9 µm at 24 °C but 279.2 ± SD 50.3 µm at 28 °C. There was no significant interaction between temperature and salinity on total body length (F1,1855 = 1.91, p = 0.167), but there was a significant interactive effect on arm length (F1,1855 = 12.0, p = 0.001) such that larvae at 24 °C had longer arms at salinity 24 than in the other treatments.
Maternal lineage had a significant effect on larval body length and arm length (F9,1855 = 13.80 and 24.02, respectively, and p < 0.0001). There were significant interactions for female × salinity (F9,1855 ≥ 19.97, p < 0.001), female × temperature (F9,1855 ≥ 16.4, p < 0.001), and female × temperature × salinity (F9,1855 ≥ 4.60, p < 0.001) on larval size. In the low-salinity, high-temperature treatment (salinity 24 and 28 °C), there were 12.2 and 41.2% differences in average total body length and arm length between the fastest and slowest growing lineages (Fig. 4e–h).
Discussions
Marine organisms are simultaneously challenged by multiple environmental stressors and understanding variability in responses between life stages, families, and species are essential for predicting community-level responses (Przeslawski et al. 2015). For the sea urchin H. crassispina, reduction in salinity even within present-day extremes could negatively impact thermal tolerance of early embryonic development, larval growth, and survival. Warming further interacted with salinity to reduce larval growth and survival. We also observed that some maternal lineages were more affected by these interactive stressors than others. These results highlight both vulnerability of such an important species in the face of climate change and the intra-specific variation which could be the basis for acclimation or adaption.
Small thermal safety factor for early development
The critical temperature (LT50) of fertilization is higher than that of the present-day extreme of 31 °C in Port Shelter, Hong Kong, based on Marine Water Quality Survey records from 1986 to 2015. However, the LT50 of blastula formation is around 31–32 °C at salinities of 24 and 32 (Fig. 2), suggesting that present-day extreme heat is already detrimental to early development. The predicted warming by 3–5 °C could significantly negatively affect the success of embryonic development, and in turn, reduce the urchin populations. This observed stage-dependent response is consistent with earlier studies on other echinoderm species in which fertilization was more thermally tolerant than subsequent development processes (Roller and Stickle 1985; Carballeira et al. 2011; Delorme and Sewell 2014; Collin and Chan 2016).
It is worth noting that only the lifting of the fertilization envelope was used to define “successful fertilization”. However, under ocean acidification conditions, the fertilization envelope can form even if fertilization has failed, e.g., partial lift-off of the fertilization envelope or hyaline blebs (Bögner et al. 2014; Bögner 2016). Our estimated fertilization success may be an over estimate and could account for the large variability of “fertilization success” observed at around 37.5 °C and the relatively lower r2 value of the regression at the control salinity of 32. This overestimation could also contribute towards a higher estimated LT50 than the blastula stage. Such between-stage differences highlight the need to investigate and compare physiological limits across developmental stages and careful investigation into the physiological regulatory mechanisms, such that the “weakest link” can be identified and targeted for conservation strategies.
Salinity further negatively affects larval development
Reduction in salinity decreased the LT50 significantly for early development and long-term exposure to low salinity impacts larval survival and growth (Figs. 2, 3). There are several possible mechanisms through which low salinity exerts pressure on larval urchins, e.g., osmotic stress increases metabolic demands (Anger 2003), oxidative damange caused by enhanced reactive oxygen species (ROS) generation (An and Choi 2010), or intracellular acidification due to low extracellular sodium ion concentration inhibiting Na+/H+ exchange (Ciapa and Philippe 2013). In mediums with low sodium ion concentrations, the cell cycle stops due to interruption to mitosis promoting factor (MPF) and extracellular regulated kinase (ERK) activities (Ciapa and Philippe 2013).
Larval survivorship was the lowest when larvae experienced a temperature of 28 °C and salinity of 24 (Fig. 3), highlighting the interactive effect of the two stressors (Table 2). During periods of intense rainfall, salinity can fall below 25 (e.g., in June 2006 and July 2014 at Station PM7 in Port Shelter, EPD), suggesting potential negative effects can already be experienced under present-day conditions. With climate change, the mean annual rainfall of Hong Kong is projected to increase by 10% (248 mm) by the end of this century (Lee et al. 2008; IPCC 2014). Such an increase in freshwater input could, therefore, interact with other climate change-related stressors, e.g., warming and acidification to further increase the risk the local H. crassispina population faces.
The results from our long-term exposure experiments may be the upper bound estimates of the impact of low salinity because larval swimming behaviors were not accounted for (Metaxas 1998; Chan 2012; Koehl and Cooper 2015). Other studies have demonstrated that larval urchins and other echinoderms respond to the presence of haloclines and would actively avoid low-salinity layers (Metaxas and Young 1998; Bashevkin et al. 2016), but in some cases individuals would venture into low-salinity layers that are sub-optimal for growth (Arellano et al. 2012). The salinity response of larval H. crassispina, however, is unknown and warrants future studies. Furthermore, Port Shelter waters are shallow and highly retentive, requiring over 20 days for complete flushing even with over ten sources of discharge draining freshwater into the bay (Mao et al. 2011). In the wet summer months, the behavioral choice, even if present, would have limited influence on the abiotic conditions larvae experience as they are retained in the bay. Therefore, the interactive, negative impacts on growth and survival of the two stressors are relevant to field conditions, and would imply a decline in urchin population under the future climate conditions.
Stress-induced blastula-like particle release
Larval cloning has been reported in various echinoderms and can take place via budding, fission, and autotomy (Eaves and Palmer 2003; Knott et al. 2003). In addition to changes in food concentration or presence of predator cues (Vickery and McClintock 2000; Vaughn 2010), our observations suggest that low salinity and high temperature might induce cloning, and in rare cases, incomplete separation. Allen et al. (2015) reported that low salinity and increased temperature at fertilization can lead to polyembryony, and that these “twins” can develop and eventually settle. While we did not expose the eggs and sperm to low salinity or high temperature, we did transfer the fertilized embryos to their respective treatments before hatching. Therefore, it is possible that the cloning we observed was a result of swelling of the hyaline layer within the fertilization envelope and/or reduced cell–cell adhesion caused by low Ca2+. Alternatively, the release of blastula-like particles was due to exogastrulation, which is also observed when larval urchins are exposed to other environmental stressors. Indeed, for H. crassispina peptides extracted from oocytes and whole embryos were shown to induce exogastrulae formation (Yamasu et al. 1995). These maternally derived exogastrulae induction peptides (EGIP) binds to a protein found on the hyaline layer, removal of which with Ca2+ and Mg2+-free seawater would inhibit the function of EGIP (Kinoshita et al. 1992; Fujita et al. 1994). Furthermore, cleavage failure during first cellular division caused by changes in chemical properties of the extracellular matrix and the subsequence increase blastomere separation could also account for the conjoined individuals (Matese et al. 1997; Bögner 2016).
Strong maternal influence on stress responses
Larvae from different maternal lineages responded differently to salinity and temperature stress in terms of survival, growth, and frequency of blastula-like particle releases (Fig. 4). For example, larvae of female 9 had relatively higher mortality rate at the control conditions (24 °C and salinity 32); however, their survivorship was comparable to other females, if not higher, when salinity is reduced to 24 or temperature was increased to 28 °C. The differences observed was unlikely due to differences in adult physiological conditions as they originated from the same site, were acclimated too identical lab conditions, and were of similar sizes. Such difference in larval responses could imply that some wild-type individuals, which experience variable conditions, perform better under stress. This maternal effect has been reported in larval echinoderms, e.g., likelihood of larval cloning differed between maternal lineages (Vaughn 2009; Chan et al. 2013). Larval response to acidification and salinity stress is also affected by pre-adaption of the adults (Roller and Stickle 1993; Chan et al. 2015). These differences are suggested to be a result of variability in genetic makeup, gene expression patterns, and/or egg provisioning (Strathmann 1987; Runcie et al. 2012; Kelly et al. 2013). The observed phenotypic plasticity in response to temperature and salinity stress may help provide additional buffering time for the population to evolve under climate change. Further quantitative investigation should address if these environmental stresses exert directional selection pressure and in turn reduce variability within a single lineage. To further assess this possibility of evolution, better understanding of the role of paternity, cost of the variability, heritability of such variations, and population turnover rate is needed (Sunday et al. 2011). Therefore, it is essential for future studies to carefully consider intra-specific variability in stress responses and their role in coping with a changing world.
Conclusion
Our results suggest that the urchin H. crassispina, an ecologically important species, is currently living close to its upper thermal limit for blastula formation, and therefore, is vulnerable to global warming. Reduction in salinity, due to increase in precipitation, will likely interact with warming to further threaten larval survival of this species. However, we observed significant intra-specific variations which could serve as a buffer to counter climate change stress. Between-stage and maternal lineage variations observed also highlighted the importance to consider various developmental stages and family groups in multiple stressor studies. Understanding such phenotypic plasticity is essential for better predictions of species, population, and community responses to global climate change.
References
Allen JD, Armstrong AF, Ziegler SL (2015) Environmental induction of polyembryony in echinoid echinoderms. Biol Bull 229:221–231
Allen J, Schrage K, Foo S, Watson S-A, Byrne M (2017) The effects of salinity and pH on fertilization, early development, and hatching in the crown-of-thorns seastar. Diversity 9:13
An MI, Choi CY (2010) Activity of antioxidant enzymes and physiological responses in ark shell, Scapharca broughtonii, exposed to thermal and osmotic stress: effects on hemolymph and biochemical parameters. Comp Biochem Physiol B Biochem Mol Biol 155:34–42
Anger K (2003) Salinity as a key parameter in the larval biology of decapod crustaceans. Invertebr Reprod Dev 43:29–45
Applebaum SL, Pan TCF, Hedgecock D, Manahan DT (2014) Separating the nature and nurture of the allocation of energy in response to global change. Integr Comp Biol 54:284–295. https://doi.org/10.1093/icb/icu062
Arellano SM, Reitzel AM, Button CA (2012) Variation in vertical distribution of sand dollar larvae relative to haloclines, food, and fish cues. J Exp Mar Biol Ecol 414:28–37
Bashevkin SM, Lee D, Driver P, Carrington E, George SB (2016) Prior exposure to low salinity affects the vertical distribution of Pisaster ochraceus (Echinodermata: Asteroidea) larvae in haloclines. Mar Ecol Prog Ser 542:123–140
Bögner D (2016) Life under climate change scenarios: sea urchins’ cellular mechanisms for reproductive success. J Mar Sci Eng 4:28
Bögner D, Bickmeyer U, Köhler A (2014) CO2-induced fertilization impairment in Strongylocentrotus droebachiensis collected in the Arctic. Helgol Mar Res 68:341
Burrows MT, Schoeman DS, Richardson AJ, García J (2014) Climate velocity and geographical limits to shifts in species’ distributions. Nature 507:492–495
Carballeira C, Martín-Díaz L, DelValls TA (2011) Influence of salinity on fertilization and larval development toxicity tests with two species of sea urchin. Mar Environ Res 72:196–203. https://doi.org/10.1016/j.marenvres.2011.08.008
Chan KYK (2012) Biomechanics of larval morphology affect swimming: insights from the sand dollars Dendraster excentricus. Integr Comp Biol 52:458–469
Chan BKK, Morritt D, Williams GA (2001) The effect of salinity and recruitment on the distribution of Tetraclita squamosa and Tetraclita japonica (Cirripedia; Balanomorpha) in Hong Kong. Mar Biol 138:999–1009
Chan KYK, Grünbaum D, Arnberg M, Thorndyke M, Dupont ST (2013) Ocean acidification induces budding in larval sea urchins. Mar Biol 160:2129–2135
Chan KYK, García E, Dupont S (2015) Acidification reduced growth rate but not swimming speed of larval sea urchins. Sci Rep. https://doi.org/10.1038/srep09764
Chiu S (1985) Feeding biology of the short-spined sea urchin Anthocidaris crassispina. In: Agassiz A (ed) Hong Kong proceedings of the fifth international echinoderm conference Balkema, Boston, pp 223–232
Ciapa B, Philippe L (2013) Intracellular and extracellular pH and Ca are bound to control mitosis in the early sea urchin embryo via ERK and MPF activities. PLoS One 8:e66113. https://doi.org/10.1371/journal.pone.0066113
Collin R, Chan KYK (2016) The sea urchin Lytechinus variegatus lives close to the upper thermal limit for early development in a tropical lagoon. Ecol Evol 6:5623–5634. https://doi.org/10.1002/ece3.2317
Craig SF, Slobodkin LB, Wray GA, Biermann CH (1997) The ‘paradox’of polyembryony: a review of the cases and a hypothesis for its evolution. Evol Ecol 11:127–143
Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11:1304–1315
Delorme NJ, Sewell MA (2014) Temperature and salinity: two climate change stressors affecting early development of the New Zealand sea urchin Evechinus chloroticus. Mar Biol 161:1999–2009. https://doi.org/10.1007/s00227-014-2480-0
Delorme NJ, Sewell MA (2016) Genotype-by-environment interactions during early development of the sea urchin Evechinus chloroticus. Mar Biol 163:215. https://doi.org/10.1007/s00227-016-2987-7
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci 105:6668–6672
Deutsch C, Ferrel A, Seibel B, Pörtner H-O, Huey RB (2015) Climate change tightens a metabolic constraint on marine habitats. Science 348:1132–1135
Ding J, Chang Y, Wang C, Cao X (2007) Evaluation of the growth and heterosis of hybrids among three commercially important sea urchins in China: Strongylocentrotus nudus, S. intermedius and Anthocidaris crassispina. Aquaculture 272:273–280. https://doi.org/10.1016/j.aquaculture.2007.07.231
Eaves AA, Palmer AR (2003) Reproduction: widespread cloning in echinoderm larvae. Nature 425:146
Folt C, Chen C, Moore M, Burnaford J (1999) Synergism and antagonism among multiple stressors. Limnol Oceanogr 44:864–877
Foo SA, Sparks KM, Uthicke S, Karelitz S, Barker M, Byrne M, Lamare M (2016) Contributions of genetic and environmental variance in early development of the Antarctic sea urchin Sterechinus neumayeri in response to increased ocean temperature and acidification. Mar Biol 163:130
Fujita Y, Yamasu K, Suyemitsu T, Ishihara K (1994) A protein that binds an exogastrula-inducing peptide, EGIP-D, in the hyaline layer of sea urchin embryos. Dev Growth Differ 36:275–280. https://doi.org/10.1111/j.1440-169X.1994.00275.x
Greenwood P, Bennett T (1981) Some effects of temperature-salinity combinations on the early development of the sea urchin Parechinus angulosus (Leske). Fertilization. J Exp Mar Biol Ecol 51:119–131
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Harley CD, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJ, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9:228–241
Helmuth B, Kingsolver JG, Carrington E (2005) Biophysics, physiological ecology, and climate change: does mechanism matter? Annu Rev Physiol 67:177–201
IPCC (2014) Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. IPCC, Geneva, Switzerland
Ishihara K, Tonegawa Y, Suyemitsu T, Kubo H (1982) The blastocoelic fluid of sea urchin embryo induces exogastrulation. J Exp Zool Part A Ecol Genet Physiol 220:227–233
Karelitz SE, Uthicke S, Foo SA, Barker MF, Byrne M, Pecorino D, Lamare MD (2017) Ocean acidification has little effect on developmental thermal windows of echinoderms from Antarctica to the tropics. Glob Change Biol 23:657–672. https://doi.org/10.1111/gcb.13452
Kelly MW, Padilla-Gamiño JL, Hofmann GE (2013) Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Glob Change Biol 19:2536–2546
Kinoshita K, Fujii Y, Fujita Y, Yamasu K, Suyemitsu T, Ishihara K (1992) Maternal exogastrula-inducing peptides (EGIPs) and their changes during development in the sea urchin Anthocidaris crassispina. Dev Growth Differ 34:661–668. https://doi.org/10.1111/j.1440-169X.1992.tb00034.x
Knott KE, Balser EJ, Jaeckle WB, Wray GA (2003) Identification of asteroid genera with species capable of larval cloning. Biol Bull 204:246–255
Koehl M, Cooper T (2015) Swimming in an unsteady world. Integr Comp Biol 55:683–697. https://doi.org/10.1093/icb/icv092
Kuo ES, Sanford E (2009) Geographic variation in the upper thermal limits of an intertidal snail: implications for climate envelope models. Mar Ecol Prog Ser 388:137–146
Lee T, Leung W, Ginn E (2008) Rainfall projections for Hong Kong based on the IPCC fourth assessment report. Hong Kong Meteorol Soc Bull 18:12–22
Lu X, Wu R (2005) UV induces reactive oxygen species, damages sperm, and impairs fertilisation in the sea urchin Anthocidaris crassispina. Mar Biol 148:51–57
Mao J-Q, Wong KT, Lee JH, Choi K (2011) Tidal flushing time of marine fish culture zones in Hong Kong. China Ocean Eng 25:625–643
Matese JC, Black S, McClay DR (1997) Regulated exocytosis and sequential construction of the extracellular matrix surrounding the sea urchin zygote. Dev Biol 186:16–26. https://doi.org/10.1006/dbio.1997.8585
McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR (2015) Marine defaunation: animal loss in the global ocean. Science 347:1255641
Metaxas A (1998) The effect of salinity on larval survival and development in the sea urchin Echinometra lucunter. Invertebr Reprod Dev 34:323–330. https://doi.org/10.1080/07924259.1998.9652667
Metaxas A, Young CM (1998) Behaviour of echinoid larvae around sharp haloclines: effects of the salinity gradient and dietary conditioning. Mar Biol 131:443–459
Morley S, Nguyen K, Peck L, Lai C-H, Tan K (2016) Can acclimation of thermal tolerance, in adults and across generations, act as a buffer against climate change in tropical marine ectotherms? J Therm Biol 68:195–199
Okazaki K (1956) Exogastrulation induced by calcium deficiency in the sea urchin, Pseudocentrotus depressus. Dev Growth Differ 3:23–36
Pechenik JA, Pearse JS, Qian P-Y (2007) Effects of salinity on spawning and early development of the tube-building polychaete Hydroides elegans in Hong Kong: not just the sperm’s fault? Biol Bull 212:151–160
Przeslawski R, Byrne M, Mellin C (2015) A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob Change Biol 21:2122–2140
Qiu J-W, Qian P-Y (1999) Tolerance of the barnacle Balanus amphitrite amphitrite to salinity and temperature stress: effects of previous experience. Mar Ecol Prog Ser 188:123–132
Roller RA, Stickle WB (1985) Effects of salinity on larval tolerance and early developmental rates of four species of echinoderms. Can J Zool 63:1531–1538. https://doi.org/10.1139/z85-227
Roller RA, Stickle WB (1993) Effects of temperature and salinity acclimation of adults on larval survival, physiology, and early development of Lytechinus variegatus (Echinodermata: Echinoidea). Mar Biol 116:583–591
Runcie DE, Garfield DA, Babbitt CC, Wygoda JA, Mukherjee S, Wray GA (2012) Genetics of gene expression responses to temperature stress in a sea urchin gene network. Mol Ecol 21:4547–4562
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Sparks KM, Foo SA, Uthicke S, Byrne M, Lamare M (2017) Paternal identity influences response of Acanthaster planci embryos to ocean acidification and warming. Coral Reefs 36:325–338. https://doi.org/10.1007/s00338-016-1505-1
Strathmann MF (1987) Reproduction and development of marine invertebrates of the northern Pacific coast: data and methods for the study of eggs, embryos, and larvae. University of Washington Press, Seattle
Sunday JM, Crim RN, Harley CDG, Hart MW (2011) Quantifying rates of evolutionary adaptation in response to ocean acidification. PLoS One 6:e22881. https://doi.org/10.1371/journal.pone.0022881
Takahashi T, Hoshi M, Asahina É (1977) Exogastrulation induced by chilling in sea urchin larvae. Dev Growth Differ 19:131–137
Todgham AE, Stillman JH (2013) Physiological responses to shifts in multiple environmental stressors: relevance in a changing world. Integr Comp Biol 53:539–544
Tomanek L, Helmuth B (2002) Physiological ecology of rocky intertidal organisms: a synergy of concepts. Integr Comp Biol 42:771–775
Urriago JD, Wong JCY, Dumont CP, Qiu J-W (2016) Reproduction of the short-spined sea urchin Heliocidaris crassispina (Echinodermata: Echinoidea) in Hong Kong with a subtropical climate. Reg Stud Mar Sci 8:445–453. https://doi.org/10.1016/j.rsma.2016.03.005
Vaschenko M, Zhang Z, Lam P, Wu R (1999) Toxic effects of cadmium on fertilizing capability of spermatozoa, dynamics of the first cleavage and pluteus formation in the sea urchin Anthocidaris crassispina (Agassiz). Mar Pollut Bull 38:1097–1104
Vaughn D (2009) Predator-induced larval cloning in the sand dollar Dendraster excentricus: might mothers matter? Biol Bull 217:103–114
Vaughn D (2010) Why run and hide when you can divide? Evidence for larval cloning and reduced larval size as an adaptive inducible defense. Mar Biol 157:1301–1312
Vickery MS, McClintock JB (2000) Effects of food concentration and availability on the incidence of cloning in planktotrophic larvae of the sea star Pisaster ochraceus. Biol Bull 199:298–304
Wai T-C, Williams GA (2005) The relative importance of herbivore-induced effects on productivity of crustose coralline algae: sea urchin grazing and nitrogen excretion. J Exp Mar Biol Ecol 324:141–156
Yamasu K, Watanabe H, Kohchi C, Soma G-I, Mizuno D-I, Akasaka K, Shimada H, Suyemitsu T, Ishihara K (1995) Molecular cloning of a cDNA that encodes the precursor to several exogastrula-inducing peptides, epidermal-growth-factor-related polypeptides of the sea urchin Anthocidaris crassispina. Eur J Biochem 228:515–523. https://doi.org/10.1111/j.1432-1033.1995.0515n.x
Zhao B (2002) Larval biology and ecology of a non-indigenous species, the slipper limpet Crepidula onyx. The Hong Kong University of Science and Technology, Hong Kong
Acknowledgements
We thank the reviewers for their inputs, Y. K. Tam and L. W. Pang for their technical assistance during this study, C. Yau, N. Dorey and J. Ngo for their input on the manuscript.
Funding
This study is supported by the Research Grant Council, University Grants Committee, Hong Kong (Project no. 26102515) to KC and partially supported by the Croucher Foundation, Hong Kong.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: M. Byrne.
Reviewed by D. Bögner and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mak, K.KY., Chan, K.Y.K. Interactive effects of temperature and salinity on early life stages of the sea urchin Heliocidaris crassispina. Mar Biol 165, 57 (2018). https://doi.org/10.1007/s00227-018-3312-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3312-4